A wound is defined as a disruption of the anatomical or functional continuity of living tissue. The majority of wound healing research is focused on the skin, which is the body’s most susceptible organ and is affected by environmental interactions. New topical formulations that are physically, chemically, and biologically stable have drawn increasing attention from scientists in recent decades. Inorganic and organic molecules are encased and interpenetrated by a liquid in the gel formulation. The purpose of these pharmaceutical formulations is to minimize adverse effects while achieving targeted drug delivery. The gel-based system should be washable, non-staining, and stable at room temperature. Being simple to administer and transfer, they have the major benefit of avoiding the hepatic first pass. The use of topical drug delivery systems is growing, and many medications have been effectively administered this way for both systemic and local action. Topical antifungal therapy is thought to be the first line of treatment for superficial fungal infections of the skin, which are very common [
1]. Compounds with antifungal activity against yeasts, molds, or both can be found naturally or artificially produced. Since both fungi and mammalian cells are eukaryotes, antifungal drugs that prevent the synthesis of proteins, RNA, and DNA may be harmful to mammalian cells [
2]. There are numerous topical antifungals with low systemic side effects and high efficacy available. The well-known azole antifungals miconazole, clotrimazole, and ketoconazole have been used widely to treat dermatophytoses. Whereas ketoconazole works well for treating seborrheic dermatitis, miconazole and clotrimazole are effective in treating cutaneous candidiasis [
3]. The two most recent azoles to come to light are efinaconazole and luliconazole. Efinaconazole has been approved for the treatment of onychomycosis, along with tavaborole, a novel agent with a unique mechanism of action. Luliconazole has been approved for the treatment of tinea corporis, tinea cruris, and tinea pedis. A broad-spectrum antifungal drug called econazole nitrate is used to treat skin infections brought on by different pathogenic dermatophyte species. It is applied to treat candidiasis [
4]. Fungal infections typically affect the mucous membranes, skin, hair, and nails, but they can also infect the lungs or other sections of your body. Some individuals, typically those with compromised immune systems, develop potentially fatal systemic candidiasis when Candida invades deeper tissues in addition to the blood. One of the most frequent species of Aspergillus that sickens immunocompromised people is
Aspergillus fumigatus. Although fungus infections of the skin, hair, and nails are normally not dangerous, they can take some time to fully heal even with treatment [
5]. Nowadays, a range of antifungal creams are used to treat various mycotic infections and dermatological conditions. Nevertheless, it has been demonstrated that a large number of these fungal infections are resistant to treatment and control measures. Furthermore, patients receiving topical treatment may experience local reactions such as burning, erythema, stinging, pruritic rash, and tenderness [
6]. Additional issues with the creams include failed stability tests, chemical stability, or physical emulsion separation brought on by the imidazole salt’s salting effect when used at concentrations of at least 1%. Topical administration of gels at pathological locations offers major advantages over cream and ointment in terms of faster absorption and direct drug release from medicine. The use of single-phase gel is common in skin care products [
7]. Econazole irritates the gastrointestinal tract if taken orally. Topical gel can maximize drug concentration at the infected site of action and avoid the first-pass effect [
8]. An ideal topical formulation should spread evenly and leave no residue behind. Carbopol
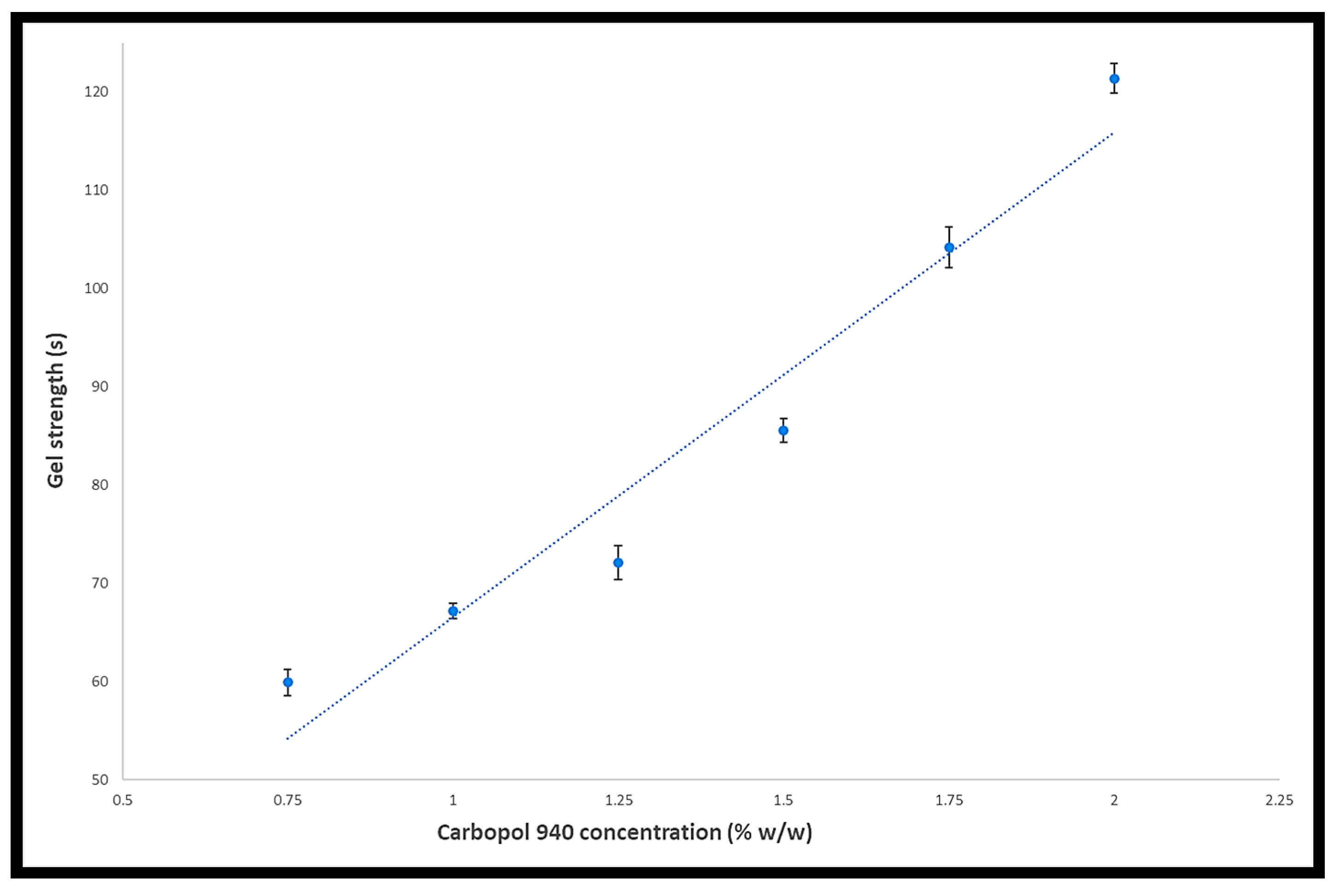
® 940 is frequently used in gel-based formulations due to its exceptional viscosity building properties (even at low concentrations) and resistance to microbial development [
9]. Econazole nitrate is commonly taken once or twice a day for 14 days, in the morning and evening, and up to 42 days in serious infections. Since econazole nitrate is a BCS class 2 drug with low solubility and high permeability, its solubility must be improved. The most difficult issue facing the pharmaceutical industry is solubility and permeability, which affects more than 65% of newly discovered medicinal agents as they fall into BCS classes 2 and 4 [
10]. The poor solubility of econazole nitrate can be solved by incorporating it in a topical gel using propylene glycol as a plasticizer, Capmul
® MCM C8 as an emollient and solubilizer, methyl and propyl paraben as preservatives, and Carbopol
® 940 as a gelling agent. The goal of this work was to develop a novel gel-based topical formulation of econazole nitrate (
Figure S1) that could be readily scaled up in a single pot to treat possible yeast and fungal infections. The physicochemical properties of the prepared formulations were evaluated, and in vitro release and mechanism studies were carried out to evaluate the behavior of the developed system.