3D-Printed Hydrogel for Diverse Applications: A Review
Abstract
:1. Introduction
2. Hydrogels: An Overview
2.1. Classification of Hydrogels
2.1.1. Natural Hydrogels
2.1.2. Synthetic Hydrogels
2.1.3. Hybrid Hydrogels
2.1.4. Crosslinked Hydrogels
2.2. Properties of Hydrogels
2.3. Designing Hydrogel Formulations for 3D Printing
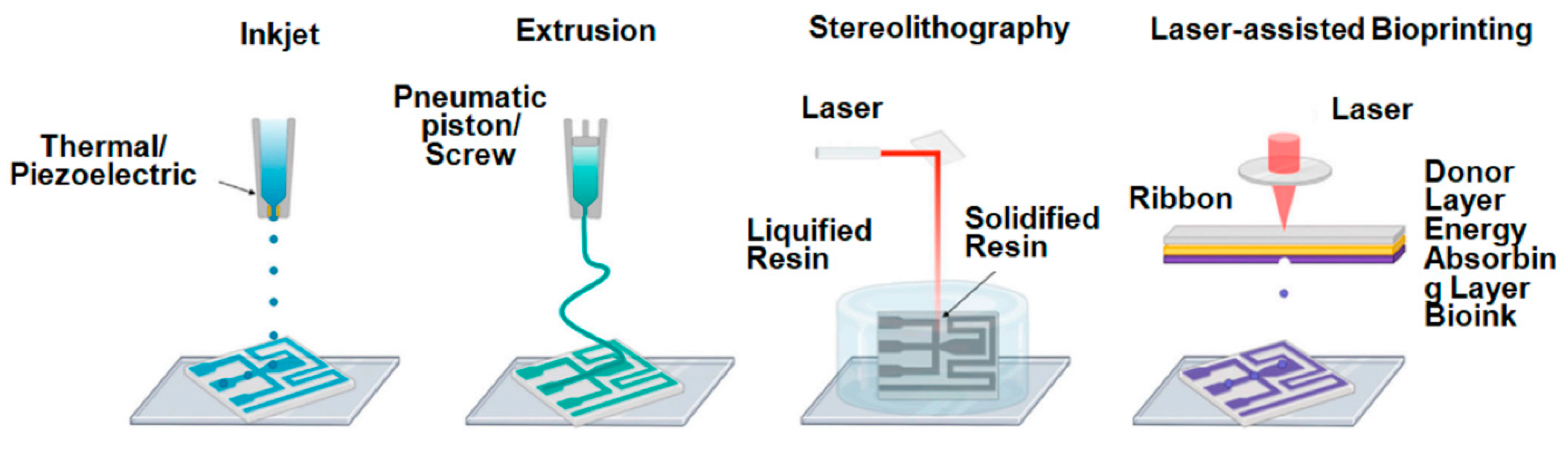
3. 3D Printing Methods for Hydrogels
3.1. Extrusion-Based Printing
3.2. Inkjet-Based Printing
3.3. Stereolithography (SLA)
3.4. Digital Light Processing (DLP)
4. Applications of 3D-Printed Hydrogels
4.1. Role of a 3D-Printed Hydrogel in Biomedical Applications
4.1.1. Tissue Engineering
4.1.2. Regenerative Medicine
4.1.3. Drug Delivery
4.2. Hydrogel 3D Printing across Industries
4.2.1. Food Industry
4.2.2. Cosmetic Industry
4.2.3. Electronics
5. Current Challenges and Future Trends in Hydrogel 3D Printing
5.1. Current Challenges
5.2. Future Trends and Advancements
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gajakosh, A.; Erannagari, S.; Kumar, R.S.; Thyagaraj, N.R.; Mallaradhya, H.M.; Rudresha, S. An Overview of 3D-Printed Smart Polymers and Composites. In Development, Properties, and Industrial Applications of 3D Printed Polymer Composites; IGI Glohal: Hershey, PA, USA, 2023; pp. 130–148. [Google Scholar]
- Blanco, I. The use of composite materials in 3D printing. J. Compos. Sci. 2020, 4, 42. [Google Scholar] [CrossRef]
- Tasneem, I.; Ariz, A.; Bharti, D.; Haleem, A.; Javaid, M.; Bahl, S. 3D printing technology and its significant applications in the context of healthcare education. J. Ind. Integr. Manag. 2023, 8, 113–130. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Yang, D. Recent advances in hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent advances in extrusion-based 3D printing for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1701161. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Hussain, C.M. Materials and Technologies for Flexible and Wearable Sensors. In Flexible and Wearable Sensors: Materials, Technologies, and Challenges; Gupta, R.K., Ed.; CRC Press: Roca Raton, FL, USA, 2023. [Google Scholar]
- Agrawal, A.; Hussain, C.M. Wearable Metal-Air Batteries. In Metal-Air Batteries: Principles, Progress, and Perspectives; Gupta, R.K., Ed.; CRC Press: Roca Raton, FL, USA, 2023; pp. 335–346. [Google Scholar]
- Wang, Z.; Jin, X.; Dai, R.; Holzman, J.F.; Kim, K. An ultrafast hydrogel photocrosslinking method for direct laser bioprinting. RSC Adv. 2016, 6, 21099–21104. [Google Scholar] [CrossRef]
- Mo, X.; Ouyang, L.; Xiong, Z.; Zhang, T. Advances in digital light processing of hydrogels. Biomed. Mater. 2022, 17, 042002. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, M.D.; Sharma, N.K.; Barhoumi, Z.; Chen, X. Printability of 3D printed hydrogel scaffolds: Influence of hydrogel composition and printing parameters. Appl. Sci. 2019, 10, 292. [Google Scholar] [CrossRef]
- Pavan Kalyan, B.G.; Kumar, L. 3D printing: Applications in tissue engineering, medical devices, and drug delivery. Aaps Pharmscitech 2022, 23, 92. [Google Scholar] [CrossRef]
- Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Shanmugam, D.K.; Dasan, A.; Mosas, K.K.A. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Zheng, Q.; Wu, Z.L. Recent advances in 3D printing of tough hydrogels: A review. Compos. Part B Eng. 2022, 238, 109895. [Google Scholar] [CrossRef]
- Xin, P.; Han, S.; Huang, J.; Zhou, C.; Zhang, J.; You, X.; Wu, J. Naturalokra-based hydrogel for chronic diabetic wound healing. Chin. Chem. Lett. 2023, 34, 108125. [Google Scholar] [CrossRef]
- Haghbin, M.; Malekshah, R.E.; Sobhani, M.; Izadi, Z.; Haghshenas, B.; Ghasemi, M.; Kalani, B.S.; Samadian, H. Fabrication and characterization of Persian gum-based hydrogel loaded with gentamicin-loaded natural zeolite: An in vitro and in silico study. Int. J. Biol. Macromol. 2023, 235, 123766. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, F.; Ranaldi, R.; Bruno, L.; Casieri, C.; Rugnini, L.; Spreti, N. Biodeterioration of stone monuments: Studies on the influence of bioreceptivity on cyanobacterial biofilm growth and on the biocidal efficacy of essential oils in natural hydrogel. Sci. Total Environ. 2023, 870, 161901. [Google Scholar] [CrossRef]
- Huang, C.; Ye, Q.; Dong, J.; Li, L.; Wang, M.; Zhang, Y.; Wang, X.; Wang, P.; Jiang, Q. Biofabrication of natural Au/bacterial cellulose hydrogel for bone tissue regeneration via in-situ fermentation. Smart Mater. Med. 2023, 4, 1–14. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, L.; Zhang, J.; Luo, H. Constructions and properties of physically cross-linked hydrogels based on natural polymers. Polym. Rev. 2023, 63, 574–612. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Du, W.; Zhao, J.; Ling, G.; Zhang, P. Chitosan-based high-strength supramolecular hydrogels for 3D bioprinting. Int. J. Biol. Macromol. 2022, 219, 545–557. [Google Scholar] [CrossRef]
- Cai, F.F.; Heid, S.; Boccaccini, A.R. Potential of Laponite® incorporated oxidized alginate–gelatin (ADA-GEL) composite hydrogels for extrusion-based 3D printing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1090–1104. [Google Scholar] [CrossRef]
- Van Velthoven, M.J.; Gudde, A.N.; Arendsen, E.; Roovers, J.P.; Guler, Z.; Oosterwijk, E.; Kouwer, P.H. Growth Factor Immobilization to Synthetic Hydrogels: Bioactive bFGF-Functionalized Polyisocyanide Hydrogels. Adv. Healthc. Mater. 2023, 12, 2301109. [Google Scholar] [CrossRef]
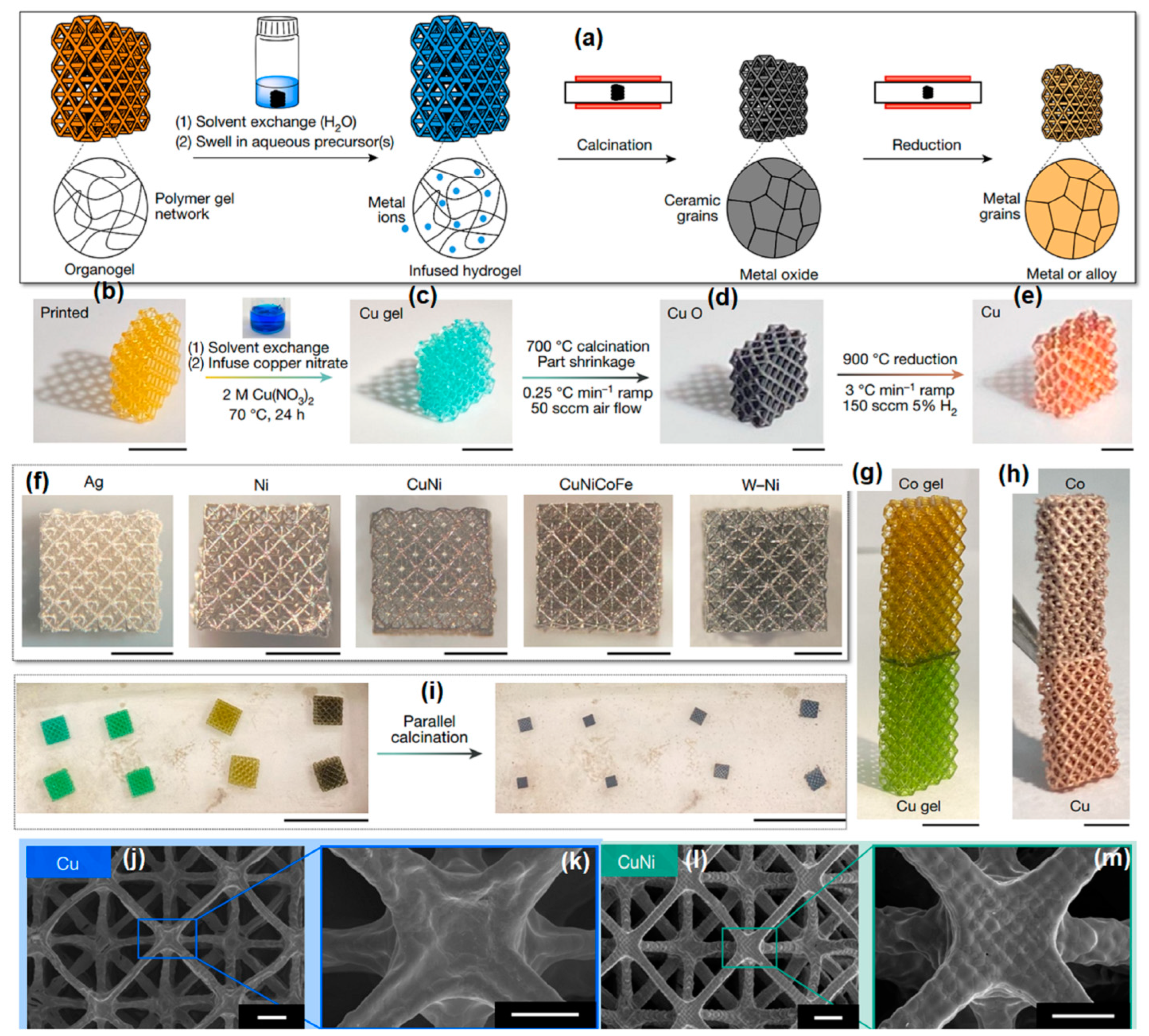
- Saccone, M.A.; Gallivan, R.A.; Narita, K.; Yee, D.W.; Greer, J.R. Additive manufacturing of micro-architected metals via hydrogel infusion. Nature 2022, 612, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D. Biodegradability of bio-based and synthetic hydrogels as sustainable soil amendments: A review. J. Appl. Polym. Sci. 2023, 140, e53655. [Google Scholar] [CrossRef]
- Meyer, L.E.; Horváth, D.; Vaupel, S.; Meyer, J.; Alcalde, M.; Kara, S. A 3D printable synthetic hydrogel as an immobilization matrix for continuous synthesis with fungal peroxygenases. React. Chem. Eng. 2023, 8, 984–988. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Li, J.; Li, J.; Zhao, S.; Liu, W.; Liu, T.; Wang, J.; Liu, Y. Nanocellulose/PEGDA aerogel scaffolds with tunable modulus prepared by stereolithography for three-dimensional cell culture. J. Biomater. Sci. Polym. Ed. 2019, 30, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Luo, Y.; Li, Z.; Tan, L.; Liu, X.; Li, C.; Zheng, Y.; Cui, Z.; Yeung, K.W.K.; Liang, Y.; et al. Antibacterial hybrid hydrogels. Macromol. Biosci. 2021, 21, 2000252. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Liao, J.; Shi, K.; Jia, Y.; Wu, Y.; Qian, Z. Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion. Bioact. Mater. 2021, 6, 2221–2230. [Google Scholar] [CrossRef]
- Choi, S.; Choi, Y.; Kim, J. Anisotropic hybrid hydrogels with superior mechanical properties reminiscent of tendons or ligaments. Adv. Funct. Mater. 2019, 29, 1904342. [Google Scholar] [CrossRef]
- Yang, X.; Yao, Y.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. 3D macroporous oxidation-resistant Ti3C2Tx MXene hybrid hydrogels for enhanced supercapacitive performances with ultralong cycle life. Adv. Funct. Mater. 2022, 32, 2109479. [Google Scholar] [CrossRef]
- Iwanaga, S.; Hamada, Y.; Tsukamoto, Y.; Arai, K.; Kurooka, T.; Sakai, S.; Nakamura, M. Design and Fabrication of Mature Engineered Pre-Cardiac Tissue Utilizing 3D Bioprinting Technology and Enzymatically Crosslinking Hydrogel. Materials 2022, 15, 7928. [Google Scholar] [CrossRef]
- Ianchis, R.; Alexa, R.L.; Gifu, I.C.; Marin, M.M.; Alexandrescu, E.; Constantinescu, R.; Serafim, A.; Nistor, C.L.; Petcu, C. Novel Green Crosslinked Salecan Hydrogels and Preliminary Investigation of Their Use in 3D Printing. Pharmaceutics 2023, 15, 373. [Google Scholar] [CrossRef]
- Farsheed, A.C.; Thomas, A.J.; Pogostin, B.H.; Hartgerink, J.D. 3D Printing of Self-Assembling Nanofibrous Multidomain Peptide Hydrogels. Adv. Mater. 2023, 35, 2210378. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the progress of hydrogel-based 3D printing: Correlating rheological properties with printing behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef]
- Amorim, P.A.; d’Ávila, M.A.; Anand, R.; Moldenaers, P.; Van Puyvelde, P.; Bloemen, V. Insights on shear rheology of inks for extrusion-based 3D bioprinting. Bioprinting 2021, 22, e00129. [Google Scholar] [CrossRef]
- Kokol, V.; Pottathara, Y.B.; Mihelčič, M.; Perše, L.S. Rheological properties of gelatine hydrogels affected by flow-and horizontally-induced cooling rates during 3D cryo-printing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126356. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, Y.W.; Jung, W.K.; Oh, J.; Nam, S.Y. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J. Mech. Behav. Biomed. Mater. 2019, 98, 187–194. [Google Scholar] [CrossRef]
- Townsend, J.M.; Beck, E.C.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 2019, 91, 126–140. [Google Scholar] [CrossRef]
- Zhou, M.; Lee, B.H.; Tan, Y.J.; Tan, L.P. Microbial transglutaminase induced controlled crosslinking of gelatin methacryloyl to tailor rheological properties for 3D printing. Biofabrication 2019, 11, 025011. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Mora-Boza, A.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; San Román, J. Glycerylphytate as an ionic crosslinker for 3D printing of multi-layered scaffolds with improved shape fidelity and biological features. Biomater. Sci. 2020, 8, 506–516. [Google Scholar] [CrossRef]
- Huang, L.; Du, X.; Fan, S.; Yang, G.; Shao, H.; Li, D.; Zhang, Y. Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores. Carbohydr. Polym. 2019, 221, 146–156. [Google Scholar] [CrossRef]
- Sheikhi, M.; Rafiemanzelat, F.; Ghodsi, S.; Moroni, L.; Setayeshmehr, M. 3D printing of jammed self-supporting microgels with alternative mechanism for shape fidelity, crosslinking and conductivity. Addit. Manuf. 2022, 58, 102997. [Google Scholar] [CrossRef]
- Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef]
- Bedell, M.L.; Torres, A.L.; Hogan, K.J.; Wang, Z.; Wang, B.; Melchiorri, A.J.; Grande-Allen, K.J.; Mikos, A.G. Human gelatin-based composite hydrogels for osteochondral tissue engineering and their adaptation into bioinks for extrusion, inkjet, and digital light processing bioprinting. Biofabrication 2022, 14, 045012. [Google Scholar] [CrossRef]
- Pose-Boirazian, T.; Martínez-Costas, J.; Eibes, G. 3D Printing: An Emerging Technology for Biocatalyst Immobilization. Macromol. Biosci. 2022, 22, 2200110. [Google Scholar] [CrossRef]
- Rothbauer, M.; Eilenberger, C.; Spitz, S.; Bachmann, B.E.M.; Kratz, S.R.A.; Reihs, E.I.; Windhager, R.; Toegel, S.; Ertl, P. Recent advances in additive manufacturing and 3D bioprinting for organs-on-A-chip and microphysiological systems. Front. Bioeng. Biotechnol. 2022, 10, 837087. [Google Scholar] [CrossRef]
- Cheng, Y.; Shi, X.; Jiang, X.; Wang, X.; Qin, H. Printability of a cellulose derivative for extrusion-based 3D printing: The application on a biodegradable support material. Front. Mater. 2020, 7, 86. [Google Scholar] [CrossRef]
- Zhou, S.; Han, C.; Ni, Z.; Yang, C.; Ni, Y.; Lv, Y. Gelatin-oxidized nanocellulose hydrogels suitable for extrusion-based 3D bioprinting. Processes 2022, 10, 2216. [Google Scholar] [CrossRef]
- Dong, L.; Bu, Z.; Xiong, Y.; Zhang, H.; Fang, J.; Hu, H.; Liu, Z.; Li, X. Facile extrusion 3D printing of gelatine methacrylate/Laponite nanocomposite hydrogel with high concentration nanoclay for bone tissue regeneration. Int. J. Biol. Macromol. 2021, 188, 72–81. [Google Scholar] [CrossRef]
- Falcone, G.; Mazzei, P.; Piccolo, A.; Esposito, T.; Mencherini, T.; Aquino, R.P.; Del Gaudio, P.; Russo, P. Advanced printable hydrogels from pre-crosslinked alginate as a new tool in semi solid extrusion 3D printing process. Carbohydr. Polym. 2022, 276, 118746. [Google Scholar] [CrossRef]
- Murphy, R.D.; Kimmins, S.; Hibbitts, A.J.; Heise, A. 3D-extrusion printing of stable constructs composed of photoresponsive polypeptide hydrogels. Polym. Chem. 2019, 10, 4675–4682. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Z.; Kang, S.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D.; He, J. Cellulose hydrogel skeleton by extrusion 3D printing of solution. Nanotechnol. Rev. 2020, 9, 345–353. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in extrusion 3D bioprinting: A focus on multicomponent hydrogel-based bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef]
- Ramezani, H.; Mohammad Mirjamali, S.; He, Y. Simulations of extrusion 3D printing of chitosan hydrogels. Appl. Sci. 2022, 12, 7530. [Google Scholar] [CrossRef]
- Suntornnond, R.; Ng, W.L.; Huang, X.; Yeow CH, E.; Yeong, W.Y. Improving printability of hydrogel-based bio-inks for thermal inkjet bioprinting applications via saponification and heat treatment processes. J. Mater. Chem. B 2022, 10, 5989–6000. [Google Scholar] [CrossRef]
- Stella, G.; Saitta, L.; Ongaro, A.E.; Cicala, G.; Kersaudy-Kerhoas, M.; Bucolo, M. Advanced Technologies in the Fabrication of a Micro-Optical Light Splitter. Micro 2023, 3, 338–352. [Google Scholar] [CrossRef]
- Saitta, L.; Arcadio, F.; Celano, G.; Cennamo, N.; Zeni, L.; Tosto, C.; Cicala, G. Design and manufacturing of a surface plasmon resonance sensor based on inkjet 3D printing for simultaneous measurements of refractive index and temperature. Int. J. Adv. Manuf. Technol. 2023, 124, 2261–2278. [Google Scholar] [CrossRef]
- Saitta, L.; Cutuli, E.; Celano, G.; Tosto, C.; Stella, G.; Cicala, G.; Bucolo, M. A Regression Approach to Model Refractive Index Measurements of Novel 3D Printable Photocurable Resins for Micro-Optofluidic Applications. Polymers 2023, 15, 26901. [Google Scholar] [CrossRef]
- Marzano, C.; Arcadio, F.; Minardo, A.; Zeni, L.; Del Prete, D.; Cicala, G.; Saitta, L. Towards V-shaped Plasmonic probes made by exploiting 3D printers and UV-cured optical adhesives for Medical applications. In Proceedings of the IEEE International Workshop on Metrology for Industry 4.0 & IoT 2023, Brescia, Italy, 6–8 June 2023. [Google Scholar]
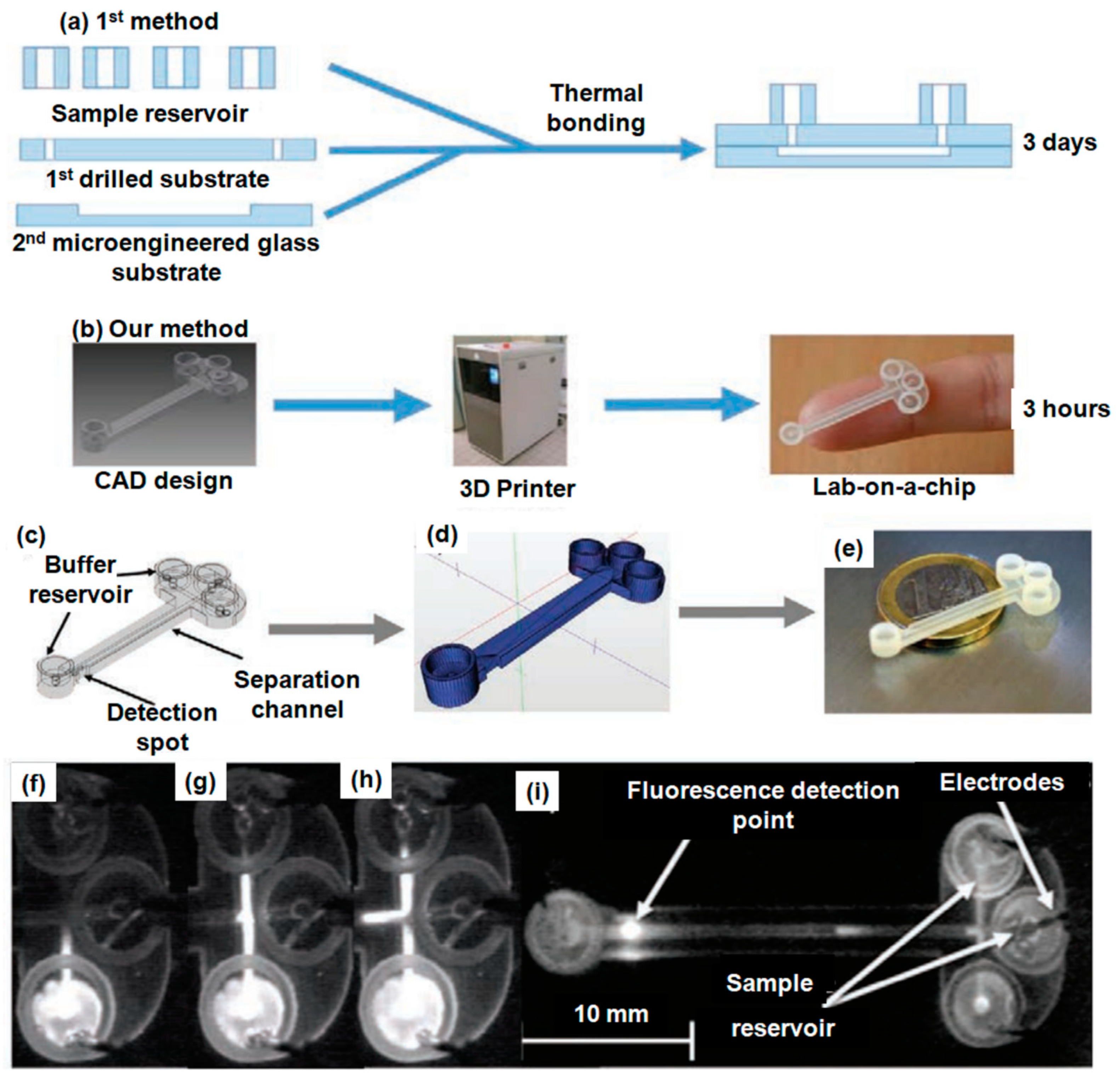
- Adamski, K.; Kubicki, W.; Walczak, R. 3D printed electrophoretic lab-on-chip for DNA separation. Procedia Eng. 2016, 168, 1454–1457. [Google Scholar] [CrossRef]
- Negro, A.; Cherbuin, T.; Lutolf, M.P. 3D inkjet printing of complex, cell-laden hydrogel structures. Sci. Rep. 2018, 8, 17099. [Google Scholar] [CrossRef]
- Tetyczka, C.; Brisberger, K.; Reiser, M.; Zettl, M.; Jeitler, R.; Winter, C.; Kolb, D.; Leitinger, G.; Spoerk, M.; Roblegg, E. Itraconazole nanocrystals on hydrogel contact lenses via inkjet printing: Implications for ophthalmic drug delivery. ACS Appl. Nano Mater. 2022, 5, 9435–9446. [Google Scholar] [CrossRef]
- Teo, M.Y.; Kee, S.; RaviChandran, N.; Stuart, L.; Aw, K.C.; Stringer, J. Enabling free-standing 3D hydrogel microstructures with microreactive inkjet printing. ACS Appl. Mater. Interfaces 2019, 12, 1832–1839. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ohta, S.; Nakamura, M.; Ito, T. 3D inkjet printing of star block copolymer hydrogels cross-linked using various metallic ions. RSC Adv. 2017, 7, 55571–55576. [Google Scholar] [CrossRef]
- Jiao, T.; Lian, Q.; Zhao, T.; Wang, H.; Li, D. Preparation, mechanical and biological properties of inkjet printed alginate/gelatin hydrogel. J. Bionic Eng. 2021, 18, 574–583. [Google Scholar] [CrossRef]
- Yoon, S.; Park, J.A.; Lee, H.R.; Yoon, W.H.; Hwang, D.S.; Jung, S. Inkjet–spray hybrid printing for 3D freeform fabrication of multilayered hydrogel structures. Adv. Healthc. Mater. 2018, 7, 1800050. [Google Scholar] [CrossRef]
- Peng, X.; Liu, T.; Zhang, Q.; Shang, C.; Bai, Q.W.; Wang, H. Surface patterning of hydrogels for programmable and complex shape deformations by ion inkjet printing. Adv. Funct. Mater. 2017, 27, 1701962. [Google Scholar] [CrossRef]
- Duffy, G.L.; Liang, H.; Williams, R.L.; Wellings, D.A.; Black, K. 3D reactive inkjet printing of poly-ɛ-lysine/gellan gum hydrogels for potential corneal constructs. Mater. Sci. Eng. C 2021, 131, 112476. [Google Scholar] [CrossRef]
- Chen, F.; Gu, S.; Zhang, Q.; Liu, T.; Liu, Z.; Kuang, T. A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods. e-Polymers 2022, 22, 332–341. [Google Scholar] [CrossRef]
- Kalossaka, L.M.; Mohammed, A.A.; Sena, G.; Barter, L.; Myant, C. 3D printing nanocomposite hydrogels with lattice vascular networks using stereolithography. J. Mater. Res. 2021, 36, 4249–4261. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: A controlled release study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef]
- Burke, G.; Devine, D.M.; Major, I. Effect of stereolithography 3D printing on the properties of PEGDMA hydrogels. Polymers 2020, 12, 2015. [Google Scholar] [CrossRef]
- Magalhães LS, S.; Santos FE, P.; Elias CD, M.V.; Afewerki, S.; Sousa, G.F.; Furtado, A.S.; Marciano, F.R.; Lobo, A.O. Printing 3D hydrogel structures employing low-cost stereolithography technology. J. Funct. Biomater. 2020, 11, 12. [Google Scholar] [CrossRef]
- Alketbi, A.S.; Shi, Y.; Li, H.; Raza, A.; Zhang, T. Impact of PEGDA photopolymerization in micro-stereolithography on 3D printed hydrogel structure and swelling. Soft Matter 2021, 17, 7188–7195. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, Y.; Zhao, Q.; Wu, J. A new stereolithographic 3D printing strategy for hydrogels with a large mechanical tunability and self-weldability. Addit. Manuf. 2022, 50, 102563. [Google Scholar] [CrossRef]
- Hosseinabadi, H.G.; Nieto, D.; Yousefinejad, A.; Fattel, H.; Ionov, L.; Miri, A.K. Ink material selection and optical design considerations in DLP 3D printing. Appl. Mater. Today 2023, 30, 101721. [Google Scholar] [CrossRef]
- Ding, H.; Dong, M.; Zheng, Q.; Wu, Z.L. Digital light processing 3D printing of hydrogels: A minireview. Mol. Syst. Des. Eng. 2022, 7, 1017–1029. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Q.; Ma, S.; Wu, J. DLP 3D printed hydrogels with hierarchical structures post-programmed by lyophilization and ionic locking. Mater. Horiz. 2023, 10, 179–186. [Google Scholar] [CrossRef]
- Dong, M.; Han, Y.; Hao, X.P.; Yu, H.C.; Yin, J.; Du, M.; Zheng, Q.; Wu, Z.L. Digital Light Processing 3D Printing of Tough Supramolecular Hydrogels with Sophisticated Architectures as Impact-Absorption Elements. Adv. Mater. 2022, 34, 2204333. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, C.; Xie, W.; Tang, A.; Liu, W. An effective DLP 3D printing strategy of high strength and toughness cellulose hydrogel towards strain sensing. Carbohydr. Polym. 2023, 315, 121006. [Google Scholar] [CrossRef] [PubMed]
- Cafiso, D.; Septevani, A.A.; Noè, C.; Schiller, T.; Pirri, C.F.; Roppolo, I.; Chiappone, A. 3D printing of fully cellulose-based hydrogels by digital light processing. Sustain. Mater. Technol. 2022, 32, e00444. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Slita, A.; Backman, O.; Gounani, Z.; Rosqvist, E.; Peltonen, J.; Willför, S.; Xu, C.; Rosenholm, J.M.; et al. Digital light processing (DLP) 3D-fabricated antimicrobial hydrogel with a sustainable resin of methacrylated woody polysaccharides and hybrid silver-lignin nanospheres. Green Chem. 2022, 24, 2129–2145. [Google Scholar] [CrossRef]
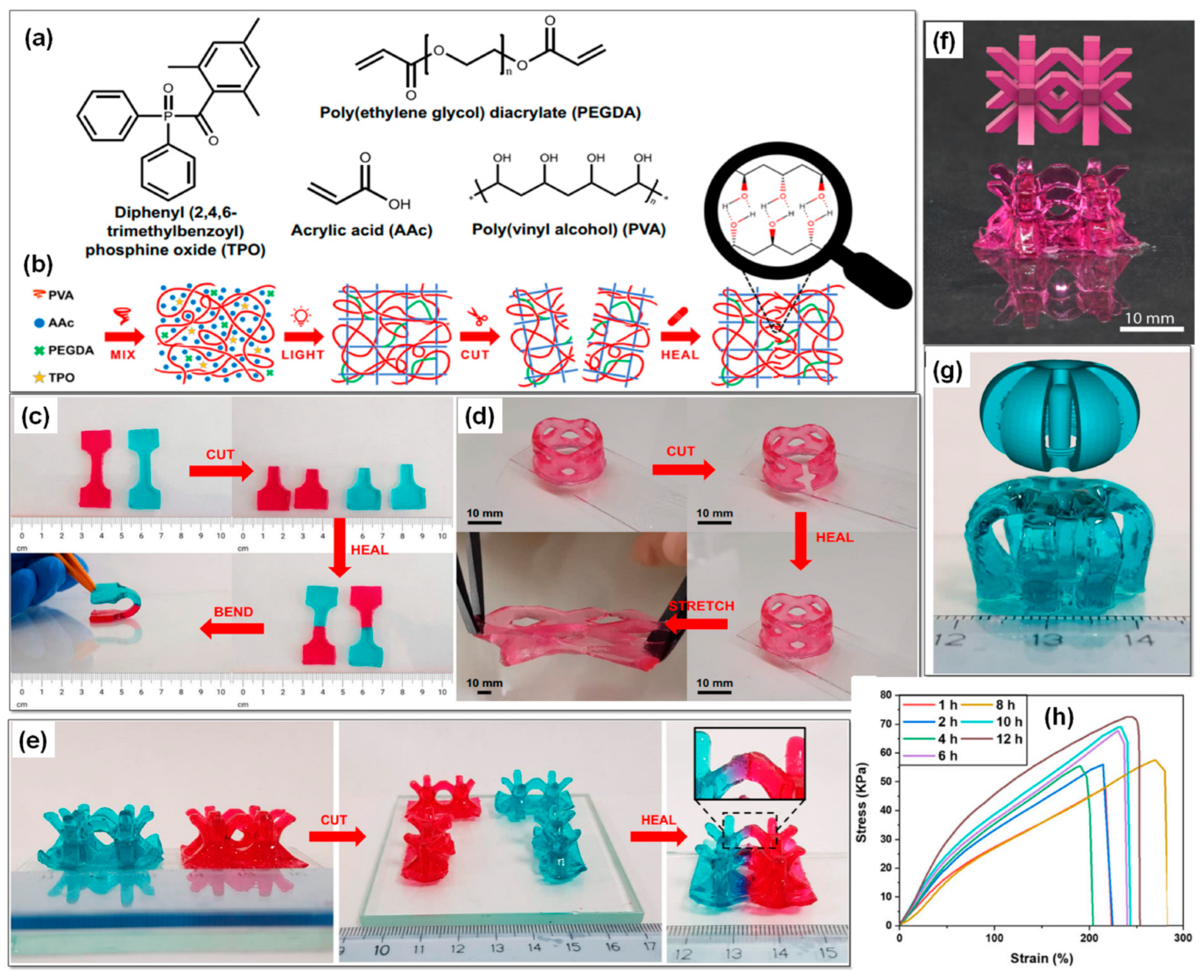
- Caprioli, M.; Roppolo, I.; Chiappone, A.; Larush, L.; Pirri, C.F.; Magdassi, S. 3D-printed self-healing hydrogels via Digital Light Processing. Nat. Commun. 2021, 12, 2462. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Huang, Y.; Yang, X.; Gou, M. Antheraea pernyi silk fibroin bioinks for digital light processing 3D printing. Int. J. Bioprinting 2023, 9, 760. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, N.; Rong, Y.; Zhu, L.; Huang, X. 3D-printed high-toughness double network hydrogels via digital light processing. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128329. [Google Scholar] [CrossRef]
- Lopez-Larrea, N.; Criado-Gonzalez, M.; Dominguez-Alfaro, A.; Alegret, N.; Agua, I.D.; Marchiori, B.; Mecerreyes, D. Digital Light 3D Printing of PEDOT-Based Photopolymerizable Inks for Biosensing. ACS Appl. Polym. Mater. 2022, 4, 6749–6759. [Google Scholar] [CrossRef]
- Athukorala, S.S.; Tran, T.S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.K.; Roy Choudhury, N. 3D Printable Electrically Conductive Hydrogel Scaffolds for Biomedical Applications: A Review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Su, C.; Chen, Y.; Tian, S.; Lu, C.; Lv, Q. Natural Materials for 3D Printing and Their Applications. Gels 2022, 8, 748. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mettu, S.; Roy Choudhury, N.; Dutta, N.K. 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Pharmaceuticals 2022, 15, 1282. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Garcia, C.N.; Gillooley, S.; Tayebi, L. 3D Printing of Hybrid-Hydrogel Materials for Tissue Engineering: A Critical Review. Regen. Eng. Transl. Med. 2023, 9, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Huang, X.; Huang, D.; Wei, X.; Chen, W. Progress in 3D printing for bone tissue engineering: A review. J. Mater. Sci. 2022, 57, 12685–12709. [Google Scholar] [CrossRef]
- Varaprasad, K.; Karthikeyan, C.; Yallapu, M.M.; Sadiku, R. The significance of biomacromolecule alginate for the 3D printing of hydrogels for biomedical applications. Int. J. Biol. Macromol. 2022, 212, 561–578. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Huang, J.; Wu, J. Novel glucose-responsive antioxidant hybrid hydrogel for enhanced diabetic wound repair. ACS Appl. Mater. Interfaces 2022, 14, 7680–7689. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Qu, X.; Zhu, W.; Xiang, M.; Yang, J.; Zhang, K.; Wei, Y.; Chen, S. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat. Commun. 2014, 5, 3774. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, Y.; Wang, Z.; Liu, H.; Le, M.; Lin, C.; Wu, G.; Wang, L.; Shi, X.; Jia, Y.G.; et al. Polymerizable rotaxane hydrogels for three-dimensional printing fabrication of wearable sensors. Nat. Commun. 2023, 14, 1331. [Google Scholar] [CrossRef]
- Deptuła, M.; Zawrzykraj, M.; Sawicka, J.; Banach-Kopeć, A.; Tylingo, R.; Pikuła, M. Application of 3D-printed hydrogels in wound healing and regenerative medicine. Biomed. Pharmacother. 2023, 167, 115416. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Simon, M.; Rafailovich, M.H. Hydrogels for regenerative medicine. In Recent Advances in Biopolymers; IntechOpen: London, UK, 2016; Volume 105. [Google Scholar]
- Cernencu, A.I.; Dinu, A.I.; Stancu, I.C.; Lungu, A.; Iovu, H. Nanoengineered biomimetic hydrogels: A major advancement to fabricate 3D-printed constructs for regenerative medicine. Biotechnol. Bioeng. 2022, 119, 762–783. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Hasirci, N.; Kilic, C.; Kömez, A.; Bahcecioglu, G.; Hasirci, V. Hydrogels in regenerative medicine. In GELS Handbook: Fundamentals, Properties and Applications Volume 2: Applications of Hydrogels in Regenerative Medicine; World Scientific: London, UK, 2016; pp. 1–52. [Google Scholar]
- Aghamirsalim, M.; Mobaraki, M.; Soltani, M.; Kiani Shahvandi, M.; Jabbarvand, M.; Afzali, E.; Raahemifar, K. 3D printed hydrogels for ocular wound healing. Biomedicines 2022, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Shamma, R.N.; Sayed, R.H.; Madry, H.; El Sayed, N.S.; Cucchiarini, M. Triblock copolymer bioinks in hydrogel three-dimensional printing for regenerative medicine: A focus on pluronic F127. Tissue Eng. Part B Rev. 2022, 28, 451–463. [Google Scholar] [CrossRef]
- Jervis, P.J. Hydrogels in Regenerative Medicine and Other Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 3270. [Google Scholar] [CrossRef]
- Tayler, I.M.; Stowers, R.S. Engineering hydrogels for personalized disease modeling and regenerative medicine. Acta Biomater. 2021, 132, 4–22. [Google Scholar] [CrossRef]
- Heo, D.N.; Castro, N.J.; Lee, S.J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using a 3D printed microstructure incorporated with a hybrid nano hydrogel. Nanoscale 2017, 9, 5055–5062. [Google Scholar] [CrossRef]
- Foyt, D.A.; Norman, M.D.; Yu, T.T.; Gentleman, E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 2018, 7, 1700939. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Barroso, N.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. 3D printable self-healing hyaluronic acid/chitosan polycomplex hydrogels with drug release capability. Int. J. Biol. Macromol. 2021, 188, 820–832. [Google Scholar] [CrossRef]
- Larush, L.; Kaner, I.; Fluksman, A.; Tamsut, A.; Pawar, A.A.; Lesnovski, P.; Magdassi, S. 3D printing of responsive hydrogels for drug-delivery systems. J. 3D Print. Med. 2017, 1, 219–229. [Google Scholar] [CrossRef]
- Aguilar-de-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D printed drug delivery systems based on natural products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, C.; Wang, Z.; Luo, Y. 3D printed core-shell hydrogel fiber scaffolds with NIR-triggered drug release for localized therapy of breast cancer. Int. J. Pharm. 2020, 580, 119219. [Google Scholar] [CrossRef]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Zhang, J.; Wu, J.P.; Kirk, T.B.; Xu, J.; Xue, W. Three-dimensional printing of shape memory hydrogels with internal structure for drug delivery. Mater. Sci. Eng. C 2018, 84, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar]
- Park, S.M.; Kim, H.W.; Park, H.J. Callus-based 3D printing for food exemplified with carrot tissues and its potential for innovative food production. J. Food Eng. 2020, 271, 109781. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Junior MD, M.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E. Hydrogels based on ozonated cassava starch: Effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, S.P.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef]
- Finny, A.S.; Jiang, C.; Andreescu, S. 3D printed hydrogel-based sensors for quantifying UV exposure. ACS Appl. Mater. Interfaces 2020, 12, 43911–43920. [Google Scholar] [CrossRef]
- Diogo, G.S.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Cell-laden biomimetically mineralized shark-skin-collagen-based 3D printed hydrogels for the engineering of hard tissues. ACS Biomater. Sci. Eng. 2020, 6, 3664–3672. [Google Scholar] [CrossRef]
- de Oliveira, R.S.; Fantaus, S.S.; Guillot, A.J.; Melero, A.; Beck, R.C.R. 3D-printed products for topical skin applications: From personalized dressings to drug delivery. Pharmaceutics 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Norton, L.; Finny, A.S.; Andreescu, S. Easy-to-use and inexpensive sensors for assessing the quality and traceability of cosmetic antioxidants. Talanta 2020, 208, 120473. [Google Scholar] [CrossRef]
- Guo, B.; Zhong, Y.; Chen, X.; Yu, S.; Bai, J. 3D printing of electrically conductive and degradable hydrogel for epidermal strain sensor. Compos. Commun. 2023, 37, 101454. [Google Scholar] [CrossRef]
- Liu, H.; Du, C.; Liao, L.; Zhang, H.; Zhou, H.; Zhou, W.; Ren, T.; Sun, Z.; Lu, Y.; Nie, Z.; et al. Approaching intrinsic dynamics of MXenes hybrid hydrogel for 3D printed multimodal intelligent devices with ultrahigh superelasticity and temperature sensitivity. Nat. Commun. 2022, 13, 3420. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, X.; Zheng, Y.; Chen, K.; Zeng, W.; Wu, X. Recent advances in the 3D printing of electrically conductive hydrogels for flexible electronics. J. Mater. Chem. C 2022, 10, 5380–5399. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, F.; Chen, L.; Wei, X.; Xue, M.; Yang, F.; Jiang, S. 3D printing hydrogels for actuators: A review. Chin. Chem. Lett. 2021, 32, 2923–2932. [Google Scholar] [CrossRef]
- Chen, L.; Duan, G.; Zhang, C.; Cheng, P.; Wang, Z. 3D printed hydrogel for soft thermo-responsive smart window. Int. J. Extrem. Manuf. 2022, 4, 025302. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, H.; Zhao, F.; Zhou, X.; Shi, W.; Yu, G. Biomass-derived hybrid hydrogel evaporators for cost-effective solar water purification. Adv. Mater. 2020, 32, 1907061. [Google Scholar] [CrossRef]
- Wang, H.; Guo, J. Recent advances in 4D printing hydrogel for biological interfaces. Int. J. Mater. Form. 2023, 16, 55. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Park, Y.J.; Bae, H. Hydrogel production platform with dynamic movement using photo-crosslinkable/temperature reversible chitosan polymer and stereolithography 4D printing technology. Tissue Eng. Regen. Med. 2020, 17, 423–431. [Google Scholar] [CrossRef]


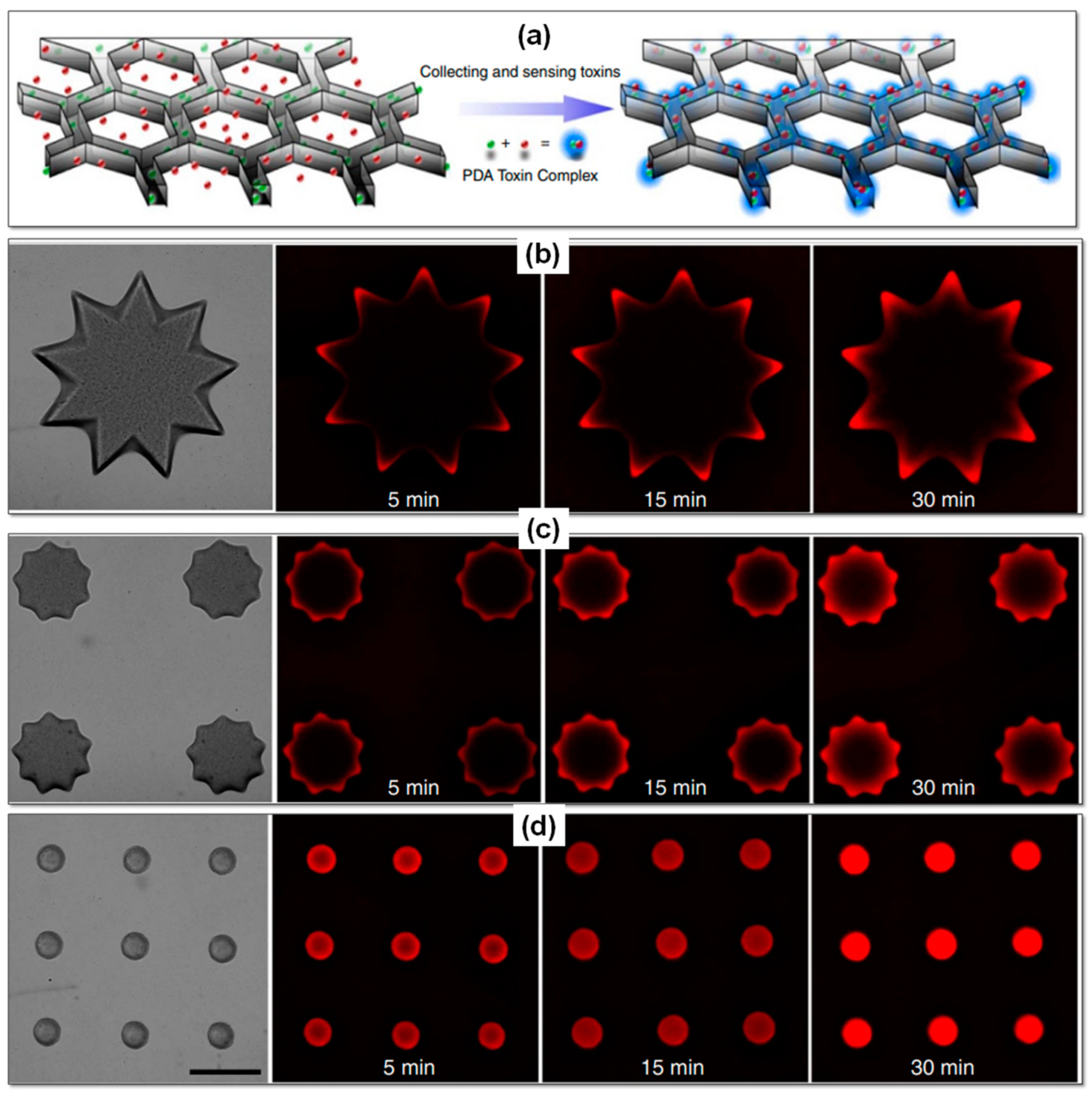
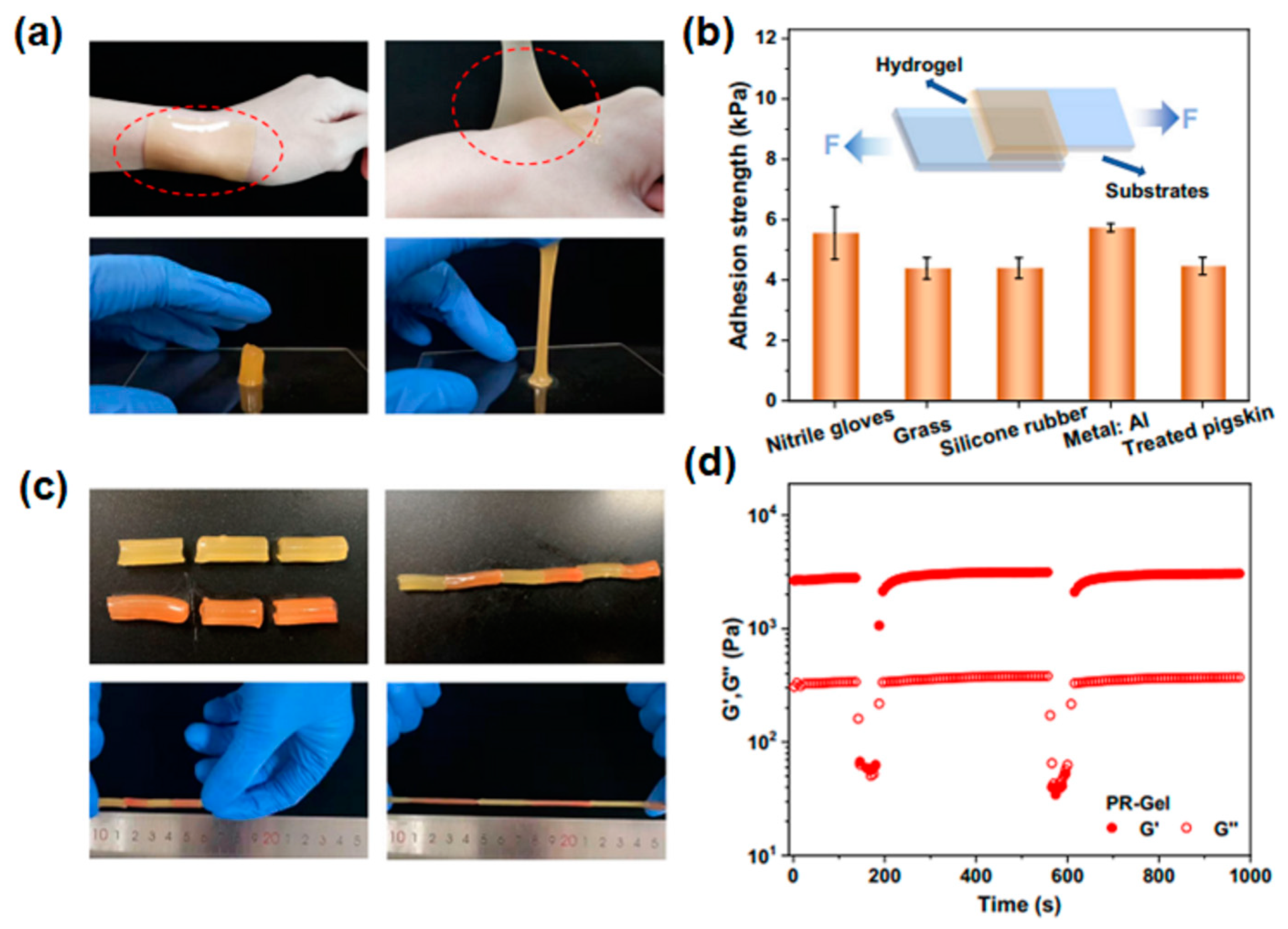
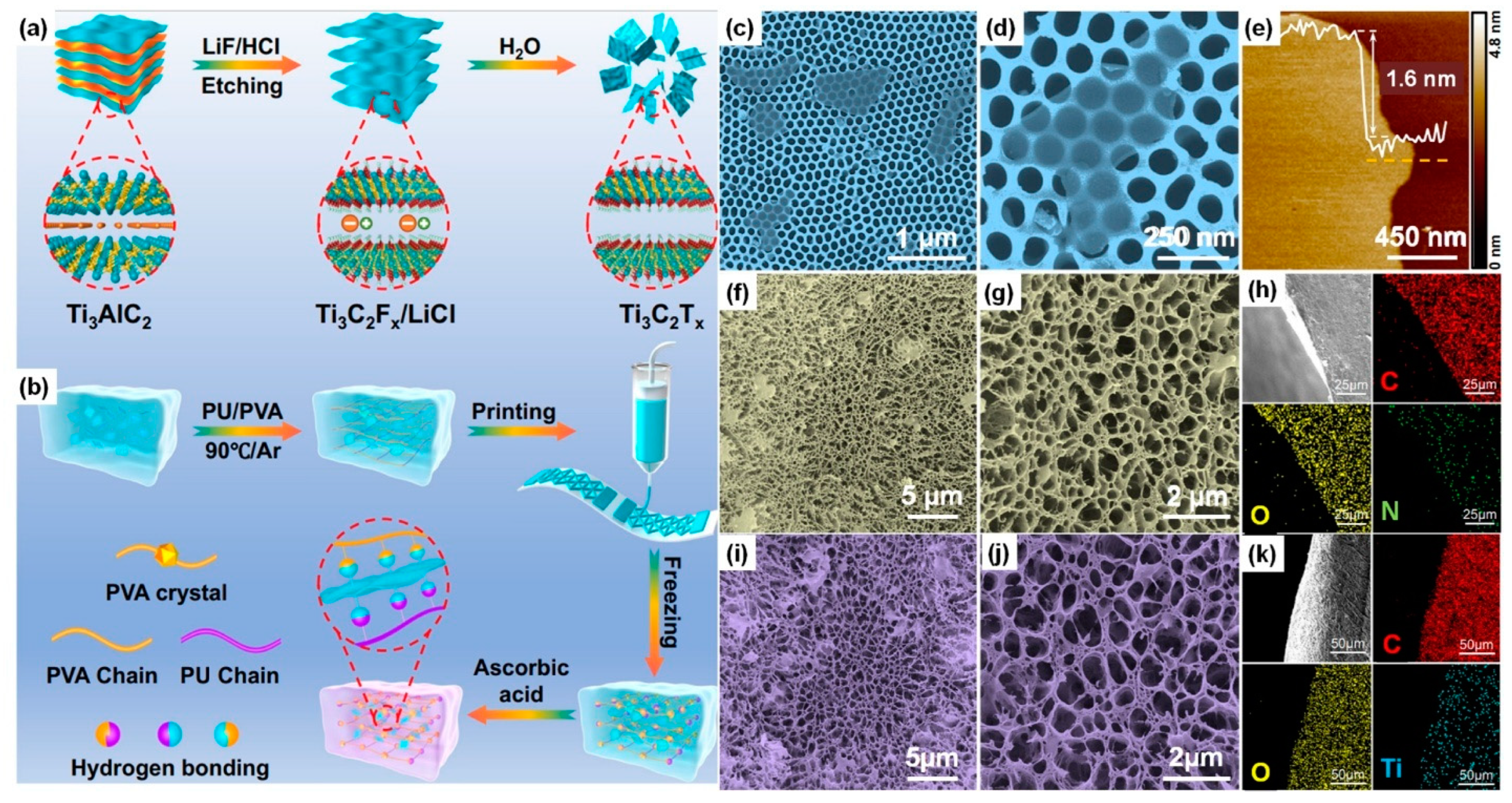
| Property | Natural Hydrogels | Synthetic Hydrogels | Hybrid Hydrogels |
|---|---|---|---|
| Preparation |
|
|
|
| Printability |
|
|
|
| Biocompatibility |
|
|
|
| Mechanical Properties |
|
|
|
| Property | Description | Applications |
|---|---|---|
| Water Absorption and Retention | Ability to absorb and retain a significant amount of water or biological fluids. | Wound dressings, contact lenses, diapers, and drug delivery systems. |
| Biocompatibility | Compatibility with living tissues, making hydrogels suitable for medical and biological applications. | Tissue engineering, drug delivery, wound healing, and surgical implants. |
| Tunable Mechanical Properties | Adjustability of mechanical characteristics like elasticity and stiffness for specific applications. | Cartilage replacements, soft tissue engineering, and drug delivery matrices. |
| Swelling Behavior | Controllable ability to swell in response to factors such as pH, temperature, or ionic strength. | Controlled drug release, biosensors, and wound dressings. |
| Permeability | Ability to allow the passage of certain substances while restricting others. | Controlled drug delivery, filtration membranes, and biosensors. |
| Responsive to Stimuli | Capability to undergo changes in volume or structure in response to external stimuli (e.g., temperature, pH, and light). | Smart drug delivery, biosensing, and controlled release systems. |
| Adhesive Properties | Ability to adhere to biological tissues, useful for applications like wound dressings and tissue adhesives. | Surgical adhesives, wound closures, and tissue engineering. |
| Versatility | Wide range of options in terms of composition and structure, allowing customization for various applications. | Tissue engineering scaffolds, wound dressings, and drug delivery systems. |
| Electrical Conductivity | Capability to conduct electricity, often achieved by incorporating conductive polymers or nanoparticles. | Biosensors, flexible electronics, and neural interfaces. |
| Parameter/Properties | Definition | Effect on 3D Printing |
|---|---|---|
| RHEOLOGICAL PROPERTIES | ||
| Viscosity | Viscosity measures a fluid’s resistance to flow. | Affects the ease of handling and deposition. |
| Shear-Thinning Behavior | Shear-thinning is the property where viscosity decreases under shear stress. | Facilitates smooth flow during printing; hydrogel becomes less viscous when subjected to shear stress, allowing for easy extrusion. |
| Thixotropy | Property where a material becomes less viscous over time under constant shear stress and recovers its viscosity when the stress is removed. | Allows the hydrogel to recover its original viscosity between printing layers, preventing spreading and maintaining structural integrity. |
| Viscoelasticity | Viscoelastic materials exhibit both viscous (flow) and elastic (deformation recovery) properties. | Affects the material’s response to stress and strain during printing and ensures the printed structure retains its shape after deposition. |
| Gelling Mechanisms | Refers to the process by which a liquid transforms into a gel or solid. | Determines speed and control of gelation, influencing the overall printing process and final construct. |
| Extrudability | The ease with which a material can be extruded or forced through a nozzle. | Affects the precision and control of material deposition during 3D printing. |
| Shape Retention | The ability of the material to maintain its intended shape after deposition. | Critical for achieving accurate and consistent layer-by-layer printing, ensuring the final structure matches the design. |
| GELATION KINETICS | ||
| Gelation Time | The time taken for a liquid to transition into a gel. | Affects the overall printing speed and duration of the 3D printing process. |
| Crosslinking Density | The concentration of crosslinks formed between polymer chains during gelation. | Affects the mechanical strength and stability of the resulting hydrogel. |
| Temperature | The degree of heat applied during the gelation process. | Affects the rate of chemical reactions or physical processes leading to gel formation. |
| Concentration of Crosslinking Agents | The amount of crosslinking agents, such as chemical initiators, present in the hydrogel formulation. | Higher concentrations typically result in faster gelation but may impact other material properties. |
| pH Level | The acidity or alkalinity of the hydrogel formulation. | Affects the ionization of functional groups and, hence, the gelation process. |
| Polymer Concentration | The concentration of polymer molecules in the hydrogel formulation. | Higher concentrations can lead to denser networks and affect the gelation time and mechanical properties. |
| Solvent Composition | The type and ratio of solvents used in the hydrogel formulation. | Solvent properties can impact the rate of gelation and the resulting structure of the hydrogel. |
| Initiator Concentration (Photochemical Gelation) | The concentration of photoinitiators in the hydrogel formulation. | Critical for photochemical gelation processes, where light triggers crosslink formation. |
| Stirring Rate (for Physical Gelation) | The speed at which the hydrogel components are mixed. | Affects the distribution of components and can influence the gelation time in physically gelled hydrogels. |
| Presence of Catalysts | Chemical substances that accelerate gelation reactions. | Help in enhancing the speed and efficiency of gelation processes. |
| PRINT FIDELITY | ||
| Layer Resolution | The thickness of each layer deposited during printing. | Finer resolutions lead to smoother surfaces and improved details. |
| Extruder Calibration | Adjusting the extruder to ensure accurate material deposition. | Prevents under- or over-extrusion, enhancing print accuracy. |
| Bed Leveling | Ensuring the print bed is perfectly level. | Prevents uneven layer heights, promoting uniform adhesion. |
| Print Speed | The speed at which the printer deposits material. | Optimizing print speed balances accuracy with efficiency; too fast can lead to errors. |
| Temperature Control | Maintaining consistent temperatures for the printer and printing material. | Fluctuations can affect material flow and layer adhesion. |
| Material Quality | The quality and consistency of the printing material. | Inconsistent materials may lead to variations in print quality. |
| Print Bed Adhesion | Ensuring the first layer adheres well to the print bed. | Proper adhesion prevents warping and helps maintain accurate layer alignment. |
| Support Structures | Temporary structures to support overhanging features. | Well-designed supports prevent deformations and maintain accuracy. |
| Cooling Systems | Fans or other cooling mechanisms to solidify layers quickly. | Proper cooling prevents overheating and improves feature definition. |
| Printer Rigidity | The stability and rigidity of the printer frame. | A stable frame reduces vibrations and ensures precise movements. |
| Print Orientation | The angle and direction in which the object is printed. | Optimal orientation minimizes overhangs and supports, improving print fidelity. |
| Filament Diameter | The diameter of the printing filament. | Accurate filament diameter ensures consistent material flow. |
| Environmental Conditions | Factors like temperature and humidity in the printing environment. | Extreme conditions can affect material properties and printing outcomes. |
| Print Design | The complexity and geometry of the printed object. | Complex designs may require specific settings for accurate printing. |
| Hydrogel Used | Applications/Remarks | Reference |
|---|---|---|
| Laponite® incorporated oxidized alginate–gelatin composite hydrogels | Integration of natural hydrogels into advanced manufacturing processes | Cai et al., (2021) [23] |
| Cellulose derivative | Biodegradable support material | Cheng, Y. et al., (2020) [53] |
| Gelatin-oxidized nanocellulose hydrogels | Bioprinting applications for tissue engineering | Zhou, S. et al., (2022) [54] |
| Gelatine methacrylate/Laponite nanocomposite hydrogel | High-concentration nanoclay for bone tissue regeneration | Dong, L. et al., (2021) [55] |
| Pre-crosslinked alginate | Advanced printable hydrogels | Falcone, G. et al., (2022) [56] |
| Photoresponsive polypeptide hydrogels | Stable constructs composed of photoresponsive polypeptide hydrogels | Murphy, R. D. et al., (2019) [57] |
| Cellulose hydrogel | Cellulose hydrogel skeleton usingextrusion 3D printing of solution | Hu, X. et al., (2020) [58] |
| Multicomponent hydrogel-based bioinks | Bioprinting for various applications using multicomponent hydrogel-based bioinks | Cui, X. et al., (2020) [59] |
| Chitosan hydrogels | Simulations of extrusion 3D printing of chitosan hydrogels | Ramezani, H. et al., (2022) [60] |
| Hydrogel Used | 3D Printing Technology | Applications/Remarks | Reference |
|---|---|---|---|
| Itraconazole nanocrystals on hydrogel | Inkjet Printing | Ophthalmic drug delivery | Tetyczka, C. et al., (2022) [68] |
| Hydrogel-based bioinks | Thermal inkjet bioprinting | Printability improvement via saponification and heat treatment processes | Suntornnond, R. et al., (2022) [61] |
| Hyaluronic acid hydrogel | Photolithography and light-cured inkjet printing methods | Comparing different methods for creating hyaluronic acid hydrogel micropatterns | Chen, F. et al., (2022) [75] |
| Hydrogel | 3D Inkjet Printing | Cell-laden structures Complex tissue engineering | Negro et al., (2018) [67] |
| Hydrogel | Microreactive Inkjet Printing | Free-standing 3D microstructures Microscale devices | Teo et al., (2019) [69] |
| Star block copolymer hydrogels | 3D Inkjet Printing | Cross-linked with metallic ions Structural materials | Nakagawa et al., (2017) [70] |
| Alginate/gelatin hydrogel | Inkjet Printing | Mechanical and biological properties Tissue engineering | Jiao et al., (2021) [71] |
| Multilayered hydrogel | Inkjet–Spray Hybrid Printing | Multilayered hydrogel structures Multifunctional constructs | Yoon et al., (2018) [72] |
| Multilayered hydrogel | Ion Inkjet Printing | Surface patterning for shape deformations Programmable structures | Peng et al., (2017) [73] |
| Poly-ɛ-lysine/gellan gum hydrogels | Reactive Inkjet Printing | Corneal constructs Ophthalmic applications | Duffy et al., (2021) [74] |
| Hydrogel Used | Applications/Remarks | Reference |
|---|---|---|
| Nanocellulose/PEGDA | 3D cell culture, tissue engineering | Tang, A. et al., (2019) [29] |
| Nanocomposite hydrogels | Creating hydrogel structures with vascular networks | Kalossaka, L.M. et al., (2021) [76] |
| Ascorbic acid-loaded hydrogels | Controlled drug release from 3D-printed hydrogels | Karakurt, I. et al., (2020) [77] |
| PEGDMA hydrogels | Investigating the impact of 3D printing on hydrogel properties | Burke, G. et al., (2020) [78] |
| Hydrogel structures | Low-cost 3D printing of hydrogel structures | Magalhães, L.S.S. et al., (2020) [79] |
| PEGDA hydrogels | Studying the impact of photopolymerization in micro-stereolithography | Alketbi, A.S. et al., (2021) [80] |
| Hydrogels with tunability | Developing hydrogels with mechanical tunability and self-welding properties | Sun, Z. et al., (2022) [81] |
| Hydrogel Used | Applications/Remarks | Reference |
|---|---|---|
| Hydrogels | Creating hydrogels with hierarchical structures | Sun, Z. et al., (2023) [84] |
| Supramolecular hydrogels | Tough supramolecular hydrogels with sophisticated architectures as impact-absorption elements | Dong, M. et al., (2022) [85] |
| Cellulose hydrogel | Cellulose hydrogel for strain sensing | Guo, Z. et al., (2023) [86] |
| Cellulose-based hydrogels | 3D printing of fully cellulose-based hydrogels usingDLP | Cafiso, D. et al., (2022) [87] |
| Antimicrobial hydrogel | Developing an antimicrobial hydrogel using sustainable resin and hybrid nanospheres | Wang, L. et al., (2022) [88] |
| Antheraea pernyi silk fibroin | Using silk fibroin bioinks for DLP 3D printing | Zhang, X. et al., (2023) [90] |
| Double-network hydrogels | 3D printing of high-toughness double network hydrogels | Xiang, Z. et al., (2022) [91] |
| PEDOT-based photopolymerizable inks | DLP 3D printing of PEDOT-based photopolymerizable inks for biosensing | Lopez-Larrea, N. et al., (2022) [92] |
| Extrusion-Based | Inkjet-Based | SLA | DLP | |
|---|---|---|---|---|
| Principle | Layer-by-layer extrusion | Droplet deposition | Photopolymerization | Photopolymerization |
| Resolution | Moderate | High | Very High | Very High |
| Material Compatibility | Wide range of hydrogel materials | Limited to specific hydrogel formulations | Limited choice due to compatibility | Limited choice due to compatibility |
| Speed | Moderate | Moderate to Fast | Moderate to Fast | Very Fast |
| Post-Processing | Often minimal | May require curing | Minimal | Minimal |
| Advantages |
|
|
|
|
| Limitations |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. https://doi.org/10.3390/gels9120960
Agrawal A, Hussain CM. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels. 2023; 9(12):960. https://doi.org/10.3390/gels9120960
Chicago/Turabian StyleAgrawal, Arpana, and Chaudhery Mustansar Hussain. 2023. "3D-Printed Hydrogel for Diverse Applications: A Review" Gels 9, no. 12: 960. https://doi.org/10.3390/gels9120960






