Advanced Drug Delivery Systems for Renal Disorders
Abstract
:1. Introduction
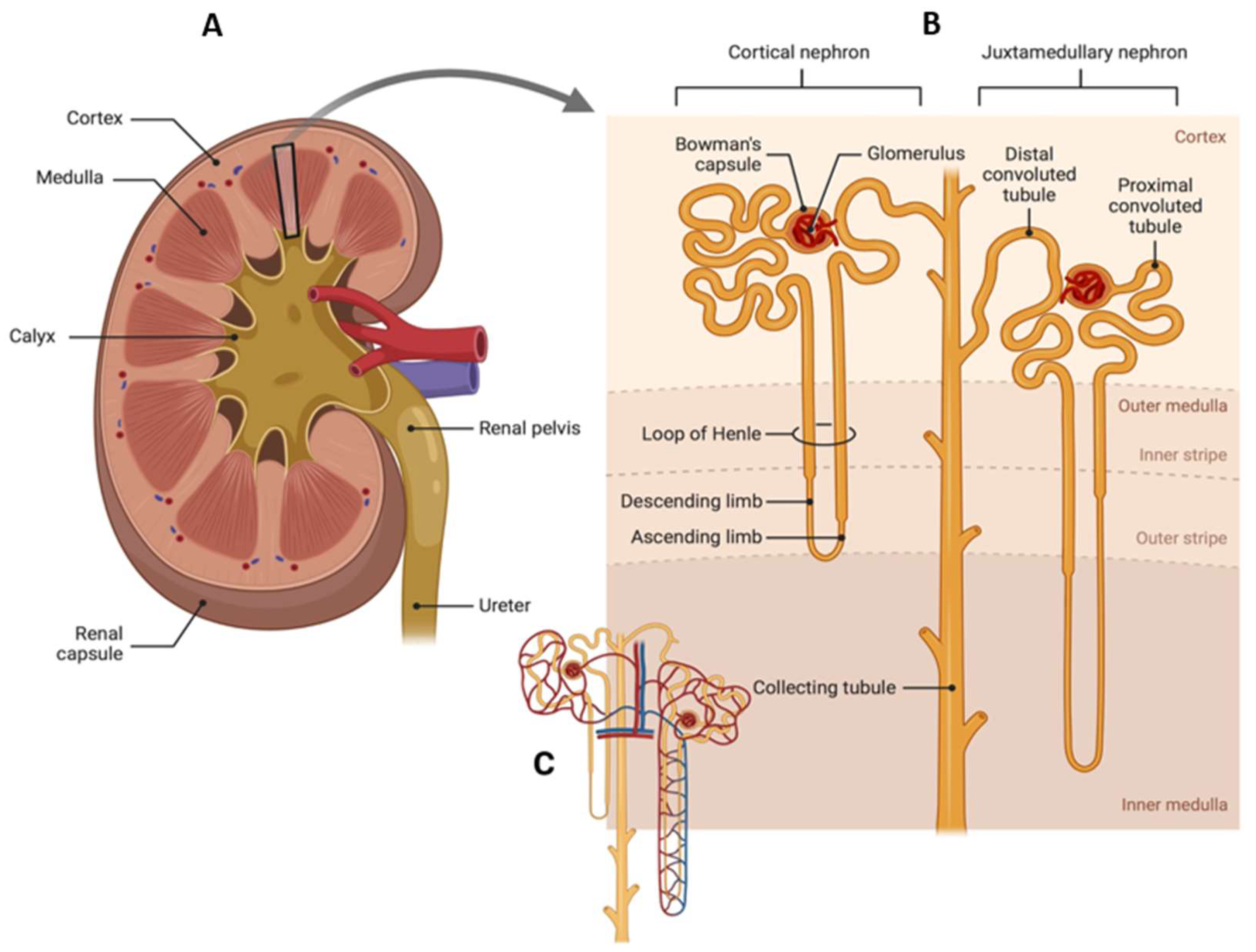
2. Kidney Anatomy and Function
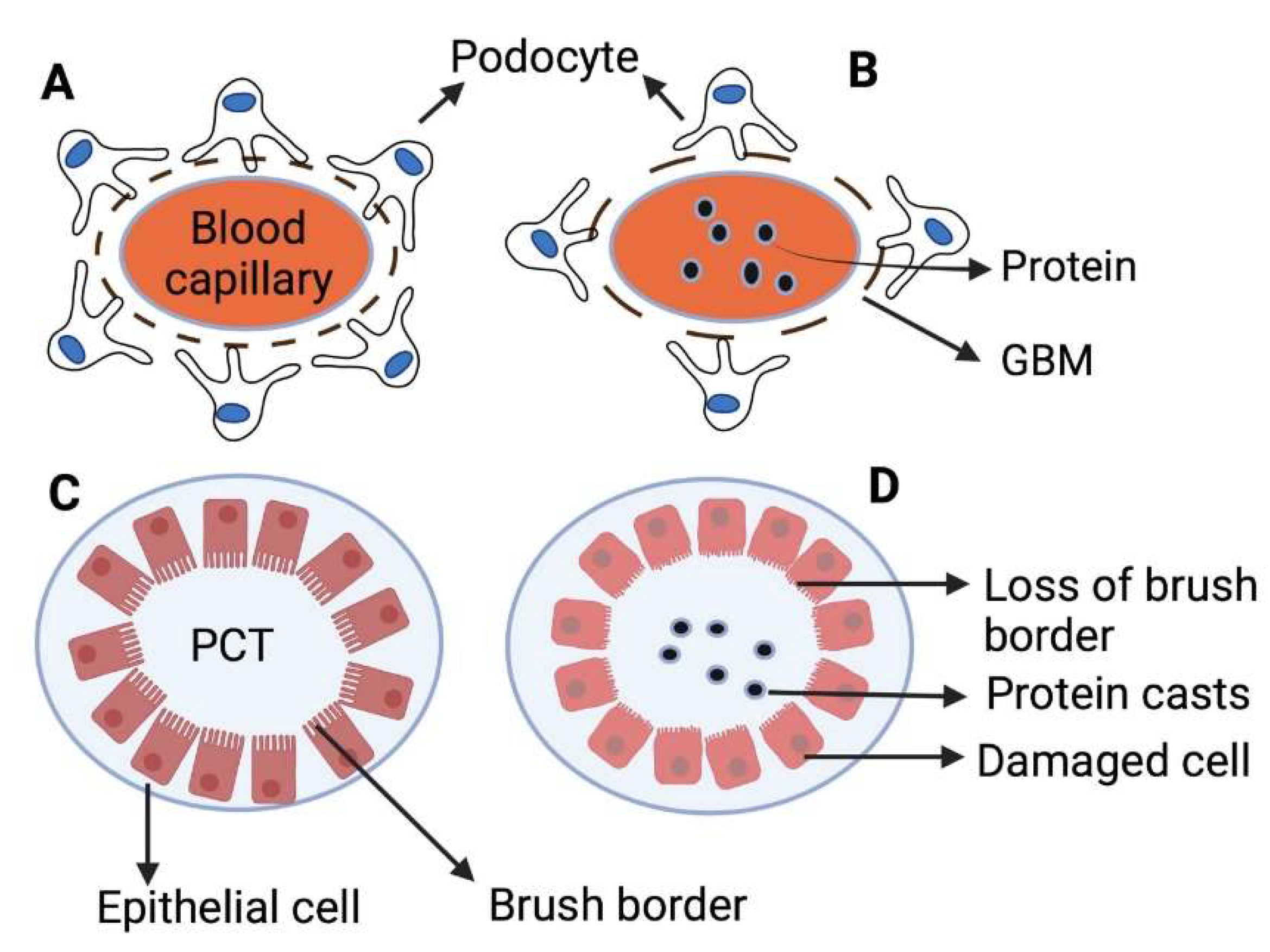
3. Renal Pathophysiology
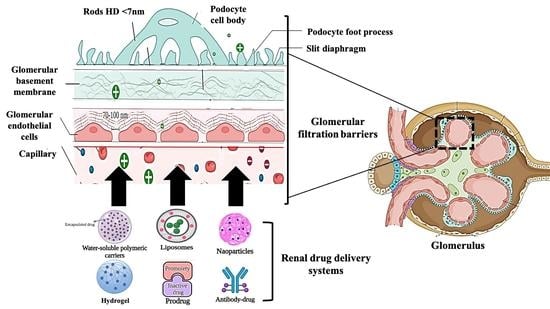
4. Kidney Drug Delivery Barriers
5. Delivery Sites of Renal Drug Delivery Systems
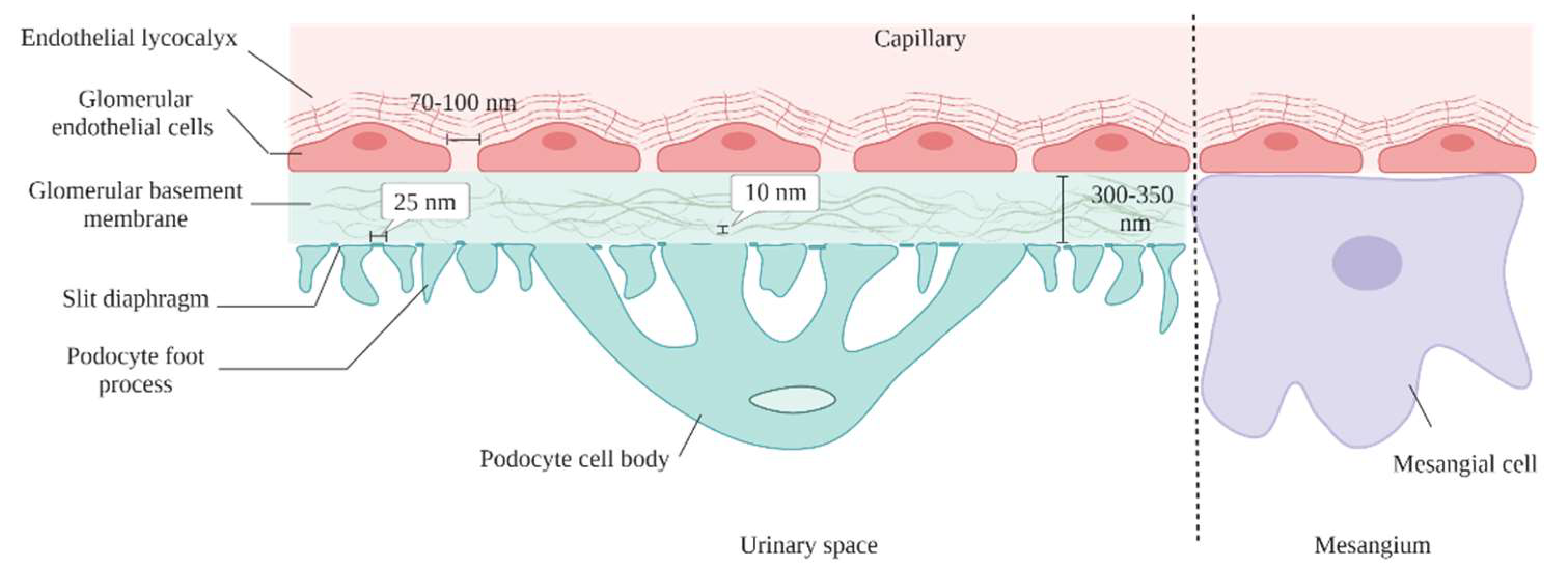
5.1. Glomerular Endothelial Cells
5.2. Glomerular Basement Membrane
5.3. Podocytes
5.4. Mesangial Cells
| Targeted Site | Target | Delivery System | Loaded Drug | Size (nm) | Disease | Refs. |
|---|---|---|---|---|---|---|
| Glomerular endothelial cells | E-selectin | Liposomes conjugated with anti-E-selectin antibodies | Dexamethas-one | 121 ± 20 | Glomerulonephritis | [64] |
| Glomerular basement membrane | - | cyclodextrin-containing polymer-based siRNA nanoparticles | siRNA | 60 to 100 | Normal | [81] |
| Mesangial cells | - | siRNA-loaded polycationic cyclodextrin nanoparticles (siRNA/CDPNPs) | siRNA | ~70 | Normal | [82] |
| Mesangial cells | - | Celastrol-albumin nanoparticles | Celastrol | 95 | (Thy1.1) Nephritis | [83] |
| Mesangial cells | - | PEG- (TRX-20)-modified liposomes | Triptolide | 100 | Membranous nephropathy | [84] |
| Podocyte | Neonatal Fc receptor | Bovine serum albumin-methylprednisolone conjugate Nanoparticles (BSA633-MP) | Prednisolone | 10 | Nephrotic syndrome | [74] |
| Podocyte | VCAM-1 receptor | Lipid-based nanocarrier SAINT-O-Somes | Rapamycin | 128 ± 4 | TNFα-activated podocytes (mimic the inflammatory condition) | [58] |
6. Nanoparticle Factors for Enhanced Renal Accumulation
6.1. Nanoparticle Size
| NPs | Size (nm) | Charge (mV) | Renal Accumulation (% ID) 24 h Post Injection * | Renal Clearance (% ID) | Refs. |
|---|---|---|---|---|---|
| [64Cu]Cu-1,4,7- triazacyclononane-triacetic acid tagged with near-infrared dye (IR800-CW)- silicon NPs | 2.4 ± 0.5 | 100% ID 24 h post injection | [87] | ||
| Glutathione-coated gold NPs (GS-AuNPs) Gold NPs coated with glutathione and cysteamine (GC-AuNPs) | GS-AuNPs = 2.1 ± 0.4 GC-AuNPs = 2.9 ± 0.3 | 40-50% ID 24 h post injection | [86] | ||
| Cysteine-coated gold NPs | 3.5 ± 0.9 | 8.8 ± 2.0 | More than 50% ID 24 h post injection | [88] | |
| Luminescent glutathione coated copper NPs (GS-CuNPs) | 2.2 | 0.6 | 78.5 ± 3.5% ID 24 h post injection | [89] | |
| Glutathione-coated silver NPs (GS-AgNPs) Glutathione-coated Au/Ag NPs (GS-Au/AgNPs) Glutathione-coated gold NPs (GS-Au) | ~3.1 | GS-AgNPs = 51.36% ID GS-Au/Ag(1)NPs = 52.99% ID GS-Au/Ag(2)NPs = 48.69% ID GS-AuNPs = 45.57% ID 48 h post injection. | [90] | ||
| Core-shell silica-based NPs (C dots) | 3.3 and 6.0 | C dots (3.3 nm) = 73% ID 48 h post injection C dots (6.0 nm) = 64% ID 48 h post injection | [91] | ||
| GS-[198Au]AuNPs | 3.0 ± 0.4 | ~50% ID 48 h post injection | [92] | ||
| Gold NPs | 1.4 | ~1.9 | [104] | ||
| Quantum dots | 5.6 | ~15% ID/g | [105] | ||
| Gold NPs | 3.1 | ~15% ID/g | [106] | ||
| Carbon nanotubes | 25 | 0.6% ID/g | [94] | ||
| PEGylated kidney-targeting peptide amphiphile micelles and PEGylated amphiphile micelles | Targeted micelles = 15 nm Non-targeted micelles = 12 nm | Targeted micelles = ~35% and non-targeted micelles = 26% of total fluorescence, in the kidneys | [95] | ||
| Copper sulfide nanodots | 5.6 | +2.9 | 95% ID 24 h post injection | [107] | |
| Silicon NPs | 2.4 | +5.4 | 100% ID 24 h post injection | [103] | |
| Gold NPs | 2.9 | −27 | 42% ID 24 h post injection | [86] | |
| Gold nanoparticles coated with cysteine (Cys-AuNPs) Gold nanoparticles coated with glycine-cysteine (Gly-Cys-AuNPs) | Cys-AuNPs = 2.69 ± 0.46 nm Gly-Cys-AuNPs = 3.12 ± 0.61 nm | Cys-AuNPs = −12.52 Gly-Cys-AuNPs = −27.33 | Cys-AuNPs = 21.5% ID 24 h post injection Gly-Cys-AuNPs = 41.6% ID 24 h post injection | [106] |
6.2. Nanoparticle Surface Charge
6.3. Nanoparticle Shape
| NPs | Shape | Size (nm) | Advantage over Control Particles | Refs. |
|---|---|---|---|---|
| Single walled carbon nanotubes | Rod | 1.2 × 100−1000 | 65% ID increase in renal clearance | [126] |
| Malleable poly(glycidyl methacrylate) (L-PGMA) | Rod | 43 | 4.2% ID/g increase in renal accumulation | [128] |
| Mesoporous silica NPs | Rod | 159 | 16% renal increase in renal accumulation | [129] |
| RNA nanosquare | Square | 10 | 66% increase in renal accumulation | [130] |
| Gold nanostar | Star | 55 | ~40% increase in renal accumulation | [131] |
6.4. Material Choice of Nanoparticles
7. Strategies of Renal Drug Delivery Systems
7.1. Small Molecule Prodrugs
7.1.1. Folate-Modified Prodrugs
7.1.2. Sugar-Modified Prodrugs
7.1.3. Amino Acid-Modified Prodrugs
7.2. Antibody Modified Carriers
7.3. Macromolecular Carriers
7.4. Water Soluble Polymeric Carriers
7.5. Nanoparticles
7.6. Liposomes
7.7. Hydrogel
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidwell, G.L.; Mahdi, F.; Shao, Q.; Logue, O.C.; Waller, J.P.; Reese, C.; Chade, A.R. A kidney-selective biopolymer for targeted drug delivery. Am. J. Physiol. Physiol. 2017, 312, F54–F64. [Google Scholar] [CrossRef] [PubMed]
- Saminathan, T.A.; Hooi, L.S.; Yusoff, M.F.M.; Ong, L.M.; Bavanandan, S.; Hasani, W.S.R.; Tan, E.Z.Z.; Wong, I.; Rifin, H.M.; Robert, T.G.; et al. Prevalence of chronic kidney disease and its associated factors in Malaysia; findings from a nationwide population-based cross-sectional study. BMC Nephrol. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, L.; Lu, H.; Kovacevic, N.; Thomas, R.; Lakshmanan, Y.; Cystatin, C. Neutrophil Gelatinase-associated Lipocalin, and Lysozyme C: Urinary Biomarkers for Detection of Early Kidney Dysfunction in Children with Urolithiasis. Urology 2020, 143, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-X.; Sun, X.; Gong, T.; Ding, H.; Fu, Y.; Zhang, Z.-R. Randomly 50%N-acetylated low molecular weight chitosan as a novel renal targeting carrier. J. Drug Target. 2007, 15, 269–278. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef]
- Ruebner, R.L.; Pradhan, M. Chronic kidney disease. In The 5-Minute Pediatric Consult, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 389, pp. 194–195. [Google Scholar]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Vanmassenhove, J.; Kielstein, J.; Jörres, A.; Van Biesen, W. Management of patients at risk of acute kidney injury. Lancet 2017, 389, 2139–2151. [Google Scholar] [CrossRef]
- Askenazi, D.; Selewski, D.; Willig, L.; Warady, B.A. Acute Kidney Injury and Chronic Kidney Disease. In Avery’s Diseases of the Newborn, 10th ed.; Massachusetts Medical Society: Waltham, MA, USA, 2018; Volume 371, pp. 1280–1300.e5. [Google Scholar]
- Sinha, A.; Bagga, A. Nephrotic Syndrome. Indian J. Pediatr. 2012, 79, 1045–1055. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Zhou, H.; Setia, O.; Liu, B.; Kanasaki, K.; Koya, D.; Dardik, A.; Fernandez-Hernando, C.; Goodwin, J. Loss of endothelial glucocorticoid receptor accelerates diabetic nephropathy. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Pesce, F.; Stea, E.D.; Rossini, M.; Fiorentino, M.; Piancone, F.; Infante, B.; Stallone, G.; Castellano, G.; Gesualdo, L. Glomerulonephritis in AKI: From Pathogenesis to Therapeutic Intervention. Front. Med. 2021, 7, 582272. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, T.; Yang, B.; Gu, H.F.; Chi, Z. Effects of ZnT8 on epithelial-to-mesenchymal transition and tubulointerstitial fibrosis in diabetic kidney disease. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Henry, T.Y. Progression of chronic renal failure. Arch. Intern. Med. 2003, 163, 1417–1429. [Google Scholar]
- Mori, K.; Mostafaei, H.; Miura, N.; Karakiewicz, P.I.; Luzzago, S.; Schmidinger, M.; Bruchbacher, A.; Pradere, B.; Egawa, S.; Shariat, S.F. Systemic therapy for metastatic renal cell carcinoma in the first-line setting: A systematic review and network meta-analysis. Cancer Immunol. Immunother. 2020, 70, 265–273. [Google Scholar] [CrossRef]
- Tang, W.; Huang, J.; Huang, X.; Han, X.; Tang, W.; Ke, G.; Xu, Q. Effect of alkaline phosphatase on sepsis-associated acute kidney injury patients: A systematic review and meta-analysis. Medicine 2020, 99, e18788. [Google Scholar] [CrossRef]
- Seyam, S.; Nordin, N.A.; Alfatama, M. Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery. Pharmaceuticals 2020, 13, 307. [Google Scholar] [CrossRef]
- de Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 20, 1469–1479. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Z.; Wang, M.; Ma, L. Current understandings and clinical translation of nanomedicines for breast cancer therapy. Adv. Drug Deliv. Rev. 2021, 180, 114034. [Google Scholar] [CrossRef]
- Alallam, B.; Altahhan, S.; Taher, M.; Mohd Nasir, M.H.; Doolaanea, A.A. Electrosprayed Alginate Nanoparticles as Crispr Plasmid Dna Delivery Carrier: Preparation, Optimization, and Characterization. Pharmaceuticals 2020, 13, 158. [Google Scholar] [CrossRef]
- Alallam, B.; Doolaanea, A.A.; Kyaw Oo, M.; Mohd Nasir, M.H.; Taher, M. Influence of Nanoparticles Surface Coating on Physicochemical Properties for CRISPR Gene Delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102910. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Akram, M.W.; Begum, R.; Wu, W.; Irfan, A. Inorganic nanoparticles for reduction of hexavalent chromium: Physicochemical aspects. J. Hazard. Mater. 2020, 402, 123535. [Google Scholar] [CrossRef]
- Thi, T.; Suys, E.; Lee, J.; Nguyen, D.; Park, K.; Truong, N. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayati, M.R.Y.; Hussein, Y.F.; Faisal, G.G.; Fuaat, A.A.; Affandi, K.A.; Abidin, M.A.Z. The Effect of Eurycoma Longifolia Jack Tongkat Ali Hydrogel on Wound Contraction and Re-Epithelialization in In Vivo Excisional Wound Model. Maced. J. Med. Sci. I 2022, 10, 634–643. [Google Scholar] [CrossRef]
- Yaseen, M.R.; Faisal, G.G.; Fuaat, A.A.; Affandi, K.A.; Alallam, B.; Mohd Nasir, M.H. Preparation of Euyrycoma Longifolia Jack (E.L) Tongkat Ali (Ta) Root Extract Hydrogel for Wound Application. Pharmacogn. J. 2021, 13, 1456–1463. [Google Scholar] [CrossRef]
- Tripathy, N.; Wang, J.; Tung, M.; Conway, C.; Chung, E.J. Transdermal Delivery of Kidney-Targeting Nanoparticles Using Dissolvable Microneedles. Cell. Mol. Bioeng. 2020, 13, 475–486. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, H.; Zhang, C. Advances in kidney-targeted drug delivery systems. Int. J. Pharm. 2020, 587. [Google Scholar] [CrossRef]
- Uthappa, U.; Arvind, O.; Sriram, G.; Losic, D.; Jung, H.Y.; Kigga, M.; Kurkuri, M.D. Nanodiamonds and their surface modification strategies for drug delivery applications. J. Drug Deliv. Sci. Technol. 2020, 60, 101993. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Y.; Li, Y.; Han, F.; Lin, W. Targeted Drug Delivery Systems for Kidney Diseases. Front. Bioeng. Biotechnol. 2021, 9, 683247. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Y.; Lin, J.; Fu, H.; Lim, L.Y.; Yuan, Z. Targeting strategies for drug delivery to the kidney: From renal glomeruli to tubules. Med. Res. Rev. 2018, 39, 561–578. [Google Scholar] [CrossRef]
- Wang, H.; Shang, Y.; Chen, X.; Wang, Z.; Zhu, D.; Liu, Y.; Zhang, C.; Chen, P.; Wu, J.; Wu, L.; et al. Delivery of MSCs with a Hybrid β-Sheet Peptide Hydrogel Consisting IGF-1C Domain and D-Form Peptide for Acute Kidney Injury Therapy. Int. J. Nanomed. 2020, 15, 4311–4324. [Google Scholar] [CrossRef]
- Qin, X.; Xu, Y.; Zhou, X.; Gong, T.; Zhang, Z.-R.; Fu, Y. An injectable micelle-hydrogel hybrid for localized and prolonged drug delivery in the management of renal fibrosis. Acta Pharm. Sin. B 2020, 11, 835–847. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Liu, Y.; Wang, C.; Chen, X.; Chang, Y.; Li, Y.; Guo, Z.; Han, Z.; Han, Z.-C.; et al. Renal subcapsular delivery of PGE2 promotes kidney repair by activating endogenous Sox9+ stem cells. Iscience 2021, 24, 103243. [Google Scholar] [CrossRef]
- O’Callaghan, C. The Renal System at a Glance; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Matovinović, M.S. 1. Pathophysiology and Classification of Kidney Diseases. EJIFCC 2009, 20, 2–11. [Google Scholar]
- Oliveira, D.; Snelling, P.; Kanellis, J.; Walker, R.; Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Nat. Rev. Dis. Prim. 2017, 3, 1–24. [Google Scholar]
- Sun, H.; Frassetto, L.; Benet, L.Z. Effects of renal failure on drug transport and metabolism. Pharmacol. Ther. 2006, 109, 1–11. [Google Scholar] [CrossRef]
- Gagliardini, E.; Benigni, A. Role of anti-TGF-β antibodies in the treatment of renal injury. Cytokine Growth Factor Rev. 2006, 17, 89–96. [Google Scholar] [CrossRef]
- Sakata, R.K.; Nunes, M.H.G. Analgesics use for kidney failure. Rev. Dor 2014, 15, 48. [Google Scholar] [CrossRef]
- Laurent, S. Antihypertensive drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Zhou, P.; Sun, X.; Zhang, Z. Kidney–targeted drug delivery systems. Acta Pharm. Sin. B 2014, 4, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Liu, L.; Xue, X.; Liang, X.-J. Nanoparticle-based drug delivery systems: What can they really do in vivo? F1000Research 2017, 6, 681. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. WITHDRAWN: Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. X 2020, 2, 100050. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Gray, A.B.; Patra, H.K. Nanomedicine: Controlling nanoparticle clearance for translational success. Trends Pharmacol. Sci. 2022, 43, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Lea-Henry, T.N.; Carland, J.; Stocker, S.; Sevastos, J.; Roberts, D.M. Clinical Pharmacokinetics in Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1085–1095. [Google Scholar] [CrossRef]
- Williams, R.M.; Shah, J.; Ng, B.D.; Minton, D.R.; Gudas, L.J.; Park, C.Y.; Heller, D.A. Mesoscale Nanoparticles Selectively Target the Renal Proximal Tubule Epithelium. Nano Lett. 2015, 15, 2358–2364. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Charbgoo, F.; Nejabat, M.; Abnous, K.; Soltani, F.; Taghdisi, S.M.; Alibolandi, M.; Shier, W.T.; Steele, T.; Ramezani, M. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J. Control. Release 2018, 272, 39–53. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef]
- Kaufman, D.P.; Basit, H.; Knohl, S.J. Physiology, Glomerular Filtration Rate; StatPearls, July 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK500032/ (accessed on 20 December 2022).
- Kamaly, N.; He, J.C.; Ausiello, D.A.; Farokhzad, N.K.O.C. Nanomedicines for renal disease: Current status and future applications. Nat. Rev. Nephrol. 2016, 12, 738–753. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Tariverdian, T.; Zarintaj, P.; Milan, P.B.; Saeb, M.R.; Kargozar, S.; Sefat, F.; Samadikuchaksaraei, A.; Mozafari, M. Nanoengineered biomaterials for kidney regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 325–344. [Google Scholar]
- Visweswaran, G.R.R.; Gholizadeh, S.; Ruiters, M.H.J.; Molema, G.; Kok, R.J.; Kamps, J.A.A.M. Targeting Rapamycin to Podocytes Using a Vascular Cell Adhesion Molecule-1 (VCAM-1)-Harnessed SAINT-Based Lipid Carrier System. PLoS ONE 2015, 10, e0138870. [Google Scholar] [CrossRef] [Green Version]
- Falkson, S.R.; Bordoni, B. Anatomy, Abdomen and Pelvis, Bowman Capsule; StatPearls Publishing: Waltham, MA, USA, 2020. [Google Scholar]
- Li, L.; Bonventre, J.V. Endothelial Glycocalyx: Not Just a Sugar Coat. Am. J. Respir. Crit. Care Med. 2016, 194, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Leeuwis, J.W.; Nguyen, T.Q.; Dendooven, A.; Kok, R.J.; Goldschmeding, R. Targeting podocyte-associated diseases. Adv. Drug Deliv. Rev. 2010, 62, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.F.; Molitoris, B.A. The Physiology of the Glomerulus. In Critical Care Nephrology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–42.e2. [Google Scholar]
- Youngman, K.R.; Lazarus, N.H.; Butcher, E.C. Lymphocyte Homing: Chemokines and Adhesion Molecules in T cell and IgA Plasma Cell Localization in the Mucosal Immune System. In Mucosal Immunology, Two-Volume Set; Academic Press: Cambridge, MA, USA, 2005; pp. 667–680. [Google Scholar] [CrossRef]
- Ásgeirsdóttir, S.A.; Zwiers, P.J.; Morselt, H.W.; Moorlag, H.E.; Bakker, H.I.; Heeringa, P.; Kok, J.W.; Kallenberg, C.G.M.; Molema, G.; Kamps, J.A.A.M. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am. J. Physiol. Physiol. 2008, 294, F554–F561. [Google Scholar] [CrossRef] [PubMed]
- Paz, D.L.; Le Meur, Y.; Renaudineau, Y. Glomerular Basement Membrane Autoantibodies. In Autoantibodies, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 553–560. [Google Scholar] [CrossRef]
- Scott, R.P.; Quaggin, S.E. Formation and Maintenance of a Functional Glomerulus. In Kidney Development, Disease, Repair and Regeneration; Academic Press: Cambridge, MA, USA, 2016; pp. 103–119. [Google Scholar] [CrossRef]
- Rabelink, T.J.; Heerspink, H.J.L.; de Zeeuw, D. The Pathophysiology of Proteinuria. In Chronic Renal Disease; Academic Press: Cambridge, MA, USA, 2015; pp. 92–105. [Google Scholar] [CrossRef]
- D’Amico, G.; Bazzi, C. Pathophysiology of proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef]
- Bolton, G.R.; Deen, W.M.; Daniels, B.S. Assessment of the charge selectivity of glomerular basement membrane using Ficoll sulfate. Am. J. Physiol. Physiol. 1998, 274, F889–F896. [Google Scholar] [CrossRef] [PubMed]
- Hoven, M.V.D.; Wijnhoven, T.; Li, J.-P.; Zcharia, E.; Dijkman, H.; Wismans, R.; Rops, A.; Lensen, J.; Heuvel, L.V.D.; van Kuppevelt, T.; et al. Reduction of anionic sites in the glomerular basement membrane by heparanase does not lead to proteinuria. Kidney Int. 2008, 73, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Okafor, A.E.; Bhattacharya, R.; Musah, S. Models of kidney glomerulus derived from human-induced pluripotent stem cells. In iPSCs in Tissue Engineering; Academic Press: Cambridge, MA, USA, 2021; pp. 329–370. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; González-Martínez, C.; Olea-Herrero, N.; Reventún, P.; Di Nunzio, M.; Sánchez-Esteban, S.; Arilla-Ferreiro, E.; Saura, M.; Bosch, R.J. Bisphenol A impaired cell adhesion by altering the expression of adhesion and cytoskeleton proteins on human podocytes. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Akilesh, S. Glomerular Disease. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; Academic Press: Cambridge, MA, USA, 2014; pp. 2734–2752. [Google Scholar]
- Wu, L.; Chen, M.; Mao, H.; Wang, N.; Zhang, B.; Zhao, X.; Qian, J.; Xing, C. Albumin-based nanoparticles as methylprednisolone carriers for targeted delivery towards the neonatal Fc receptor in glomerular podocytes. Int. J. Mol. Med. 2017, 39, 851–860. [Google Scholar] [CrossRef]
- Davidson, A.; Berthier, C.; Kretzler, M. Pathogenetic Mechanisms in Lupus Nephritis. J. Am. Soc. Nephrol. 2013, 24, 237–255. [Google Scholar] [CrossRef]
- Ballow, M.C. Immunoglobulin Therapy. In Clinical Immunology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1143–1153.e1. [Google Scholar] [CrossRef]
- van Asbeck, A.H.; Dieker, J.; Boswinkel, M.; van der Vlag, J.; Brock, R. Kidney-targeted therapies: A quantitative perspective. J. Control. Release 2020, 328, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, Y.; Cina, D.; Quaggin, S.E. Glomerular Cell Biology. In Seldin and Geibisch’s The Kidney; Academic Press: Cambridge, MA, USA, 2013; Volume 1, pp. 721–755. [Google Scholar]
- Boim, M.A.; Teixeira, V.P.C.; Schor, N. The Physiology of the Glomerular Tuft. Crit. Care Nephrol. 2009, 122–128. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Choi, C.H.J.; Han, H.; Davis, M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Gale, A.; Wu, P.; Ma, R.; Davis, M.E. siRNA Delivery to the Glomerular Mesangium Using Polycationic Cyclodextrin Nanoparticles Containing siRNA. Nucleic Acid Ther. 2015, 25, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Luo, S.; Du, Z.; Zhou, M.; Li, P.; Fu, Y.; Sun, X.; Huang, Y.; Zhang, Z. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Yuan, Z.-X.; Jia, L.; Lim, L.Y.; Lin, J.-C.; Shu, G.; Zhao, L.; Ye, G.; Liang, X.-X.; Ji, H.; Fu, H.-L. Renal-targeted delivery of triptolide by entrapment in pegylated TRX-20-modified liposomes. Int. J. Nanomed. 2017, 12, 5673–5686. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, C.; Liu, L.; Zhang, S.; Sun, S.; Hankins, J.D.; Sun, X.; Zheng, J. Interactions of Renal-Clearable Gold Nanoparticles with Tumor Microenvironments: Vasculature and Acidity Effects. Angew. Chem. Int. Ed. 2017, 56, 4314–4319. [Google Scholar] [CrossRef]
- Singh, G.; Ddungu, J.L.Z.; Licciardello, N.; Bergmann, R.; De Cola, L.; Stephan, H. Ultrasmall silicon nanoparticles as a promising platform for multimodal imaging. Faraday Discuss. 2019, 222, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Long, M.; Qin, Y.; Sun, X.; Zheng, J. Luminescent Gold Nanoparticles with Efficient Renal Clearance. Angew. Chem. Int. Ed. 2011, 50, 3168–3172. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, S.; Zhou, C.; Hao, G.; Liu, J.; Ramezani, S.; Yu, M.; Sun, X.; Zheng, J. Renal Clearance and Degradation of Glutathione-Coated Copper Nanoparticles. Bioconjugate Chem. 2015, 26, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Peng, C.; Xu, J.; Du, B.; Wang, Q.; Iii, R.D.V.; Yu, M.; Kim, M.J.; Zheng, J. Tailoring Renal Clearance and Tumor Targeting of Ultrasmall Metal Nanoparticles with Particle Density. Angew. Chem. Int. Ed. 2016, 55, 16039–16043. [Google Scholar] [CrossRef]
- Burns, A.A.; Vider, J.; Ow, H.; Herz, E.; Medina, O.P.; Baumgart, M.; Larson, S.M.; Wiesner, U.; Bradbury, M. Fluorescent Silica Nanoparticles with Efficient Urinary Excretion for Nanomedicine. Nano Lett. 2008, 9, 442–448. [Google Scholar] [CrossRef]
- Zhou, C.; Hao, G.; Thomas, P.; Liu, J.; Yu, M.; Sun, S.; Öz, O.K.; Sun, X.; Zheng, J. Near-Infrared Emitting Radioactive Gold Nanoparticles with Molecular Pharmacokinetics. Angew. Chem. Int. Ed. 2012, 51, 10118–10122. [Google Scholar] [CrossRef]
- Du, B.; Jiang, X.; Das, A.; Zhou, Q.; Yu, M.; Jin, R.; Zheng, J. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017, 12, 1096–1102. [Google Scholar] [CrossRef]
- Lacerda, L.; Soundararajan, A.; Singh, R.; Pastorin, G.; Al-Jamal, K.; Turton, J.; Frederik, P.; Herrero, M.A.; Li, S.; Bao, A.; et al. Dynamic Imaging of Functionalized Multi-Walled Carbon Nanotube Systemic Circulation and Urinary Excretion. Adv. Mater. 2007, 20, 225–230. [Google Scholar] [CrossRef]
- Wang, J.; Poon, C.; Chin, D.; Milkowski, S.; Lu, V.; Hallows, K.R.; Chung, E.J. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 2018, 11, 5584–5595. [Google Scholar] [CrossRef]
- Jiang, D.; Im, H.-J.; Boleyn, M.E.; England, C.G.; Ni, D.; Kang, L.; Engle, J.W.; Huang, P.; Lan, X.; Cai, W. Efficient renal clearance of DNA tetrahedron nanoparticles enables quantitative evaluation of kidney function. Nano Res. 2018, 12, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Xu, C.; Tian, H.; Chen, X. A disassembling strategy overcomes the EPR effect and renal clearance dilemma of the multifunctional theranostic nanoparticles for cancer therapy. Biomaterials 2019, 197, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, J.; Li, Y.; Nie, Y.; Zhu, D.; Wang, R.; Liu, J.; Gao, J.; Liu, N.; He, N.; et al. IGF-1 C Domain–Modified Hydrogel Enhances Cell Therapy for AKI. J. Am. Soc. Nephrol. 2016, 27, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, Y.; Liu, Y.; Xu, Z. circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J. Cell. Physiol. 2019, 234, 21249–21259. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Shah, J.; Tian, H.S.; Chen, X.; Geissmann, F.; Jaimes, E.A.; Heller, D.A. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension 2018, 71, 87–94. [Google Scholar] [CrossRef]
- Naumenko, V.; Nikitin, A.; Kapitanova, K.; Melnikov, P.; Vodopyanov, S.; Garanina, A.; Valikhov, M.; Ilyasov, A.; Vishnevskiy, D.; Markov, A.; et al. Intravital microscopy reveals a novel mechanism of nanoparticles excretion in kidney. J. Control. Release 2019, 307, 368–378. [Google Scholar] [CrossRef]
- Wyss, P.P.; Lamichhane, S.P.; Abed, A.; Vonwil, D.; Kretz, O.; Huber, T.B.; Sarem, M.; Shastri, V.P. Renal clearance of polymeric nanoparticles by mimicry of glycan surface of viruses. Biomaterials 2019, 230, 119643. [Google Scholar] [CrossRef]
- Deng, X.; Zeng, T.; Li, J.; Huang, C.; Yu, M.; Wang, X.; Tan, L.; Zhang, M.; Li, A.; Hu, J. Kidney-targeted triptolide-encapsulated mesoscale nanoparticles for high-efficiency treatment of kidney injury. Biomater. Sci. 2019, 7, 5312–5323. [Google Scholar] [CrossRef]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schäffler, M.; Takenaka, S.; Möller, W.; Schmid, G.; Simon, U.; et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef]
- Liang, X.; Wang, H.; Zhu, Y.; Zhang, R.; Cogger, V.C.; Liu, X.; Xu, Z.P.; Grice, J.E.; Roberts, M.S. Short- and Long-Term Tracking of Anionic Ultrasmall Nanoparticles in Kidney. ACS Nano 2016, 10, 387–395. [Google Scholar] [CrossRef]
- Ning, X.; Peng, C.; Li, E.S.; Xu, J.; Iii, R.D.V.; Yu, M.; Zheng, J. Physiological stability and renal clearance of ultrasmall zwitterionic gold nanoparticles: Ligand length matters. APL Mater. 2017, 5, 053406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, J.; Liang, S.; Sood, A.K.; Liang, D.; Li, C. CuS Nanodots with Ultrahigh Efficient Renal Clearance for Positron Emission Tomography Imaging and Image-Guided Photothermal Therapy. ACS Nano 2015, 9, 7085–7096. [Google Scholar] [CrossRef]
- Musah, S.; Mammoto, A.; Ferrante, T.C.; Jeanty, S.S.F.; Hirano-Kobayashi, M.; Mammoto, T.; Roberts, K.; Chung, S.; Novak, R.; Ingram, M.; et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H.; El-Boubbou, K.; Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; et al. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Gravier, J.; Bao, K.; Wada, H.; Lee, J.H.; Baek, Y.; El Fakhri, G.; Gioux, S.; Rubin, B.P.; Coll, J.-L.; et al. Renal Clearable Organic Nanocarriers for Bioimaging and Drug Delivery. Adv. Mater. 2016, 28, 8162–8168. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, K.; Zhang, X.; Chung, E.J. The effect of size, charge, and peptide ligand length on kidney targeting by small, organic nanoparticles. Bioeng. Transl. Med. 2020, 5, 10173. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef]
- Debayle, M.; Balloul, E.; Dembele, F.; Xu, X.; Hanafi, M.; Ribot, F.; Monzel, C.; Coppey, M.; Fragola, A.; Dahan, M.; et al. Zwitterionic polymer ligands: An ideal surface coating to totally suppress protein-nanoparticle corona formation? Biomaterials 2019, 219, 119357. [Google Scholar] [CrossRef]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef]
- Williams, R.M.; Jaimes, E.A.; Heller, D.A. Nanomedicines for kidney diseases. Kidney Int. 2016, 90, 740–745. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Davis, M.E. Targeting Therapeutics to the Glomerulus With Nanoparticles. Adv. Chronic Kidney Dis. 2013, 20, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Culver, K.S.B.; Shin, Y.J.; Rotz, M.W.; Meade, T.J.; Hersam, M.C.; Odom, T.W. Shape-Dependent Relaxivity of Nanoparticle-Based T1 Magnetic Resonance Imaging Contrast Agents. J. Phys. Chem. C 2016, 120, 22103–22109. [Google Scholar] [CrossRef] [PubMed]
- Rampersaud, S.; Fang, J.; Wei, Z.; Fabijanic, K.; Silver, S.; Jaikaran, T.; Ruiz, Y.; Houssou, M.; Yin, Z.; Zheng, S.; et al. The Effect of Cage Shape on Nanoparticle-Based Drug Carriers: Anticancer Drug Release and Efficacy via Receptor Blockade Using Dextran-Coated Iron Oxide Nanocages. Nano Lett. 2016, 16, 7357–7363. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- Calderon, A.J.; Bhowmick, T.; Leferovich, J.; Burman, B.; Pichette, B.; Muzykantov, V.; Eckmann, D.M.; Muro, S. Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers. J. Control. Release 2011, 150, 37–44. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Jarsch, I.K.; Daste, F.; Gallop, J.L. Membrane curvature in cell biology: An integration of molecular mechanisms. J. Cell Biol. 2016, 214, 375–387. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Ruggiero, A.; Villa, C.H.; Bander, E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Manova-Todorova, K.; Deen, W.M.; Scheinberg, D.A.; McDevitt, M.R. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA 2010, 107, 12369–12374. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Omidi, M.; Choolaei, M.; Nazarzadeh, M.; Yadegari, A.; Haghierosadat, F.; Oroojalian, F.; Azhdari, M. Electromechanical Properties of Vertically Aligned Carbon Nanotube. Adv. Mater. Res. 2013, 705, 332–336. [Google Scholar]
- Zhang, Z.; Liu, C.; Li, C.; Wu, W.; Jiang, X. Shape Effects of Cylindrical versus Spherical Unimolecular Polymer Nanomaterials on in Vitro and in Vivo Behaviors. Research 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Jasinski, D.L.; Li, H.; Guo, P. The Effect of Size and Shape of RNA Nanoparticles on Biodistribution. Mol. Ther. 2017, 26, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Talamini, L.; Violatto, M.B.; Cai, Q.; Monopoli, M.P.; Kantner, K.; Krpetić, Ž.; Perez-Potti, A.; Cookman, J.; Garry, D.; Silveira, C.P.; et al. Influence of Size and Shape on the Anatomical Distribution of Endotoxin-Free Gold Nanoparticles. ACS Nano 2017, 11, 5519–5529. [Google Scholar] [CrossRef]
- Decuzzi, P.; Lee, S.; Bhushan, B.; Ferrari, M. A Theoretical Model for the Margination of Particles within Blood Vessels. Ann. Biomed. Eng. 2005, 33, 179–190. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [Green Version]
- Markovic, M.; Ben-Shabat, S.; Dahan, A. Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 2020, 12, 1031. [Google Scholar] [CrossRef]
- Dufès, C. Brain Delivery of Peptides and Proteins. In Peptide and Protein Delivery; Academic Press: Cambridge, MA, USA, 2011; pp. 105–122. [Google Scholar] [CrossRef]
- Huttunen, K.M.; Raunio, H.; Rautio, J. Prodrugs—From Serendipity to Rational Design. Pharmacol. Rev. 2011, 63, 750–771. [Google Scholar] [CrossRef]
- Mathias, C.J.; Hubers, D.; Low, P.S.; Green, M.A. Synthesis of [99mTc]DTPA-Folate and Its Evaluation as a Folate-Receptor-Targeted Radiopharmaceutical. Bioconjugate Chem. 2000, 11, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.P.; Mathias, C.J.; Yang, Z.; Low, P.S.; Marmion, M.; A Green, M. Synthesis and evaluation of 99mTc(CO)3-DTPA-folate as a folate-receptor-targeted radiopharmaceutical. Nucl. Med. Biol. 2002, 29, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, J.; Lantrip, D.A.; Waters, D.J.; Mathias, C.J.; Green, M.A.; Fuchs, P.L.; Low, P.S. Design and Synthesis of [111In]DTPA−Folate for Use as a Tumor-Targeted Radiopharmaceutical. Bioconjugate Chem. 1997, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duong, A.; James, L.; Wyslouzil, B.E. Electrospray Production of Nanoparticles for Drug/Nucleic Acid Delivery. In The Delivery of Nanoparticles; Intech Europe: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Shan, L.; Zhuo, X.; Zhang, F.; Dai, Y.; Zhu, G.; Yung, B.C.; Fan, W.; Zhai, K.; Jacobson, O.; Kiesewetter, D.O.; et al. A paclitaxel prodrug with bifunctional folate and albumin binding moieties for both passive and active targeted cancer therapy. Theranostics 2018, 8, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Susaki, H.; Okuno, S.; Yamada, H.; Watanabe, H.K.; Sugiyama, Y. Specific renal delivery of sugar-modified low-molecular-weight peptides. J. Pharmacol. Exp. Ther. 1999, 288, 888–897. [Google Scholar]
- Suzuki, K.; Susaki, H.; Okuno, S.; Sugiyama, Y. Renal drug targeting using a vector “alkylglycoside”. J. Pharmacol. Exp. Ther. 1999, 288, 57–64. [Google Scholar]
- Shirota, K.; Kato, Y.; Suzuki, K.; Sugiyama, Y. Characterization of novel kidney-specific delivery system using an alkylglucoside vector. J. Pharmacol. Exp. Ther. 2001, 299, 459–467. [Google Scholar]
- Lin, Y.; Sun, X.; Gong, T.; Zhang, Z.R. Prednisolone succinate–glucosamine conjugate: Synthesis, characterization and in vitro cellular uptake by kidney cell lines. Chin. Chem. Lett. 2012, 23, 25–28. [Google Scholar] [CrossRef]
- Liang, Z.; Gong, T.; Sun, X.; Tang, J.Z.; Zhang, Z. Chitosan oligomers as drug carriers for renal delivery of zidovudine. Carbohydr. Polym. 2012, 87, 2284–2290. [Google Scholar] [CrossRef]
- Liu, D.; Shu, G.; Jin, F.; Qi, J.; Xu, X.; Du, Y.; Yu, H.; Wang, J.; Sun, M.; You, Y.; et al. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci. Adv. 2020, 6, eabb7422. [Google Scholar] [CrossRef]
- Wilk, S.; Mizoguchi, H.; Orlowski, M. Gamma-Glutamyl dopa: A kidney-specific dopamine precursor. J. Pharmacol. Exp. Ther. 1978, 206, 227–232. [Google Scholar] [PubMed]
- Mizoguchi, H.; Orlowski, M.; Wilk, S.; Green, J.P. γ-glutamyl dopa and γ-glutamyl dopamine: Effect of plasma glucose levels. Eur. J. Pharmacol. 1979, 57, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Pestana, M.; Soares-Da-Silva, P. The renal handling of dopamine originating from l-DOPA and γ-glutamyl-l-DOPA. Br. J. Pharmacol. 1994, 112, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; He, Q.; Zhang, Z.-R.; Hu, B.; Liu, S.-W. [Kidney-targeting characteristics of N-acetyl-L-glutamic prednisolone prodrug]. Yao Xue Xue Bao = Acta Pharm. Sin. 2003, 38, 627–630. [Google Scholar]
- Scott, R.P.; Quaggin, S.E. The cell biology of renal filtration. J. Cell Biol. 2015, 209, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Durigutto, P.; Sblattero, D.; Biffi, S.; De Maso, L.; Garrovo, C.; Baj, G.; Colombo, F.; Fischetti, F.; Di Naro, A.F.; Tedesco, F.; et al. Targeted Delivery of Neutralizing Anti-C5 Antibody to Renal Endothelium Prevents Complement-Dependent Tissue Damage. Front. Immunol. 2017, 8, 01093. [Google Scholar] [CrossRef]
- Akizawa, H.; Uehara, T.; Arano, Y. Renal uptake and metabolism of radiopharmaceuticals derived from peptides and proteins. Adv. Drug Deliv. Rev. 2008, 60, 1319–1328. [Google Scholar] [CrossRef]
- Grünberg, J.; Novak-Hofer, I.; Honer, M.; Zimmermann, K.; Knogler, K.; Blaüenstein, P.; Ametamey, S.; Maecke, H.R.; Schubiger, P.A. In vivo Evaluation of 177Lu- and 67/64Cu-Labeled Recombinant Fragments of Antibody chCE7 for Radioimmunotherapy and PET Imaging of L1-CAM-Positive Tumors. Clin. Cancer Res. 2005, 11, 5112–5120. [Google Scholar] [CrossRef]
- Behr, T.M.; Becker, W.S.; Sharkey, R.M.; Juweid, M.E.; Dunn, R.M.; Bair, H.J.; Wolf, F.G.; Goldenberg, D.M. Reduction of renal uptake of monoclonal antibody fragments by amino acid infusion. J. Nucl. Med. 1996, 37, 829–833. [Google Scholar]
- Baines, R.J.; Brunskill, N.J. The Molecular Interactions between Filtered Proteins and Proximal Tubular Cells in Proteinuria. Nephron Exp. Nephrol. 2008, 110, e67–e71. [Google Scholar] [CrossRef]
- Wang, S.-N.; LaPage, J.; Hirschberg, R. Glomerular ultrafiltration and apical tubular action of IGF-I, TGF-β, and HGF in nephrotic syndrome. Kidney Int. 1999, 56, 1247–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Peterson, N.; Hanna, R.N.; Kuszpit, K.; White, J.; Allen, K.L.; Barnes, A.; Rickert, K.W.; Shan, L.; Wu, H.; et al. Antibody Fragment F(ab′)2 Targeting Caveolae-Associated Protein PV1 for Selective Kidney Targeting and Retention. Mol. Pharm. 2010, 17, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Kvirkvelia, N.; McMenamin, M.; Gutierrez, V.I.; Lasareishvili, B.; Madaio, M.P. Human anti-α3(IV)NC1 antibody drug conjugates target glomeruli to resolve nephritis. Am. J. Physiol. Physiol. 2015, 309, F680-4. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.V.; Pippin, J.W.; Kaiser, C.; Krofft, R.D.; Brinkkoetter, P.T.; Hudkins, K.L.; Kerjaschki, D.; Reiser, J.; Alpers, C.E.; Shankland, S.J. Novel siRNA Delivery System to Target Podocytes In Vivo. PLoS ONE 2010, 5, e9463. [Google Scholar] [CrossRef]
- Maack, T.; Johnson, V.; Kau, S.T.; Figueiredo, J.; Sigulem, D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: A review. Kidney Int. 1979, 16, 251–270. [Google Scholar] [CrossRef]
- Kok, R.J.; Grijpstra, F.; Nederhoed, K.H.; Moolenaar, F.; De Zeeuw, D.; Meijer, D.K.F.; Kok, F.G.R.J. Renal Drug Delivery With Low-Molecular-Weight Proteins: The Effect of Charge Modifications on the Body Distribution of Drug-Lysozyme Conjugates. Drug Deliv. 1999, 6, 1–8. [Google Scholar] [CrossRef]
- Kok, R.J.; Haas, M.; Moolenaar, F.; De Zeeuw, D.; Meijer, D.K.F. Drug delivery to the kidneys and the bladder with the low molecular weight protein lysozyme. Ren. Fail. 1998, 20, 211–217. [Google Scholar] [CrossRef]
- Dolman, M.; Harmsen, S.; Storm, G.; Hennink, W.; Kok, R. Drug targeting to the kidney: Advances in the active targeting of therapeutics to proximal tubular cells. Adv. Drug Deliv. Rev. 2010, 62, 1344–1357. [Google Scholar] [CrossRef]
- Kamada, H.; Tsutsumi, Y.; Sato-Kamada, K.; Yamamoto, Y.; Yoshioka, Y.; Okamoto, T.; Nakagawa, S.; Nagata, S.; Mayumi, T. Synthesis of a poly(vinylpyrrolidone-co -dimethyl maleic anhydride) co-polymer and its application for renal drug targeting. Nat. Biotechnol. 2003, 21, 399–404. [Google Scholar] [CrossRef]
- Kodaira, H.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Kaneda, Y.; Yamamoto, Y.; Tsunoda, S.-I.; Okamoto, T.; Mukai, Y.; Shibata, H.; et al. The targeting of anionized polyvinylpyrrolidone to the renal system. Biomaterials 2004, 25, 4309–4315. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Sato-Kamada, K.; Okamoto, T.; Mukai, Y.; Shibata, H.; Nakagawa, S.; Mayumi, T. Poly(vinylpyrrolidone-co-dimethyl maleic acid) as a novel renal targeting carrier. J. Control. Release 2004, 95, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.W.; Prossnitz, A.N.; Eng, D.G.; Cheng, Y.; Subrahmanyam, N.; Pippin, J.W.; Lamm, R.J.; Ngambenjawong, C.; Ghandehari, H.; Shankland, S.J.; et al. Glomerular Disease Augments Kidney Accumulation of Synthetic Anionic Polymers. Biomaterials 2018, 178, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.V.; Chang, H.-M.; Yanagawa, N.; Hamon, M. Nanotechnology, Nanomedicine, and the Kidney. Appl. Sci. 2021, 11, 7187. [Google Scholar] [CrossRef]
- Gao, S.; Hein, S.; Dagnæs-Hansen, F.; Weyer, K.; Yang, C.; Nielsen, R.; Christensen, E.I.; Fenton, R.; Kjems, J. Megalin-Mediated Specific Uptake of Chitosan/siRNA Nanoparticles in Mouse Kidney Proximal Tubule Epithelial Cells Enables AQP1 Gene Silencing. Theranostics 2014, 4, 1039–1051. [Google Scholar] [CrossRef]
- Wang, J.; Chin, D.; Poon, C.; Mancino, V.; Pham, J.; Li, H.; Ho, P.-Y.; Hallows, K.R.; Chung, E.J. Oral delivery of metformin by chitosan nanoparticles for polycystic kidney disease. J. Control. Release 2020, 329, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, H.; Zhou, W.; Chen, M.; Yao, Y.; Zhang, Z.; Tan, N. Kidney targeted delivery of asiatic acid using a FITC labeled renal tubular-targeting peptide modified PLGA-PEG system. Int. J. Pharm. 2020, 584, 119455. [Google Scholar] [CrossRef]
- Yu, H.; Lin, T.; Chen, W.; Cao, W.; Zhang, C.; Wang, T.; Ding, M.; Zhao, S.; Wei, H.; Guo, H.; et al. Size and temporal-dependent efficacy of oltipraz-loaded PLGA nanoparticles for treatment of acute kidney injury and fibrosis. Biomaterials 2019, 219, 119368. [Google Scholar] [CrossRef]
- Tang, J.; Chen, J.-Y.; Liu, J.; Luo, M.; Wang, Y.-J.; Wei, X.-W.; Gao, X.; Wang, B.-L.; Liu, Y.-B.; Yi, T.; et al. Calcium phosphate embedded PLGA nanoparticles: A promising gene delivery vector with high gene loading and transfection efficiency. Int. J. Pharm. 2012, 431, 210–221. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, M.; Su, Z.; Xie, Y.; Chen, M.; Zong, L.; Gao, Y.; Li, H.; Qi, J.; Zhao, Q.; et al. Kidney-specific drug delivery system for renal fibrosis based on coordination-driven assembly of catechol-derived chitosan. Biomaterials 2014, 35, 7157–7171. [Google Scholar] [CrossRef]
- Akita, H.; Ishiba, R.; Togashi, R.; Tange, K.; Nakai, Y.; Hatakeyama, H.; Harashima, H. A neutral lipid envelope-type nanoparticle composed of a pH-activated and vitamin E-scaffold lipid-like material as a platform for a gene carrier targeting renal cell carcinoma. J. Control. Release 2015, 200, 97–105. [Google Scholar] [CrossRef]
- Marom, O.; Nakhoul, F.; Tisch, U.; Shiban, A.; Abassi, Z.; Haick, H. Gold nanoparticle sensors for detecting chronic kidney disease and disease progression. Nanomedicine 2012, 7, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Mansur, A.A.P.; Curti, E.; De Almeida, M.V. Functionalized-chitosan/quantum dot nano-hybrids for nanomedicine applications: Towards biolabeling and biosorbing phosphate metabolites. J. Mater. Chem. B 2013, 1, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.V.; Keliher, E.J.; Core, A.B.; Brown, D.; Weissleder, R. Characterizing the Interactions of Organic Nanoparticles with Renal Epithelial Cells in Vivo. ACS Nano 2015, 9, 3641–3653. [Google Scholar] [CrossRef]
- Ordikhani, F.; Kasinath, V.; Uehara, M.; Akbarzadeh, A.; Yilmam, O.A.; Dai, L.; Aksu, H.; Jung, S.; Jiang, L.; Li, X.; et al. Selective trafficking of light chain-conjugated nanoparticles to the kidney and renal cell carcinoma. Nano Today 2020, 35, 100990. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ghose, T.; Faulkner, G.; Kralovec, J.; Mezei, M. Targeting of methotrexate-containing liposomes with a monoclonal antibody against human renal cancer. Cancer Res. 1989, 49, 3976–3984. [Google Scholar]
- Morimoto, K.; Kondo, M.; Kawahara, K.; Ushijima, H.; Tomino, Y.; Miyajima, M.; Kimura, J. Advances in Targeting Drug Delivery to Glomerular Mesangial Cells by Long Circulating Cationic Liposomes for the Treatment of Glomerulonephritis. Pharm. Res. 2007, 24, 946–954. [Google Scholar] [CrossRef]
- Tuffin, G.; Waelti, E.; Huwyler, J.; Hammer, C.; Marti, H.-P. Immunoliposome Targeting to Mesangial Cells: A Promising Strategy for Specific Drug Delivery to the Kidney. J. Am. Soc. Nephrol. 2005, 16, 3295–3305. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Zhang, J.; Liu, Q.; Wu, T.; Zhou, J.; Huang, H.; Tang, Q.; Huang, C.; Huang, Y.; et al. Targeted delivery of celastrol to renal interstitial myofibroblasts using fibronectin-binding liposomes attenuates renal fibrosis and reduces systemic toxicity. J. Control. Release 2020, 320, 32–44. [Google Scholar] [CrossRef]
- McFetridge, M.L.; Del Borgo, M.P.; Aguilar, M.-I.; Ricardo, S.D. The use of hydrogels for cell-based treatment of chronic kidney disease. Clin. Sci. 2018, 132, 1977–1994. [Google Scholar] [CrossRef]
- Gao, J.; Liu, R.; Wu, J.; Liu, Z.; Li, J.; Zhou, J.; Hao, T.; Wang, Y.; Du, Z.; Duan, C.; et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials 2012, 33, 3673–3681. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, Y.; Chen, X.; Midgley, A.C.; Wang, Z.; Zhu, D.; Wu, J.; Chen, P.; Wu, L.; Wang, X.; et al. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano 2020, 14, 0c05681. [Google Scholar] [CrossRef]
- Hou, J.; Pan, Y.; Zhu, D.; Fan, Y.; Feng, G.; Wei, Y.; Wang, H.; Qin, K.; Zhao, T.; Yang, Q.; et al. Targeted delivery of nitric oxide via a ‘bump-and-hole’-based enzyme–prodrug pair. Nat. Chem. Biol. 2018, 15, 151–160. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, J.; Wang, H.; Hezam, K.; Zhao, X.; Huang, H.; Chen, S.; Han, Z.; Han, Z.-C.; Guo, Z.; et al. Enhanced therapeutic effects of MSC-derived extracellular vesicles with an injectable collagen matrix for experimental acute kidney injury treatment. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhou, Y.; Liu, S.; Li, L.; Chen, Y.; Cheng, J.; Lu, Y.; Liu, J. Control release of mitochondria-targeted antioxidant by injectable self-assembling peptide hydrogel ameliorated persistent mitochondrial dysfunction and inflammation after acute kidney injury. Drug Deliv. 2018, 25, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, M.V.; Hauser, P.V.; Zieris, A.; Carvalhosa, R.; Bussolati, B.; Freudenberg, U.; Camussi, G.; Werner, C. Growth factor delivery from hydrogel particle aggregates to promote tubular regeneration after acute kidney injury. J. Control. Release 2013, 167, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef]
- Cho, S.-H.; Noh, J.-R.; Cho, M.Y.; Go, M.-J.; Kim, Y.-H.; Kang, E.S.; Lee, C.-H.; Lim, Y.T. An injectable collagen/poly(γ-glutamic acid) hydrogel as a scaffold of stem cells and α-lipoic acid for enhanced protection against renal dysfunction. Biomater. Sci. 2016, 5, 285–294. [Google Scholar] [CrossRef]
- Ghaly, T.; Rabadi, M.M.; Weber, M.; Rabadi, S.M.; Bank, M.; Grom, J.M.; Fallon, J.T.; Goligorsky, M.S.; Ratliff, B.B. Hydrogel-embedded endothelial progenitor cells evade LPS and mitigate endotoxemia. Am. J. Physiol. Physiol. 2011, 301, F802–F812. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Ghaly, T.; Brudnicki, P.; Yasuda, K.; Rajdev, M.; Bank, M.; Mares, J.; Hatzopoulos, A.K.; Goligorsky, M.S. Endothelial progenitors encapsulated in bioartificial niches are insulated from systemic cytotoxicity and are angiogenesis competent. Am. J. Physiol. Physiol. 2010, 299, F178–F186. [Google Scholar] [CrossRef]
- Zullo, J.A.; Nadel, E.P.; Rabadi, M.M.; Baskind, M.J.; Rajdev, M.A.; Demaree, C.M.; Vasko, R.; Chugh, S.S.; Lamba, R.; Goligorsky, M.S.; et al. The Secretome of Hydrogel-Coembedded Endothelial Progenitor Cells and Mesenchymal Stem Cells Instructs Macrophage Polarization in Endotoxemia. STEM CELLS Transl. Med. 2015, 4, 852–861. [Google Scholar] [CrossRef]
- Caldas, H.C.; Fernandes, I.M.M.; Kawasaki-Oyama, R.S.; Baptista, M.A.S.F.; Plepis, A.; Martins, V.; Coimbra, T.M.; Goloni-Bertollo, E.; Braile, D.M.; Abbud-Filho, M. Effect of stem cells seeded onto biomaterial on the progression of experimental chronic kidney disease. Exp. Biol. Med. 2011, 236, 746–754. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Kuipers, H.F.; Frymoyer, A.R.; Ishak, H.D.; Bollyky, J.B.; Wight, T.N.; Bollyky, P.L. 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front. Immunol. 2015, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Singampalli, K.L.; Parikh, U.M.; Yu, L.; Keswani, S.G.; Wang, X. Hyaluronan, a double-edged sword in kidney diseases. Pediatr. Nephrol. 2021, 37, 735–744. [Google Scholar] [CrossRef] [PubMed]


| Materials | Size (nm) | Characteristics | Target | Application | Refs. |
|---|---|---|---|---|---|
| PLGA | 207 ± 5 | Calcium phosphate embedded for plasmid(p)DNA delivery | Kidney | Promising vectors for gene delivery | [175] |
| Chitosan | 38–45 | Catechol-derived low molecular weight chitosan/Doxorubicin | Kidney | Renal fibrosis | [176] |
| Liposome | 100–150 | pDNA-encapsulating/SS-cleavable and pH-activated lipid | Kidney | Renal cell carcinoma | [177] |
| Gold | - | Nanoparticle arrays as biosensor | Kidney | Chronic kidney disease | [178] |
| Cationic cyclodextrin | 60–100 | cationic cyclodextrin-containing polymer (CDP)-based siRNA nanoparticles | Glomerular basement membrane | Nucleic acid delivery | [81] |
| Functionalized chitosan | 2.2–3.6 | Functionalized chitosan/quantum dot nano-hybrids | Phosphate metabolites | Treating hyperphosphataemic patients Kidney failure | [179] |
| low molecular weight chitosan | 75 ± 25 | Chitosan/siRNA nanoparticles | Proximal tubule epithelial cells (PTECs) | Knockdown of specific genes in (ptecs) Kidney diseases | [171] |
| Chitosan | 150 | Metformin-loaded chitosan nanoparticles | (Intestines) improve oral bioavailability of metformin | Polycystic kidney disease Chronic kidney disease | [172] |
| PLGA-PEG | 200 | FITC-labelled renal tubular-targeting peptide modified PLGA-PEG nanoparticles | Renal proximal tubules | Chronic kidney disease | [173] |
| PLGA | 207 ± 5 | Calcium phosphate-embedded PLGA nanoparticles | Embryonic kidney cells | Gene delivery | [175] |
| PLGA-PEG | 347.6 ± 21.0 | Poly(lactic-co-glycolic acid) conjugated to polyethylene glycol (PLGA-PEG) nanoparticles | Proximal tubule cells | Targeted drug delivery of renal tubules | [100] |
| Gold | 75 ± 25 | PEGylated Gold-based nanoparticles | Mesangium of the kidney | Kidney diseases | [80] |
| Dextran dendrimer | 5 | Dextran-based nanoparticles poly(amido amine) dendrimer nanoparticles | Renal tubular epithelial cells | - | [180] |
| PEG-PLGA | 77.8 ± 0.5 | Lambda light chains (LCs) attached to PEGylated polylactic-co-glycolic acid (PLGA) nanoparticles | Proximal tubule epithelial cells | Management of non-oncologic/oncologic renal disorders | [181] |
| Hydrogel Carrier | Cargo | Route/Target | Refs. |
|---|---|---|---|
| Hyaluronic acid/collagen/polyethylene glycol hydrogel | Mesenchymal stem cells, and endothelial progenitor cells | Intracapsular injection | [186] |
| Chitosan hydrogel | Mesenchymal stem cells | Intracapsular injection | [98] |
| Self-assembling peptide hydrogel | Mesenchymal stem cells | Intracapsular injection | [33] |
| Biotin/chitosan hydrogels | Mesenchymal stem cells-derived extracellular vesicles | Intracapsular injection | [187] |
| (Arginine-Glycine-Aspartate) peptide hydrogel | Mesenchymal stem cells -derived extracellular vesicles | Intracapsular injection | [188] |
| Chitosan hydrogel | Nitric oxide-donor enzyme-prodrug system | Intracapsular injection | [189] |
| Collagen hydrogel | Extracellular vesicles | Intracapsular injection | [190] |
| Peptide hydrogel | Mitochondria antioxidants Mito-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) | Intracapsular injection/ mitochondria | [191] |
| PEG hydrogel | Fibroblast growth factor and murine epidermal growth factor | Intracapsular injection | [192] |
| Collagen hydrogel | Prostaglandin E2 | Intracapsular injection | [35] |
| Micelle-hyaluronic acid hydrogel | Celastrol/anti-transforming growth factor-β1 antibody | Intracapsular injection | [34] |
| Folate-conjugated micelle nanoparticles into polyvinyl alcohol MN patches patch | Rhodamine B | Folate receptor targeting | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alallam, B.; Choukaife, H.; Seyam, S.; Lim, V.; Alfatama, M. Advanced Drug Delivery Systems for Renal Disorders. Gels 2023, 9, 115. https://doi.org/10.3390/gels9020115
Alallam B, Choukaife H, Seyam S, Lim V, Alfatama M. Advanced Drug Delivery Systems for Renal Disorders. Gels. 2023; 9(2):115. https://doi.org/10.3390/gels9020115
Chicago/Turabian StyleAlallam, Batoul, Hazem Choukaife, Salma Seyam, Vuanghao Lim, and Mulham Alfatama. 2023. "Advanced Drug Delivery Systems for Renal Disorders" Gels 9, no. 2: 115. https://doi.org/10.3390/gels9020115