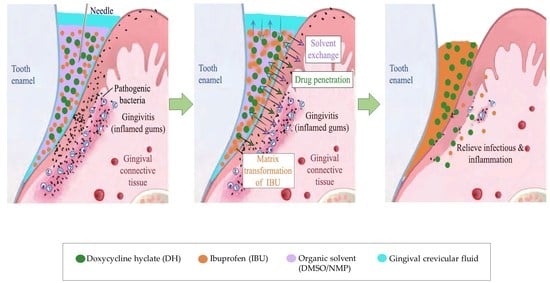
Physicochemical and Bioactivity Characteristics of Doxycycline Hyclate-Loaded Solvent Removal-Induced Ibuprofen-Based In Situ Forming Gel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.1.1. Physical Appearance
2.1.2. Density, pH, and Viscosity
2.1.3. Surface Tension and Contact Angle
2.1.4. Water Tolerance
2.1.5. In Situ Gel Transformation
2.1.6. Injectability Properties
2.1.7. Mechanical Properties
2.1.8. In Vitro Drug Release
2.1.9. Surface Topography
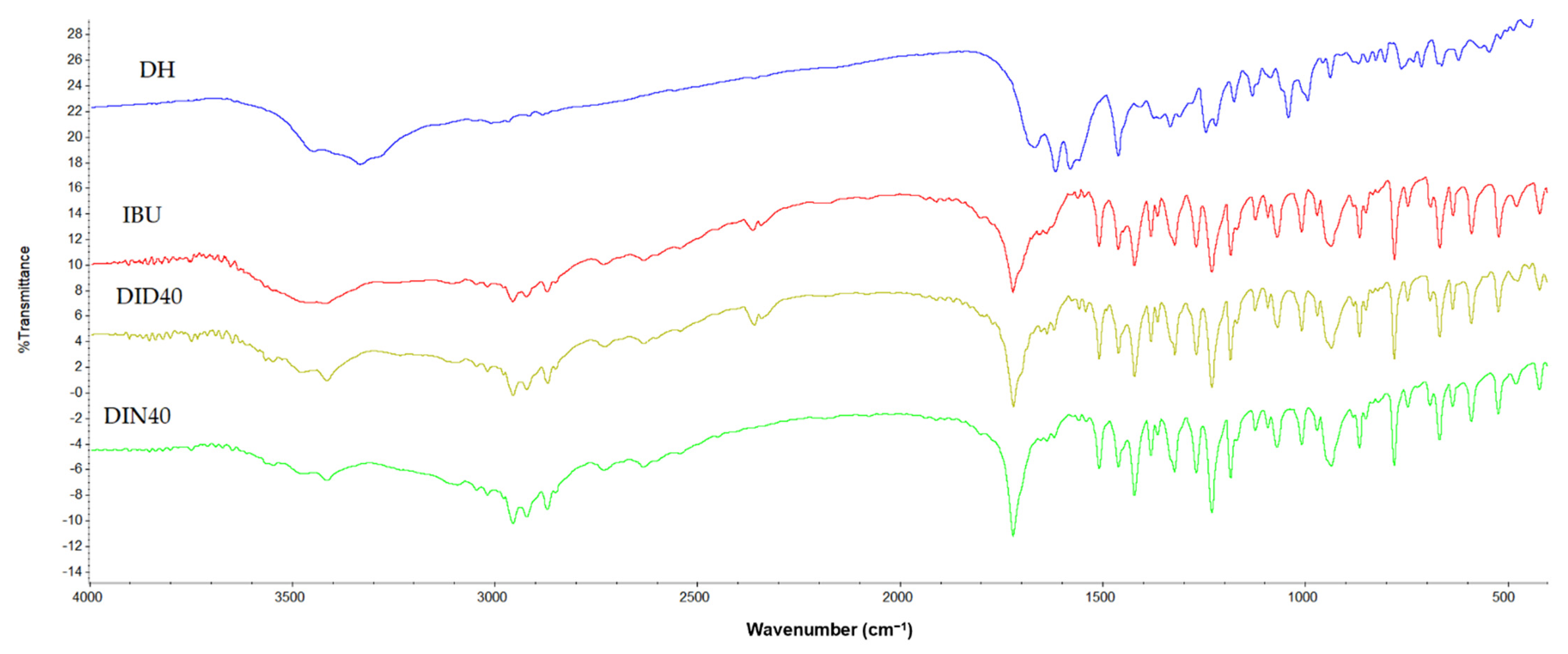
2.1.10. Differential Scanning Calorimeter (DSC), Thermogravimetric Analysis (TGA), Powder X-ray Diffraction (PXRD), and FOURIER Transform Infrared Spectroscopy (FTIR)
2.2. Bioactivities Studies
2.2.1. Antimicrobial Activities
2.2.2. Anti-Inflammatory Activities
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the ISG
4.3. Physicochemical Study
4.3.1. pH, Density, and Viscosities
4.3.2. Surface Tension and Contact Angle
4.3.3. Water Tolerance Measurement
4.3.4. Gel Formation Study
4.3.5. Interfacial Phenomena of Formulation–Aqueous Phase
4.3.6. Injectability and Mechanical Properties
4.3.7. In Vitro Drug Release Studies
4.3.8. Scanning Electron Microscopy (SEM)
4.3.9. Differential Scanning Calorimetry (DSC), Thermogravimetry (TGA), Powder X-ray Diffractometry (PXRD), and Fourier Transform Infrared (FTIR) Spectroscopy
4.4. Bioactivity Studies
4.4.1. Antimicrobial Activity
4.4.2. Anti-Inflammatory Study
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Baehni, P.; Tonetti, M.S. Group 1 of the European Workshop on P: Conclusions and consensus statements on periodontal health, policy and education in Europe: A call for action—Consensus view. Consensus report of the 1st European Workshop on Periodontal Education. Eur. J. Dent. Educ. 2010, 14 (Suppl. S1), 2–3. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.K.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Susin, C. Periodontitis epidemiology: Is periodontitis under- recognized, over- diagnosed, or both? Periodontology 2000 2017, 75, 45–51. [Google Scholar] [CrossRef]
- Rooney, J.; Wade, W.G.; Sprague, S.V.; Newcombe, R.G.; Addy, M. Adjunctive effects to nonsurgical periodontal therapy of systemic metronidazole and amoxycillin alone and combined: A placebo controlled study. J. Clin. Periodontol. 2002, 29, 342–350. [Google Scholar] [CrossRef]
- Guerrero, A.; Griffiths, G.S.; Nibali, L.; Suvan, J.; Moles, D.R.; Laurell, L.; Tonetti, M.S. Adjunctive benefits of systemic amoxicillin and metronidazole in non- surgical treatment of generalized aggressive periodontitis: A randomized placebo- controlled clinical trial. J. Clin. Periodontol. 2005, 32, 1096–1107. [Google Scholar] [CrossRef]
- Kornman, K.S. Controlled- release local delivery antimicrobials in periodontics: Prospects for the future. J. Periodontol. 1993, 64, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Chuenbarn, T.; Chantadee, T.; Phaechamud, T. Doxycycline hyclate-loaded Eudragit® RS PO in situ-forming microparticles for periodontitis treatment. J. Drug. Deliv. Sci. Technol. 2022, 71, 103294. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Sirirak, J.; Tuntarawongsa, S.; Okonogi, S.; Phaechamud, T. Design and comparative evaluation of vancomycin HCl-loaded rosin-based in situ forming gel and microparticles. Gels 2022, 8, 231. [Google Scholar] [CrossRef]
- Khaing, E.M.; Mahadlek, J.; Okonogi, S.; Phaechamud, T. Lime peel oil–incorporated rosin-based antimicrobial in situ forming gel. Gels 2022, 8, 169. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-based antisolvent-induced in situ forming matrix for crevicular pocket delivery of vancomycin hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Khaing, E.M.; Intaraphairot, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Natural-resin in-situ-forming gels: Physicochemical characteristics and bioactivities. Pharm. Sci. Asia 2021, 48, 461–470. [Google Scholar] [CrossRef]
- Schwach-Abdellaoui, K.; Vivien-Castioni, N.; Gurny, R. Local delivery of antimicrobial agents for the treatment of periodontal disease. Eur. J. Pharm. Biopharm. 2000, 50, 83–99. [Google Scholar] [CrossRef]
- Javali, M.A.; Vandana, K.L. A comparative evaluation of atrigel delivery system (10% doxycycline hyclate) Atridox with scaling and root planing and combination therapy in treatment of periodontitis: A clinical study. J. Indian. Soc. Periodontol. 2012, 16, 43–48. [Google Scholar] [PubMed]
- Singh, T.; McMillan, H.; Jones, D. Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J. Control. Release 2013, 176, 8–23. [Google Scholar]
- Phaechamud, T.; Jantadee, T.; Mahadlek, J.; Charoensuksai, P.; Pichayakorn, W. Characterization of antimicrobial agent loaded eudragit RS solvent exchange-induced in situ forming gels for periodontitis treatment. AAPS Pharm. Sci. Tech. 2017, 18, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Mahadlek, J.; Chuenbarn, T. In situ forming gel comprising bleached shellac loaded with antimicrobial drugs for periodontitis treatment. Mater. Des. 2016, 89, 294–303. [Google Scholar] [CrossRef]
- Phaechamud, T.; Setthajindalert, O. Cholesterol in situ forming gel loaded with doxycycline hyclate for intra-periodontal pocket delivery. Eur. J. Pharm. 2017, 99, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Mahadlek, J. Solvent exchange-induced in situ forming gel comprising ethyl cellulose-antimicrobial drugs. Int. J. Pharm. 2015, 494, 381–392. [Google Scholar] [CrossRef]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T. Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian. J. Pharm. Sci. 2018, 13, 131–142. [Google Scholar] [CrossRef]
- Senarat, S.; Lwin, W.W.; Mahadlek, J.; Phaechamud, T. Doxycycline hyclate-loaded in situ forming gels composed from bleached shellac, Ethocel, and Eudragit RS for periodontal pocket delivery. Saudi Pharm. J. 2021, 29, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Chantadee, T.; Sirirak, J.; Hoshino, T.; Phaechamud, T. Augmentative molecular aspect for phase inversion of vancomycin hydrochloride-loaded fatty acid in situ forming matrices. Mater. Des. 2021, 199, 109429. [Google Scholar] [CrossRef]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Saturated fatty acid-based in situ forming matrices for localized antimicrobial delivery. Pharmaceutics 2020, 12, 808. [Google Scholar] [CrossRef]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Vancomycin HCl-loaded lauric acid in situ-forming gel with phase inversion for periodontal pocket delivery. J. Drug. Deliv. Sci. Technol. 2020, 57, 101615. [Google Scholar] [CrossRef]
- Grimling, B.; Meler, J.; Szcześniak, M.; Pluta, J.; Górniak, A. The study of physicochemical properties of solid dispersions of ibuprofen in the presence of chitosan. Prog. Chem. Appl. Chitin. Deriv. 2015, 20, 64–72. [Google Scholar] [CrossRef]
- Irvine, J.; Afrose, A.; Islam, N. Formulation and delivery strategies of ibuprofen: Challenges and opportunities. Drug. Dev. Ind. Pharm. 2018, 44, 173–183. [Google Scholar] [CrossRef]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 16, 275–342. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Gu, J.; Dong, Z.; Mei, B. Ibuprofen rescues abnormalities in periodontal tissues in conditional presenilin 1 and presenilin 2 double knockout mice. Int. J. Mol. Sci. 2013, 14, 18457–18469. [Google Scholar] [CrossRef]
- Cochran, D.L. Inflammation and bone loss in periodontal disease. J. Periodontol. 2008, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Agossa, K.; Delepierre, A.; Lizambard, M.; Delcourt-Debruyne, E.; Siepmann, J.; Siepmann, F.; Neut, C. In-situ forming implants for dual controlled release of chlorhexidine and ibuprofen for periodontitis treatment: Microbiological and mechanical key properties. J. Drug. Deliv. Sci. Technol. 2020, 60, 101956. [Google Scholar] [CrossRef]
- Maddiboyina, B.; Krishna, A.; Sri, R.; Kalyani, B.; Jabeena, S.; Sharmila, S.; Lackshmibai, V. Preparation and evaluation of ibuprofen in-situ periodontal gel. Int. J. Allied. Med. Sci. Clin. 2020, 8, 67–75. [Google Scholar]
- Yang, R.; Hong, Y.; Wang, Y.; Zhao, L.; Shen, L.; Feng, Y. The embodiment of the strategy of “using active chemicals as excipients” in compound preparation. J. Pharm. Investig. 2021, 52, 1–22. [Google Scholar] [CrossRef]
- Li, X.; Fan, R.; Wang, Y.; Wu, M.; Tong, A.; Shi, J.; Xiang, M.; Zhou, L.; Guo, G. In situ gel-forming dual drug delivery system for synergistic combination therapy of colorectal peritoneal carcinomatosis. RSC Adv. 2015, 5, 101494–101506. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, X.; Meng, Y.; Chen, L.; Zhang, Q. Development of a dual drugs loaded hydrogel delivery system for enhanced cancer therapy: In situ forming, degradation and synergistic antitumor efficiency. J. Mater. Chem. B. 2017, 5, 8487–8497. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Mabrouk, M.; Amer, M.S.; Elkasabgy, N.A. Dual-Drug Delivery via Zein in situ forming implants augmented with titanium-doped bioactive glass for bone regeneration: Preparation, in vitro characterization, and in vivo evaluation. Pharmaceutics 2022, 14, 274. [Google Scholar] [CrossRef]
- Lizambard, M.; Menu, T.; Fossart, M.; Bassand, C.; Agossa, K.; Huck, O.; Neut, C.; Siepmann, F. In-situ forming implants for the treatment of periodontal diseases: Simultaneous controlled release of an antiseptic and an anti-inflammatory drug. Int. J. Pharm. 2019, 15, 118833. [Google Scholar] [CrossRef]
- Batool, F.; Agossa, K.; Lizambard, M.; Petit, C.; Bugueno, I.M.; Delcourt-Debruyne, E.; Benkirane-Jessel, N.; Tenenbaum, H.; Siepmann, J.; Siepmann, F.; et al. In-situ forming implants loaded with chlorhexidine and ibuprofen for periodontal treatment: Proof of concept study in vivo. Int. J. Pharm. 2019, 5, 118564. [Google Scholar] [CrossRef] [PubMed]
- Bello, O.S.; Alagbada, T.C.; Alao, O.C.; Olatunde, A.M. Sequestering a non-steroidal anti-inflammatory drug using modified orange peels. Appl. Water. Sci. 2020, 10, 172. [Google Scholar] [CrossRef]
- Doxycycline|564-25-0—ChemicalBook. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8663342.htm (accessed on 10 October 2022).
- Abroodi, M.; Bagheri, A.; Razavizadeh, B.M. Investigation of surface tension and surface properties of alkanolamine–alcohol mixtures at T = 313.15 K and P = 90.6 kPa. J. Mol. Liq. 2019, 287, 110924. [Google Scholar] [CrossRef]
- Ershad, A.L.; Rajabi-Siahboomi, A.; Missaghi, S.; Kirby, D.; Mohammed, A.R. Multi-analytical framework to assess the in vitro swallowability of solid oral dosage forms targeting patient. Pharmaceutics 2021, 13, 411. [Google Scholar] [CrossRef]
- Golmaghani-Ebrahimi, E.; Bagheri, A.; Fazli, M. The influence of temperature on surface concentration and interaction energy between components in binary liquid systems. J. Chem. Thermodyn. 2020, 146, 10615. [Google Scholar] [CrossRef]
- Mehtala, P.; Pashley, D.H.; Tjaderhane, L. Effect of dimethyl sulfoxide on dentin collagen. Dent. Mater. 2017, 33, 915–922. [Google Scholar] [CrossRef]
- Ozdogan, A.I.; Ilarslan, Y.D.; Kosemehmetoglu, K.; Akca, G.; Kutlu, H.B.; Comerdov, E.; Iskit, A.B.; Şenel, S. In vivo evaluation of chitosan based local delivery systems for atorvastatin in treatment of periodontitis. Int. J. Pharm. 2018, 550, 470–476. [Google Scholar] [CrossRef]
- Qazi, M.J.; Schlegel, S.J.; Backus, E.G.H.; Bonn, M.; Bonn, D.; Shahidzadeh, N. Dynamic surface tension of surfactants in the presence of high salt concentrations. Langmuir 2020, 36, 7956–7964. [Google Scholar] [CrossRef]
- Srichan, T.; Phaechamud, T. Designing solvent exchange-induced in situ forming gel from aqueous insoluble polymers as matrix base for periodonttitis treatment. AAPS Pharm. Sci. Tech. 2017, 18, 194–201. [Google Scholar] [CrossRef]
- Phaechamud, T.; Chanyaboonsub, N.; Setthajindalert, O. Doxycycline hyclate-loaded bleached shellac in situ forming microparticle for intraperiodontal pocket local delivery. Eur. J. Pharm. Sci. 2016, 93, 360–370. [Google Scholar] [CrossRef]
- Yu, Z.W.; Quinn, P.J. Dimethyl sulphoxide: A review of its applications in cell biology. Biosci. Rep. 1994, 14, 259–281. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Ye, H.; Guo, Z.P. Recent progress in designing stable composite lithium anodes with improved wettability. Adv. Sci. 2020, 7, 2002212. [Google Scholar] [CrossRef] [PubMed]
- CRC Press. CRC Handbook of Chemistry and Physics, 87th ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-8493-0487-3. [Google Scholar]
- Yang, L.-J.; Yang, X.-Q.; Huang, K.-M.; Jia, G.-Z.; Shang, H. Dielectric properties of binary solvent mixtures of dimethyl sulfoxide with water. Int. J. Mol. Sci. 2009, 10, 1261–1270. [Google Scholar] [CrossRef]
- Sangster, J. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Senarat, S.; Charoenteeraboon, J.; Praphanwittaya, P.; Phaechamud, T. Phase behavior of doxycycline hyclate-incorporated bleached shellac in-situ forming gel/microparticle after solvent movement. Key Eng. Mater. 2020, 859, 21–26. [Google Scholar] [CrossRef]
- Garzón, L.C.; Martínez, F. Temperature dependence of solubility for ibuprofen in some organic and aqueous solvents. J. Sol. Chem. 2004, 33, 1379–1395. [Google Scholar] [CrossRef]
- Rungseevijitprapa, W.; Bodmeier, R. Injectability of biodegradable in situ forming microparticle systems (ism). Eur. J. Pharmaceut. Sci. 2009, 36, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Huang, X.; Xie, Y.; Chen, J.; Huang, Y.; Wang, B.; Wang, H.; Pan, X.; Wu, C. An injectable in situ gel with cubic and hexagonal nanostructures for local treatment of chronic periodontitis. Drug. Deliv. 2017, 24, 1148–1158. [Google Scholar] [CrossRef]
- Rodríguez-Burford, C.; Oelschlager, D.K.; Talley, L.I.; Barnes, M.N.; Partridge, E.E.; Grizzle, W.E. The use of dimethylsulfoxide as a vehicle in cell culture experiments using ovarian carcinoma cell lines. Biotech. Histochem. 2003, 78, 17–21. [Google Scholar] [CrossRef]
- Poet, T.S.; Kirman, C.R.; Bader, M.; van Thriel, C.; Gargas, M.L.; Hinderliter, P.M. Quantitative risk analysis for N-methyl pyrrolidone using physiologically based pharmacokinetic and benchmark dose modeling. Toxicol. Sci. 2009, 113, 468–482. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-loaded solvent exchange-induced in situ forming beta-cyclodextrin gel and microparticle for periodontal pocket delivery. Mater. Sci. Eng. C. 2020, 117, 111275. [Google Scholar] [CrossRef]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–439. [Google Scholar] [CrossRef]
- Reza, M.S.; Quadir, M.A.; Haider, S.S. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J. Pharm. Sci. 2003, 6, 282–291. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichayakorn, W.; Chaiya, P.; Chinpaisal, C.; Phaechamud, T. Natural rubber blends for floating theophylline beads. Int. J. Biol. Macromol. 2013, 224, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing Limited: Sawston, UK, 2015; ISBN 978-0-08-100092-2. [Google Scholar]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Qian, H.; Chen, D.; Xu, X.; Li, R.; Yan, G.; Fan, T. FDM 3D-printed sustained-release gastric-floating verapamil hydrochloride formulations with cylinder, capsule and hemisphere shapes, and low infill percentage. Pharmaceutics 2022, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Ambrogi, V.; Rubini, D.; Giovagnoli, S.; Ricci, M.; Blasi, P.; Rossi, C. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J. Control. Release 2004, 95, 521–533. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release i. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Phaechamud, T.; Setthajindalert, O. Antimicrobial in-situ forming gels based on bleached shellac and different solvents. J. Drug Deliv. Sci. Technol. 2018, 46, 285–293. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Tuntarawongsa, S. Peppermint oil/doxycycline hyclate-loaded eudragit RS in situ forming gel for periodontitis treatment. J. Pharm. Investig. 2018, 48, 451–464. [Google Scholar] [CrossRef]
- Bannach, G.; Arcaro, R.; Ferroni, D.C.; Siqueira, A.B.; Treu-Filho, O.; Ionashiro, M.; Schnitzler, E. Thermoanalytical study of some anti-anflammatory analgesic agents. J. Therm. Anal. Calorim. 2010, 102, 163–170. [Google Scholar] [CrossRef]
- Hami, H.; Abbas, R.; Jasim, A.; Abdul abass, D.; Abed, M.A.; Maryoosh, A.A. Kinetics study of removal doxycycline drug from aqueous solution using aluminum oxide surface. Egypt. J. Chem. 2019, 62, 91–101. [Google Scholar] [CrossRef]
- Carolina Kogawa, A.; de Mello, N.P. Quantification of doxycycline in raw material by an eco-friendly method of infrared spectroscopy. Pharm. Anal. Acta. 2015, 7, 463–466. [Google Scholar] [CrossRef]
- Akesson, B. International Programme on Chemical Safety. N-Methyl-2-pyrrolidone; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Büchter, A.; Meyer, U.; Kruse-Lösler, B.; Joos, U.; Kleinheinz, J. Sustained release of doxycycline for the treatment of peri-implantitis: Randomised controlled trial. Br. J. Oral Maxillofac. Surg. 2004, 42, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Micromedex. Dimethyl Sulfoxide; Thomson Micromedex: Greenwood Village, CO, USA, 2020. [Google Scholar]
- Phaechamud, T.; Mahadlek, J.; Charoenteeraboon, J.; Choopun, S. Characterization and antimicrobial activity of N-methyl-2-pyrrolidone-loaded ethylene oxide-propylene oxide block copolymer thermosensitive gel. Indian J. Pharm. Sci. 2012, 74, 498. [Google Scholar] [CrossRef]
- Obad, J.; Šušković, J.; Kos, B. Antimicrobial activity of ibuprofen: New perspectives on an “old” non-antibiotic drug. Eur. J. Pharm. Sci. 2015, 71, 93–98. [Google Scholar] [CrossRef]
- Al-Janabi, A.A. In vitro antibacterial activity of ibuprofen and acetaminophen. J. Glob. Infect. Dis. 2010, 2, 105–108. [Google Scholar] [CrossRef]
- Seymour, R.A.; Heasman, P.A. Pharmacological control of periodontal disease. II. Antimicrobial agents. J. Dent. 1995, 23, 5–14. [Google Scholar] [CrossRef]
- Amel, Y.; Bouziane, D.; Leila, M.; Ahmed, B. Microbiological study of periodontitis in the west of Algeria. West. Indian. Med. J. 2015, 5, 7–12. [Google Scholar]
- Bevilacqua, L.; De Biasi, M.; Lorenzon, M.G.; Frattini, C.; Angerame, D. Volumetric analysis of gingival crevicular fluid and peri-implant sulcus fluid in healthy and diseased sites: A cross-sectional split-mouth pilot study. Open. Dent. J. 2016, 10, 131–138. [Google Scholar] [CrossRef]
- Charoensuksai, P.; Mahadlek, J.; Phaechamud, T.; Charoenteeraboon, J. Doxycycline and metronidazole exhibit a synergistic antibacterial activity against Porphyromonas gingivalis. Thai. Pharmaceut. Health Sci. J. 2016, 11, 92–97. [Google Scholar]
- Larsen, T. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycycline and metronidazole. Oral Microbiol. Immunol. 2002, 17, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Oettinger-Barak, O.; Dashper, S.G.; Catmull, D.V.; Adams, G.G.; Sela, M.N.; Machtei, E.E.; Reynolds, E.C. Antibiotic susceptibility of Aggregatibacter Actinomycetemcomitans JP2 in a biofilm. J. Oral Microbiol. 2013, 5, 20320. [Google Scholar] [CrossRef]
- Leelaprakash, G.; Dass, S.M. In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int. J. Drug Dev. Res. 2011, 3, 189–196. [Google Scholar]
- Kumarasinghe, N.; Dharmadeva, S.; Galgamuwa, L.; Prasadinie, C. In vitro anti-inflammatory activity of Ficus racemosa L. bark using albumin denaturation method. Int. Q. J. Res. Ayurveda 2018, 39, 239. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T.; Buckley, M. Doxycycline Is anti-Inflammatory and inhibits Staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob. Agents Chemother. 2003, 47, 3630–3633. [Google Scholar] [CrossRef] [Green Version]
- Chaiya, P.; Senarat, S.; Phaechamud, T.; Narakornwit, W. In vitro anti-inflammatory activity using thermally inhibiting protein denaturation of egg albumin and antimicrobial activities of some organic solvents. Mater. Today Proc. 2022, 65, 2290–2295. [Google Scholar] [CrossRef]
- Roche-Molina, M.; Hardwick, B.; Sanchez-Ramos, C.; Sanz-Rosa, D.; Gewert, D.; Cruz, F.M.; Gonzalez-Guerra, A.; Andres, V.; Palma, J.A.; Ibanez, B.; et al. The pharmaceutical solvent N-methyl-2-pyrrolidone (NMP) attenuates inflammation through Krüppel-like factor 2 activation to reduce atherogenesis. Sci. Rep. 2020, 10, 11636. [Google Scholar] [CrossRef]
- Ghayor, C.; Gjoksi, B.; Siegenthaler, B.; Weber, F.E. N-methyl pyrrolidone (NMP) inhibits lipopolysaccharide-induced inflammation by suppressing NF-κB signaling. Inflamm. Res. 2015, 64, 527–536. [Google Scholar] [CrossRef] [Green Version]







| Formula | Density (g/cm3) | pH | Viscosity (cP) | Surface Tension (mN/m) | Contact Angle (Degree) | ||
|---|---|---|---|---|---|---|---|
| Glass Slide | Agarose Gel | Paraffin | |||||
| ID10 | 1.0845 ± 0.0012 | 7.66 ± 0.06 | 4.02 ± 0.23 | 40.18 ± 0.41 | 18.75 ± 0.75 | 18.56 ± 3.62 | 55.17 ± 1.63 |
| ID20 | 1.0758 ± 0.0020 | 6.99 ± 0.04 | 5.73 ± 0.37 | 36.32 ± 1.17 | 16.98 ± 3.86 | 16.10 ± 1.45 | 50.71 ± 2.49 |
| ID30 | 1.0678 ± 0.006 | 6.38 ± 0.10 | 7.87 ± 0.17 | 35.94 ± 0.26 | 15.16 ± 1.19 | 16.50 ± 0.98 | 46.74 ± 4.26 |
| ID40 | 1.0586 ± 0.0011 | 5.87 ± 0.04 | 12.28 ± 0.07 b | 35.10 ± 0.87 | 15.21 ± 0.25 | 15.98 ± 0.56 | 50.57 ± 4.76 |
| IN10 | 1.0264 ± 0.0008 | 8.26 ± 0.19 | 3.80 ± 0.06 | 39.42 ± 0.19 | 12.43 ± 2.32 | 26.01 ± 0.64 | 50.04 ± 0.87 |
| IN20 | 1.0260 ± 0.0003 | 7.50 ± 0.20 | 5.95 ± 0.45 | 38.39 ± 0.76 | 11.51 ± 1.04 | 27.95 ± 0.65 | 49.03 ± 1.74 |
| IN30 | 1.0253 ± 0.0006 | 6.55 ± 0.08 | 8.17 ± 0.27 | 37.41 ± 0.69 | 16.63 ± 1.65 | 20.83 ± 0.61 | 47.42 ± 2.62 |
| IN40 | 1.0245 ± 0.0009 | 5.79 ± 0.07 | 13.86 ± 0.10 b | 36.91 ± 0.24 | 15.68 ± 0.51 | 18.69 ± 0.60 | 51.30 ± 1.40 |
| DID40 | 1.0690 ± 0.0000 | 4.93 ± 0.16 | 18.61 ± 0.19 c | 34.81 ± 1.88 | 15.42 ± 0.80 | 17.22 ± 0.82 | 53.60 ± 3.08 |
| DIN40 | 1.0260 ± 0.0004 | 5.04 ± 0.08 | 20.82 ± 0.37 c | 36.73 ± 0.89 | 15.31 ± 1.31 | 20.05 ± 0.60 | 52.74 ± 0.71 |
| NMP | 1.0265 ± 0.0008 | 10.08 ± 0.58 a | 2.04 ± 0.13 | 39.31 ± 0.28 | 31.08 ± 0.40 d | 7.12 ± 1.51 e | 54.94 ± 1.31 f |
| DMSO | 1.0935 ± 0.0007 | 10.02 ± 0.11 a | 1.98 ± 0.09 | 43.95 ± 0.13 | 33.98 ± 1.25 d | 4.30 ± 1.19 e | 64.70 ± 0.81 f |
| Formula | Deionized Water Amount (Mean ± S.D. (μL)) | % Water Tolerance (Mean ± S.D.) | ||
|---|---|---|---|---|
| 25 °C | 37 °C | 25 °C | 37 °C | |
| ID10 | 893.33 ± 9.43 | 1046.67 ± 24.94 | 26.27 ± 0.20 | 29.45 ± 0.49 |
| ID20 | 726.67 ± 9.43 | 806.67 ± 24.94 | 22.47 ± 0.23 | 24.34 ± 0.57 |
| ID30 | 593.33 ± 24.94 | 846.67 ± 24.94 | 19.13 ± 0.65 | 25.24 ± 0.55 |
| ID40 | 480.00 ± 16.33 | 613.33 ± 24.94 | 16.06 ± 0.46 | 19.65 ± 0.64 |
| IN10 | 1300.00 ± 28.28 | 1733.33 ± 24.94 | 34.14 ± 0.49 | 40.87 ± 0.35 |
| IN20 | 1126.67 ± 9.43 | 1440.00 ± 28.28 | 31.00 ± 0.18 | 36.48 ± 0.46 |
| IN30 | 766.67 ± 24.94 | 1133.33 ± 18.86 | 23.41 ± 0.58 | 31.13 ± 0.36 |
| IN40 | 566.67 ± 18.86 | 766.67 ± 24.94 | 18.43 ± 0.50 | 23.41 ± 0.58 |
| DID40 | 463.04 ± 10.27 | 604.38 ± 17.22 | 12.92 ± 1.04 | 17.02 ± 0.44 |
| DIN40 | 550.41 ± 24.16 | 748.11 ± 20.16 | 15.03 ± 0.70 | 20.79 ± 0.41 |
| Formulation | Injectability Properties | Mechanical Properties | ||||
|---|---|---|---|---|---|---|
| Injection Force (N) | AUC of Injection (N∙mm.) | Maximum Force (N) | Remaining Force (N) | Adhesion Force (N) | Mechanical Properties | |
| DMSO | 0.734 ± 0.047 a | 12.339 ± 0.375 c | - | - | - | - |
| ID40 | 1.837± 0.010 | 33.882 ± 0.663 | 0.736 ± 0.001 | 0.275 ± 0.009 | 0.083 ± 0.002 | 0.374 ± 0.013 |
| DID40 | 2.365 ± 0.013 | 43.242 ± 0.200 | 1.678 ± 0.161 | 0.792 ± 0.093 | 0.091 ± 0.001 | 0.471 ± 0.019 |
| NMP | 0.904 ± 0.171 b | 12.195 ± 0.369 d | - | - | - | - |
| IN40 * | 1.930 ± 0.053 | 36.291 ± 0.081 | - | - | 0.130 ± 0.003 | - |
| DIN40 | 2.543 ± 0.022 | 48.357 ± 0.560 | 2.623 ± 0.079 | 1.477± 0.057 | 0.087 ± 0.009 | 0.563 ± 0.005 |
| Formulation | Modelling | Criteria for Model Selection | Kinetic Parameters | ||||
|---|---|---|---|---|---|---|---|
| R2 | AIC | MSC | |||||
| DD | Zero order | 0.4415 | 32.6951 | −1.0092 | k0 = 396.66 | ||
| First order | 0.8925 | 39.1268 | 1.1125 | k1 = 11.8593 | |||
| Higuchi | 0.7001 | 47.2998 | −0.2496 | kH = 135.2473 | |||
| Korsmeyer-Peppas | 0.9845 | 23.8737 | 2.6192 | kKP = 105.0155 | n = 0.1965 | ||
| Hixson | 0.7484 | 46.1530 | −0.0585 | kHC = 1.9307 | |||
| Hopfenberg | 0.8656 | 41.1295 | 0.7787 | kHB = 0.0038 | n = 4411.0392 | ||
| Peppas-Sahlin | 0.9905 | 13.9208 | 4.6098 | k1 = 180.6271 | k2 = 96.0516 | m = 0.3160 | |
| DID40 | Zero order | 0.4050 | 24.4736 | −1.0740 | k0 = 136.33 | ||
| First order | 0.0682 | 33.3778 | −1.5290 | k1 = 1.0087 | |||
| Higuchi | 0.2194 | 61.0878 | −1.2085 | kH = 31.3751 | |||
| Korsmeyer-Peppas | 0.9345 | 57.4259 | 1.2889 | kKP = 29.8363 | n = 0.0861 | ||
| Hixson | 0.0042 | 33.7183 | −1.5971 | kHC = 1.9307 | |||
| Hopfenberg | 0.2707 | 25.6048 | −1.3568 | kHB = 0.0026 | n = 734.1810 | ||
| Peppas-Sahlin | 0.9871 | 13.5315 | 3.4335 | k1 = 53.9199 | k2 = −22.6506 | m = 0.2636 | |
| DN | Zero order | 0.0736 | 43.8395 | −1.4003 | k0 = 218.1449 | ||
| First order | 0.8795 | 40.8805 | 0.8518 | k1 = 9.4428 | |||
| Higuchi | 0.7863 | 36.4714 | 0.0734 | kH = 144.0345 | |||
| Korsmeyer-Peppas | 0.9789 | 24.8168 | 2.4043 | kKP = 101.2309n = 0.2166 | n = 0.2166 | ||
| Hixson | 0.7086 | 55.0420 | −0.0523 | kHC = 1.4516 | |||
| Hopfenberg | 0.8563 | 50.0978 | 0.6541 | kHB = 0.0030 | n = 5010.6982 | ||
| Peppas-Sahlin | 0.9940 | 17.4275 | 3.8821 | k1 = 224.8520 | k2 = −157.0385 | m = 0.4227 | |
| DIN40 | Zero order | 0.2189 | 38.5772 | −1.1486 | k0 = 144.8828 | ||
| First order | 0.2704 | 110.9836 | −0.4770 | k1 = 1.2346 | |||
| Higuchi | 0.1722 | 113.6096 | −0.6790 | kH = 42.5461 | |||
| Korsmeyer-Peppas | 0.9796 | 40.7243 | 2.8788 | kKP = 63.3992 | n = 0.1984 | ||
| Hixson | 0.3735 | 46.0956 | −0.8243 | kHC = 0.5602 | |||
| Hopfenberg | 0.2039 | 112.9868 | −0.6311 | kHB = 0.0004 | n = 3235.7009 | ||
| Peppas-Sahlin | 0.9857 | 60.2321 | 3.4269 | k1 = 75.0866 | k2 = −11.5256 | m = 0.2402 | |
| Formulation | Modelling | Criteria for Model Selection | Kinetic Parameters | ||||
|---|---|---|---|---|---|---|---|
| R2 | AIC | MSC | |||||
| DID40 | Zero order | 0.3656 | 86.5737 | 0.1331 | k0 = 6.4001 | ||
| First order | 0.5031 | 83.3354 | 0.3822 | k1 = 0.0818 | |||
| Higuchi | 0.8787 | 64.9331 | 1.7978 | kH = 14.0786 | |||
| Korsmeyer-Peppas | 0.9433 | 53.1028 | 2.7078 | kKP = 16.2576 kKP = 29.8363 | n = 0.3875 | ||
| Hixson | 0.4610 | 84.4003 | 0.3003 | kHC = 0.0251 | |||
| Hopfenberg | 0.4576 | 85.3405 | 0.2280 | kHB = 0.0001 | n = 750.6823 | ||
| Peppas-Sahlin | 0.9474 | 52.4558 | 2.7576 | k1 = 10.363 | k2 = 5.713 | m = 0.282 | |
| DIN40 | Zero order | 0.3184 | 99.2511 | 0.0064 | k0 = 9.907 | ||
| First order | 0.5583 | 92.6213 | 0.5164 | k1 = 0.1514 | |||
| Higuchi | 0.8885 | 70.2564 | 2.2367 | kH = 21.8944 | |||
| Korsmeyer-Peppas | 0.9637 | 60.2584 | 3.0058 | kKP = 25.729 | n = 0.364 | ||
| Hixson | 0.4912 | 94.8484 | 0.3450 | kHC = 0.0440 | |||
| Hopfenberg | 0.5178 | 94.6278 | 0.3620 | kHB = 0.000 | n = 1493.131 | ||
| Peppas-Sahlin | 0.9665 | 60.0905 | 3.0187 | k1 = 20.2566 | k2 = 5.3191 | m = 0.3400 | |
| Formulation | TGA Parameter | DSC Parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset Temp (°C) | Degradation Temp (°C) | End Temp (°C) | Onset Temp(°C) | T Peak (°C) | Enthalpy (∆H) (J/g) | |||||||
| Peak 1 | Peak 2 | Peak 1 | Peak 2 | Peak 1 | Peak 2 | Peak 1 | Peak 2 | Peak 1 | Peak 2 | Peak 1 | Peak 2 | |
| DH | 157.11 | 168.66 | 160.72 | 222.07 | 168.30 | 225.07 | 151.71 | 186.66 | 163.66 | 202.32 | 59.63 | 71.64 |
| IBU | 217.6 | - | 232.25 | - | 250.84 | - | 75.48 | 225.55 | 78.67 | 246.81 | 92.99 | 101.74 |
| DIN | 208.5 | - | 229.58 | - | 238.80 | - | 74.56 | 198.42 | 78.68 | 237.30 | 79.59 | 300.70 |
| DID | 199.15 | - | 228.69 | - | 232.80 | - | 74.15 | 200.9 | 77.01 | 231.29 | 72.10 | 207.95 |
| Formula | Clear Zone Diameter (mm.) (Mean ± S.D.) | |||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 6538 | S. aureus ATCC 4430 | S. aureus ATCC 6532 | S. aureus ATCC 25923 | E. coli ATCC 8739 | C. albicans ATCC 10231 | P. gingivalis ATCC 33277 | A. actinomycetemcomitans ATCC 29522 | |
| NMP | 15.7 ± 1.5 | 16.7 ± 0.6 | 16.0 ± 1.0 | 14.3 ± 0.6 | 15.3 ± 0.6 | 29.7 ± 0.6 j | 17.0 ± 1.0 | 47.7 ± 2.1 n |
| DN | 23.0 ± 1.0 a | 25.7 ± 0.6 c | 25.7 ± 0.6 d | 25.7 ± 0.6 f | 20.7 ± 1.2 h | 29.7 ± 1.2 j | 27.3 ± 1.5l | 48.0 ± 2.0 n |
| DIN 40 | 26. 0± 1.0 a | 27.3 ± 0.6 c | 27.3 ± 0.6 d | 27.0 ± 1.0 f | 21.3 ± 1.5 h | 27.7 ± 1.5 j | 27.0 ± 1.0 l | 47.0 ± 1.0 n |
| DMSO | 13.0 ± 1.0 | 12.7 ± 1.2 | 14.0 ± 1.0 | 12.3 ± 0.6 | 12.7 ± 0.6 | 21.0 ± 1.0 k | 14.3 ± 0.6 | 24.0 ± 1.0 |
| DD | 27.7 ± 0.6 b | 30.7 ± 0.6 d | 32.0 ± 1.0 e | 31.0 ± 1.0 g | 22.7 ± 0.6 i | 22.3 ± 1.2 k | 28.7 ± 0.6 m | 41.7 ± 1.5 o |
| DID 40 | 29.7 ± 0.6 b | 31.0 ± 1.0 d | 32.3 ± 0.6 e | 31.7 ± 0.6 g | 23.3 ± 0.6 i | 22.7 ± 0.6k | 30.7 ± 0.6 m | 40.0 ± 1.0 o |
| Formulation Code | IBU (% w/w) | DH (% w/w) | Organic Solvent (Adjust to 100% w/w) |
|---|---|---|---|
| ID10 | 10 | - | DMSO |
| ID20 | 20 | - | DMSO |
| ID30 | 30 | - | DMSO |
| ID40 | 40 | - | DMSO |
| IN10 | 10 | - | NMP |
| IN20 | 20 | - | NMP |
| IN30 | 30 | - | NMP |
| IN40 | 40 | - | NMP |
| DID40 | 40 | 5 | DMSO |
| DIN40 | 40 | 5 | NMP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puyathorn, N.; Senarat, S.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical and Bioactivity Characteristics of Doxycycline Hyclate-Loaded Solvent Removal-Induced Ibuprofen-Based In Situ Forming Gel. Gels 2023, 9, 128. https://doi.org/10.3390/gels9020128
Puyathorn N, Senarat S, Lertsuphotvanit N, Phaechamud T. Physicochemical and Bioactivity Characteristics of Doxycycline Hyclate-Loaded Solvent Removal-Induced Ibuprofen-Based In Situ Forming Gel. Gels. 2023; 9(2):128. https://doi.org/10.3390/gels9020128
Chicago/Turabian StylePuyathorn, Napaphol, Setthapong Senarat, Nutdanai Lertsuphotvanit, and Thawatchai Phaechamud. 2023. "Physicochemical and Bioactivity Characteristics of Doxycycline Hyclate-Loaded Solvent Removal-Induced Ibuprofen-Based In Situ Forming Gel" Gels 9, no. 2: 128. https://doi.org/10.3390/gels9020128