DOE-Assisted Formulation, Optimization, and Characterization of Tioconazole-Loaded Transferosomal Hydrogel for the Effective Treatment of Atopic Dermatitis: In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
2. Results
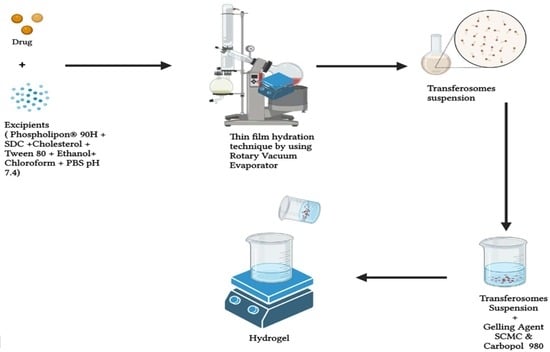
2.1. Design of Tioconazole Transferosomes Suspension (TTFs)
2.2. Optimization of Formulation Variable
2.3. Drug and Excipients Interaction Study
2.4. In Vitro Permeation Study of Tioconazole Transferosomes (TTFs)
2.5. Characterization and Optimization of Tioconazole Transferosomal Hydrogel (TTFsH)
2.6. In Vitro Drug Release Study
2.7. In Vivo Study of Scratching Score and Erythema Score
2.8. Histopathological Examination
3. Discussion
4. Material
4.1. Chemicals
4.2. Animals
4.3. Design of Experiments and Statistical Data Analysis (DOE Version 13)
4.4. Formulation of Transferosome Suspension (TTFs)
4.5. Characterization of Tioconazole-Loaded Transferosomes Suspension (TTFs)
Drug and Excipients Interaction Study
4.6. In Vitro Permeation Studies of TFs
4.7. Formulation of TTF-Loaded Hydrogel (TTFsH)
4.8. Characterization of TTFsH
4.8.1. In Vitro Drug Release Study
4.8.2. In Vivo Study of Scratching Score and Erythema Score
4.8.3. Histopathological Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| TZ | Tioconazole |
| SCMC | Sodium carboxymethyl cellulose |
| TFs | Transferosomes |
| TTFs | Tioconazole transferosomes suspension |
| TTFsH | Tioconazole transferosomes suspension loaded hydrogel |
| AD | Atopic dermatitis |
| DOE | Design of experiments and statistical data analysis |
| PDI | Polydispersity index |
| EE | Entrapment efficiency |
| FT-IR | Fourier transform infrared |
| DSC | Differential scanning colorimetry |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| BCS | Biopharmaceutically classified system |
| CPCSFA | Committee for the Purpose of Control And Supervision of Experiments on Animals |
| DNCB | 1-cholro-2,4-dinitrobenzene |
| SD | Sprague Dawley |
| IAEC | Institutional Animal Ethical Committee |
| SD | Standard deviation |
| R2 | Regression coefficient |
Appendix A. Legend of Figures
| Figure Number | Description |
| Figure 1a–c | Three-dimension surface response plot showing the effect of Phospholipon®90H concentration with respect to sodium deoxycholate concentration over the particle size, PDI, and % EE, respectively. |
| Figure 1d–f | Contour plot showing the effect of Phospholipon®90H concentration with respect to sodium deoxycholate concentration over the particle size, PDI, and % EE, respectively. |
| Figure 1g | Overlay plot of optimized area for batch B4 (in yellow). |
| Figure 2 | Vesicle size and zeta potential of the optimum batch of TTFs. Batch B4 had a vesicle size of 171.4 nm ± 9.03 (a) and a zeta potential of −26.13 (b), which facilitated the cross of the stratum corneum and stability to the TFs vesicles. FE-SEM images of B4 optimized batch of Tz-loaded transferosomes suspension (TTFs) (c and d), which was spherical, well identified, unilamellar nanovesicles. |
| Figure 3 | FTIR overlay of Tz, TTFs, and TTFsH indicated the coordination of Tz to phospholipon®90H and a sodium deoxycholate complex. (a) DSC thermogram of Tz and TTFs predicted the amorphous or partially amorphous state of Tz in TFs, which led to a high-energy state and high disorder, resulting in enhanced solubility (b). |
| Figure 4 | In vitro permeation studies of Tz-loaded TFs in saline phosphate buffer, pH 6.5, using Franz diffusion cell with freshly shredded dorsal rat skin. |
| Figure 5 | Cumulative % Tz release from different batches of TTFsH. The F2 batch showed the highest % Tz release and followed a Higuchi drug release mechanism. It showed an initial burst release followed by slow and sustained release of Tz. |
| Figure 6 | Four weeks of treatment with TTFsH (low dose) resulted in significant recovery of the skin compared to the marketed formulation, as shown in decreased scratching score (a) and erythema score (b). Clinical features of SD rat skin after the topical treatment of hydrogel base, marketed formulation (Mkt. formulation), TTFsH (low dose), and TTFsH (high dose) on DNCB-induced AD (c). |
| Figure 7 | Histopathology of (a) normal skin tissue, (b) model group (induced by DNCB), (c) tissue treated with hydrogel base, (d) tissue treated with marketed formulation, (e) tissue treated with TTFsH (low dose), and (f) tissue treated with TTFsH (high dose). |
Appendix B. Legend of Tables
| Table Number | Description |
| Table 1 | Independent and dependent variables for the multilevel factorial design. |
| Table 2 | Different concentrations of phospholipon ®90H and sodium deoxycholate in TFs formulation using 32 factorial design. |
| Table 3 | Summary of results of regression analysis for responses Y1, Y2,, and Y3 |
| Table 4 | Characterization of prepared tioconazole transferosomes suspension (TTFs). |
| Table 5 | Formulation of tioconazole-loaded transferosomes suspension (TTFs) |
| Table 6 | Physiochemical parameters of transferosome-loaded hydrogel (TTFsH) with ± SD |
| Table 7 | The calculated correlation coefficients for the in vitro release of Tz from transferosomes employing different kinetic orders or systems. |
| Table 8 | Hydrogel base batches with different concentrations of gelling agent (Carbopol 980 or sodium CMC) |
References
- Brenninkmeijer, E.E.A.; Schram, M.E.; Leeflang, M.M.G.; Bos, J.D.; Spuls, P.I. Diagnostic criteria for atopic dermatitis: A systematic review. Br. J. Dermatol. 2008, 158, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L. Atopic dermatitis: A review of topical treatment options. Curr. Med. Res. Opin. 2010, 26, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wokovich, A.M.; Prodduturi, S.; Doub, W.H.; Hussain, A.S.; Buhse, L.F. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur. J. Pharm. Biopharm. 2006, 64, 1–8. [Google Scholar] [CrossRef]
- Jevons, S.; Gymer, G.E.; Brammer, K.W.; Cox, D.A.; Leeming, M.R. Antifungal activity of tioconazole (UK-20,349), a new imidazole derivative. Antimicrob. Agents Chemother. 1979, 15, 597–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, C.; Johnsen, K.; Kiser, J. Ag Nanocluster Formation Using a Cytosine Oligonucleotide Template. Bone 2008, 23, 1–7. [Google Scholar]
- Jøhnke, H.; Vach, W.; Norberg, L.A.; Bindslev-Jensen, C.; Høst, A.; Andersen, K.E. A comparison between criteria for diagnosing atopic eczema in infants. Br. J. Dermatol. 2005, 153, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E. Transfersomes for transdermal drug delivery. Expert. Opin. Drug Deliv. 2006, 3, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Vasudevan, D.; Biju Mukund, V.; Jose, S. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv. J. Deliv. Target Ther. Agents 2006, 13, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.; Anand, S.; Koul, V. Electroporation of polymeric nanoparticles: An alternative technique for transdermal delivery of insulin. Drug Dev. Ind. Pharm. 2010, 36, 1303–1311. [Google Scholar] [CrossRef]
- Sailor, G.; Seth, A.K.; Parmar, G.; Chauhan, S.; Javia, A. Formulation and in vitro evaluation of berberine containing liposome optimized by 32 full factorial designs. J. Appl. Pharm. Sci. 2015, 5, 23–28. [Google Scholar] [CrossRef] [Green Version]
- González-Rodríguez, M.L.; Barros, L.B.; Palma, J.; González-Rodríguez, P.L.; Rabasco, A.M. Application of statistical experimental design to study the formulation variables influencing the coating process of lidocaine liposomes. Int. J. Pharm. 2007, 337, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Bragagni, M.; Mennini, N.; Maestrelli, F.; Cirri, M.; Mura, P. Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Deliv. 2012, 19, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.A.; Aboul-Einien, M.H.; El Taweel, M.M. Dry Gel Containing Optimized Felodipine-Loaded Transferosomes: A Promising Transdermal Delivery System to Enhance Drug Bioavailability. AAPS Pharm. Sci. Tech. 2018, 19, 2155–2173. [Google Scholar] [CrossRef]
- Djekic, L.; Martinović, M.; Dobričić, V.; Čalija, B.; Medarević, Đ.; Primorac, M. Comparison of the Effect of Bioadhesive Polymers on Stability and Drug Release Kinetics of Biocompatible Hydrogels for Topical Application of Ibuprofen. J. Pharm. Sci. 2019, 108, 1326–1333. [Google Scholar] [CrossRef]
- Rogerson, A.; Cummings, J.; Florence, A.T. Adriamycin-loaded niosomes: Drug entrapment, stability and release. J. Microencapsul. 1987, 4, 321–328. [Google Scholar] [CrossRef]
- Amnuaikit, T.; Limsuwan, T.; Khongkow, P.; Boonme, P. Vesicular carriers containing phenylethyl resorcinol for topical delivery system; liposomes, transfersomes and invasomes. Asian J. Pharm. Sci. 2018, 13, 472–484. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Vasilieva, E.A.; Kuznetsov, D.M.; Lenina, O.A.; Filippov, S.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Enhancement of the Transdermal Delivery of Nonsteroidal Anti-inflammatory Drugs Using Liposomes Containing Cationic Surfactants. ACS Omega 2022, 7, 25741–25750. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Valizadeh, H.; Zakeri-Milani, P. Application of response surface methodology in development of sirolimus liposomes prepared by thin film hydration technique. BioImpacts 2013, 3, 75–81. [Google Scholar] [CrossRef]
- Mahmood, S.; Taher, M.; Mandal, U.K. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int. J. Nanomed. 2014, 9, 4331–4346. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Sen, S.O.; Maji, R.; Nayak, A.K.; Sen, K.K. Transferosomal gel for transdermal delivery of risperidone: Formulation optimization and ex vivo permeation. J. Drug Deliv. Sci. Technol. 2017, 38, 59–71. [Google Scholar] [CrossRef]
- Kharwade, R.S.; Mahajan, N.M. Formulation and Evaluation of Nanostructured Lipid Carriers Based Anti-Inflammatory Gel for Topical Drug Delivery System. Asian J. Pharm. Clin. Res. 2019, 12, 286–291. [Google Scholar] [CrossRef]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of dendrimers on solubility of hydrophobic drug molecules. Front. Pharmacol. 2017, 8, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Mendhe, A.A.; Kharwade, R.S.; Mahajan, U.N. Dissolution enhancement of poorly water-soluble drug by cyclodextrins inclusion complexation. Int. J. Appl. Pharm. 2016, 8. [Google Scholar]
- Zaafarany, E.G.M.; Awad, G.A.S.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Ramos Campos, E.V.; Proença, P.L.D.F.; Doretto-Silva, L.; Andrade-Oliveira, V.; Fraceto, L.F.; de Araujo, D.R. Trends in nanoformulations for atopic dermatitis treatment. Expert Opin. Drug Deliv. 2020, 17, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Hussain, Z.; Thu, H.E.; Khan, S.; Katas, H.; Ahmed, T.A.; Tripathy, M.; Leng, J.; Qin, H.L.; Bukhari, S.N.A. Drug nanocarrier, the future of atopic diseases: Advanced drug delivery systems and smart management of disease. Colloids Surf. B Biointerfaces 2016, 147, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Eouani, C.; Piccerelle, P.; Prinderre, P.; Bourret, E.; Joachim, J. In-vitro comparative study of buccal mucoadhesive performance of different polymeric films. Eur. J. Pharm. Biopharm. 2001, 52, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Motawaa, A.M.; Samaha, M.W. Nanovesicular carrier-mediated transdermal delivery of tadalafil: I-formulation and physicsochemical characterization. Drug Dev. Ind. Pharm. 2015, 41, 714–721. [Google Scholar] [CrossRef]
- Ramteke, S.; Haigune, N.; More, S.; Pise, S.; Pise, A.; Kharwade, R. Flaxseed Mucilage Hydrogel based Floating Drug Delivery System: Design and Evaluation. Res. J. Pharm. Technol. 2022, 15, 1549–1554. [Google Scholar] [CrossRef]
- Kharwade, R.; Mahajan, N.; More, S.; Warokar, A.; Dhobley, A.; Palve, D. Effect of PEGylation on drug uptake, biodistribution, and tissue toxicity of efavirenz–ritonavir loaded PAMAM G4 dendrimers. Pharm. Dev. Technol. 2023, 28, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Li, Z.A. Review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Kharwade, R.; Nair, H.; Masurkar, D.; Pise, A.; More, S.; Pise, S. Formulation and Evaluation of Chronomodulated Pulsatile Drug Delivery System for Nocturnal Hyperacidity. Res. J. Pharm. Technol. 2022, 15, 1449–1454. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Altenbach, M.; Schnyder, N.; Zimmermann, C.; Imanidis, G. Quantitative assessment of tissue retention, lipophilicity, ionic valence and convective transport of permeant as factors affecting iontophoretic enhancement. J. Drug Deliv. Sci. Technol. 2006, 16, 91–98. [Google Scholar] [CrossRef]
- Khan, I.; Needham, R.; Yousaf, S.; Houacine, C.; Islam, Y.; Bnyan, R.; Sadozai, S.K.; Elrayess, M.A.; Elhissi, A. Journal of Drug Delivery Science and Technology Impact of phospholipids, surfactants and cholesterol selection on the performance of transfersomes vesicles using medical nebulizers for pulmonary drug delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102822. [Google Scholar] [CrossRef]
- Singh, S.; Verma, D.; Mirza, M.A.; Das, A.K.; Dudeja, M.; Anwer, M.K.; Sultana, Y.; Talegaonkar, S.; Iqbal, Z. Development and optimization of ketoconazole loaded nano-transfersomal gel for vaginal delivery using Box-Behnken design: In vitro, ex vivo characterization and antimicrobial evaluation. J. Drug Deliv. Sci. Technol. 2017, 39, 95–103. [Google Scholar] [CrossRef]
- Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol. Biol. 2017, 1522, 17–22. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Nanosized nasal emulgel of resveratrol: Preparation, optimization, in vitro evaluation and in vivo pharmacokinetic study. Drug Dev. Ind. Pharm. 2019, 45, 1624–1634. [Google Scholar] [CrossRef]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Formulation and optimisation of novel transfersomes for sustained release of local anaesthetic. J. Pharm. Pharmacol. 2019, 71, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharwade, R.; More, S.; Suresh, E.; Warokar, A.; Mahajan, N. Improvement in Bioavailability and Pharmacokinetic Characteristics of Efavirenz with Booster Dose of Ritonavir in PEGylated PAMAM G4 Dendrimers. AAPS Pharm. Sci. Tech. 2022, 23, 177. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Yu, C.; Lin, H.; Zhou, X. Development of tacrolimus-loaded transfersomes for deeper skin penetration enhancement and therapeutic effect improvement in vivo. Asian J. Pharm. Sci. 2013, 8, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.A.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, Q.; Wang, Q.; Sun, C.; Zhu, Y.; Niu, Y.; Yu, J.; Xu, X. Formulation, Characterization, and Pharmacokinetic Studies of 6-Gingerol-Loaded Nanostructured Lipid Carriers. AAPS Pharm. Sci. Tech. 2018, 19, 3661–3669. [Google Scholar] [CrossRef]
- Betz, G.; Imboden, R.; Imanidis, G. Interaction of liposome formulations with human skin in vitro. Int. J. Pharm. 2001, 229, 117–129. [Google Scholar] [CrossRef]
- Madison, K.C.; Swartzendruber, D.C.; Wertz, P.W.; Downing, D.T. Presence of intact intercellular lipid lamellae in the upper layers of the stratum corneum. J. Investig. Dermatol. 1987, 88, 714–718. [Google Scholar] [CrossRef] [Green Version]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Characterization and In Vitro Skin Permeation of Meloxicam-Loaded Liposomes versus Transfersomes. J. Drug Deliv. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro-in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef]
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm. J. 2012, 20, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Hui, P.C.L.; Wat, E.; Ng, F.S.F.; Kan, C.W.; Wang, X.; Wong, E.C.W.; Hu, H.; Chan, B.; Lau, C.B.S.; et al. In vitro drug release and percutaneous behavior of poloxamer-based hydrogel formulation containing traditional Chinese medicine. Colloids Surf. B Biointerfaces 2016, 148, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.R. Lecithin-microemulsion based organogels as topical drug delivery system (TDDS). Int. J. Curr. Res. Rev. 2011, 3, 22–33. [Google Scholar]
- Jain, S.; Patel, N.; Madan, P.; Lin, S. Formulation and rheological evaluation of ethosome-loaded carbopol hydrogel for transdermal application. Drug Dev. Ind. Pharm. 2016, 42, 1315–1324. [Google Scholar] [CrossRef]
- Li, J.; Duan, N.; Song, S.; Nie, D.; Yu, M.; Wang, J.; Xi, Z.; Li, J.; Sheng, Y.; Xu, C.; et al. Transfersomes improved delivery of ascorbic palmitate into the viable epidermis for enhanced treatment of melasma. Int. J. Pharm. 2021, 608, 121059. [Google Scholar] [CrossRef]
- El Afify, M.S.; Zein El Dein, E.A.; Elsadek, B.E.M.; Mohamed, M.A.; El-Gizawy, S.A. Development and optimization of a novel drug free nanolipid vesicular system for treatment of osteoarthritis. Drug Dev. Ind. Pharm. 2018, 44, 767–777. [Google Scholar] [CrossRef]
- Al Shuwaili, A.H.; Rasool, B.K.A.; Abdulrasool, A.A. Optimization of elastic transfersomes formulations for transdermal delivery of pentoxifylline. Eur. J. Pharm. Biopharm. 2016, 102, 101–114. [Google Scholar] [CrossRef]
- Ostertagová, E.; Ostertag, O. Methodology and Application of Oneway ANOVA. Am. J. Mech. Eng. 2013, 1, 256–261. [Google Scholar]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, T.; Hikita, I.; Asakawa, M.; Hirasawa, T.; Deguchi, M.; Matsutani, T.; Oku, H.; Horikawa, T.; Arimura, A. Spontaneous scratching behaviour in DS-Nh mice as a possible model for pruritus in atopic dermatitis. Immunology 2006, 118, 293–301. [Google Scholar] [CrossRef]
- Fule, R.; Kaleem, M.; Asar, T.O.; Rashid, M.A.; Shaik, R.A.; Eid, B.G.; Nasrullah, M.Z.; Ahmad, A.; Kazmi, I. Formulation, Optimization and Evaluation of Cytarabine-Loaded Iron Oxide Nanoparticles: From In Vitro to In Vivo Evaluation of Anticancer Activity. Nanomaterials 2023, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, S.; Arami, S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. Biomed. Res. Int. 2013, 2013, 616810. [Google Scholar] [CrossRef] [PubMed]









| Independent Variables Concentration | Variable Levels | ||
|---|---|---|---|
| Low (0) | Medium (1) | High (2) | |
| X1 = Phospholipon ®90H (mg) | 50 | 100 | 150 |
| X2 = Sodium deoxycholate (mg) | 15 | 30 | 45 |
| Dependent Variables Goal | |||
| Y1 = Particle size | Minimum in nanoscale | ||
| Y2 = PDI | Minimum range from 0 to 1 | ||
| Y3 = Entrapment Efficiency | Maximum | ||
| Formulation Code | X1 = Phospholipon ®90H (mg) | X2 = Sodium Deoxycholate (mg) |
|---|---|---|
| B1 | 100 | 15 |
| B2 | 100 | 45 |
| B3 | 50 | 30 |
| B4 | 50 | 45 |
| B5 | 100 | 30 |
| B6 | 150 | 30 |
| B7 | 150 | 15 |
| B8 | 150 | 45 |
| B9 | 50 | 15 |
| Model | R2 Value | Adjusted R2 | Predicted R2 | F-Value | S.D. | CV% |
|---|---|---|---|---|---|---|
| Y1 | 0.96 | 0.94 | 0.72 | 21.09 | 7.68 | 4.05 |
| Y2 | 0.94 | 0.92 | 0.84 | 26.03 | 0.78 | 0.83 |
| Y3 | 0.92 | 0.96 | 0.89 | 23.07 | 1.12 | 1.93 |
| Formulation Code | Vesicle Size (nm) (Y1) | Flux (µg/cm2/h) (Y2) | %EE (Y3) | Zeta Potential (mV) | Polydispersity Index Y2 (PDI) |
|---|---|---|---|---|---|
| B1 | 130.42 ± 10.07 | 32.61 ± 0.12 | 72.55 ± 1.47 | −19.46 ± 1.09 | 0.54 ± 0.09 |
| B2 | 331.43 ± 14.41 | 34.92 ± 0.62 | 44.47 ± 2.90 | −26.14 ± 2.04 | 1.03 ± 0.12 |
| B3 | 223.35 ± 19.27 | 43.04 ± 0.34 | 88.12 ± 3.05 | −18.84 ± 1.34 | 1.06 ± 0.22 |
| B4 | 171.41 ± 9.03 | 48.23 ± 0.42 | 93.89 ± 2.41 | −20.73 ± 1.23 | 0.36 ± 0.02 |
| B5 | 263.85 ± 12.14 | 42.72 ± 0.18 | 44.86 ± 1.97 | −14.02 ± 1.14 | 0.45 ± 0.04 |
| B6 | 109.42 ± 9.07 | 35.51 ± 0.31 | 64.11 ± 1.54 | −0.14 ± 0.12 | 0.32 ± 0.02 |
| B7 | 169.82 ± 10.18 | 33.72 ± 0.41 | 80.89 ± 2.54 | −0.87 ± 0.17 | 1.02 ± 0.14 |
| B8 | 272.92 ± 9.47 | 32.93 ± 0.81 | 53.05 ± 1.43 | −23.44 ± 1.47 | 0.19 ± 0.02 |
| B9 | 207.62 ± 11.02 | 31.12 ± 0.11 | 88.12 ± 1.34 | −19.34 ± 1.27 | 1.02 ± 0.03 |
| Ingredients | Quantity |
|---|---|
| Drug (Tioconazole) | 500 mg |
| Cholesterol | 10 mg |
| Phospholipon®90H | 50 mg |
| Tween 80 | 1 mL |
| Sodium Deoxycholate | 45 mg |
| Ethanol | 2 mL |
| Chloroform | 1 mL |
| PBS (pH 7.4) | q.s.10 mL |
| Formulation Batches | Homogeneity | pH | Viscosity (Pa/s) | Spreadability (cm) | Flux (µg/cm2/h) | % Drug Content |
|---|---|---|---|---|---|---|
| F1 | Poor | 6.1 ± 0.16 | 3.6 ± 0.98 | 27.1 ± 0.12 | 41.08 ± 1.34 | 92.87 ± 1.47 |
| F2 | Excellent | 6.5 ± 0.36 | 4.9 ± 0.36 | 26.6 ± 0.24 | 47.23 ± 0.82 | 95.72 ± 2.90 |
| F3 | Good | 6.3 ± 0.16 | 5.8 ± 0.57 | 21.7 ± 0.33 | 44.63 ± 0.85 | 89.87 ± 1.66 |
| F4 | Good | 7.1 ± 0.23 | 5.4 ± 0.46 | 27.6 ± 0.60 | 32.61 ± 1.02 | 90.73 ± 2.41 |
| F5 | Excellent | 6.6 ± 0.17 | 6.4 ± 0.71 | 26.8 ± 0.24 | 30.42 ± 2.52 | 93.67 ± 1.90 |
| F6 | Good | 6.6 ± 0.16 | 7.9 ± 0.56 | 22.6 ± 0.37 | 30.14 ± 0.95 | 89.45 ± 1.48 |
| Transferosomes Batches | Zero Order (R2) | First Order (R2) | Higuchi Model (R2) | Korsmeyer–Peppas Model (R2) |
|---|---|---|---|---|
| F1 | 0.8041 | 0.6927 | 0.9647 | 0.0981 |
| F2 | 0.8172 | 0.6701 | 0.9678 | 0.0890 |
| F3 | 0.8609 | 0.6781 | 0.9731 | 0.0822 |
| F4 | 0.8371 | 0.6902 | 0.9726 | 0.0811 |
| F5 | 0.8161 | 0.7012 | 0.9801 | 0.0922 |
| F6 | 0.8091 | 0.7011 | 0.9756 | 0.0840 |
| Formulation Batches | Sodium MetaBisulphite (mg) | Sodium CMC (g) | Carbopol 980 (g) | Triethanolamine (in Drops) | Methyl Paraben (mg) | Propyl Paraben (mg) | Distilled Water (q.s) (mL) |
|---|---|---|---|---|---|---|---|
| F1 | 100 | 1 | - | - | 10 | 1 | qs to 100 |
| F2 | 100 | 1.5 | - | - | 10 | 1 | qs to 100 |
| F3 | 100 | 2 | - | - | 10 | 1 | qs to 100 |
| F4 | 100 | - | 1 | 2 | 10 | 1 | qs to 100 |
| F5 | 100 | - | 1.5 | 4 | 10 | 1 | qs to 100 |
| F6 | 100 | - | 2 | 6 | 10 | 1 | qs to 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharwade, R.; Ali, N.; Gangane, P.; Pawar, K.; More, S.; Iqbal, M.; Bhat, A.R.; AlAsmari, A.F.; Kaleem, M. DOE-Assisted Formulation, Optimization, and Characterization of Tioconazole-Loaded Transferosomal Hydrogel for the Effective Treatment of Atopic Dermatitis: In Vitro and In Vivo Evaluation. Gels 2023, 9, 303. https://doi.org/10.3390/gels9040303
Kharwade R, Ali N, Gangane P, Pawar K, More S, Iqbal M, Bhat AR, AlAsmari AF, Kaleem M. DOE-Assisted Formulation, Optimization, and Characterization of Tioconazole-Loaded Transferosomal Hydrogel for the Effective Treatment of Atopic Dermatitis: In Vitro and In Vivo Evaluation. Gels. 2023; 9(4):303. https://doi.org/10.3390/gels9040303
Chicago/Turabian StyleKharwade, Rohini, Nemat Ali, Purushottam Gangane, Kapil Pawar, Sachin More, Muzaffar Iqbal, Abid R. Bhat, Abdullah F. AlAsmari, and Mohammed Kaleem. 2023. "DOE-Assisted Formulation, Optimization, and Characterization of Tioconazole-Loaded Transferosomal Hydrogel for the Effective Treatment of Atopic Dermatitis: In Vitro and In Vivo Evaluation" Gels 9, no. 4: 303. https://doi.org/10.3390/gels9040303