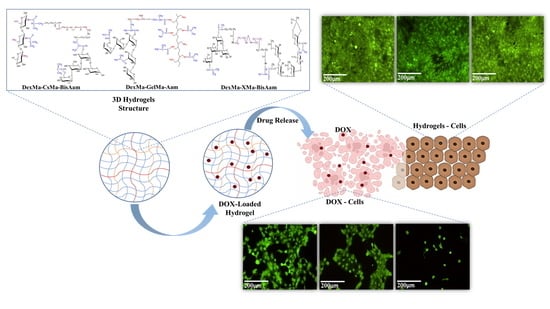
New Methacrylated Biopolymer-Based Hydrogels as Localized Drug Delivery Systems in Skin Cancer Therapy
Abstract
:1. Introduction
2. Results and Discussion
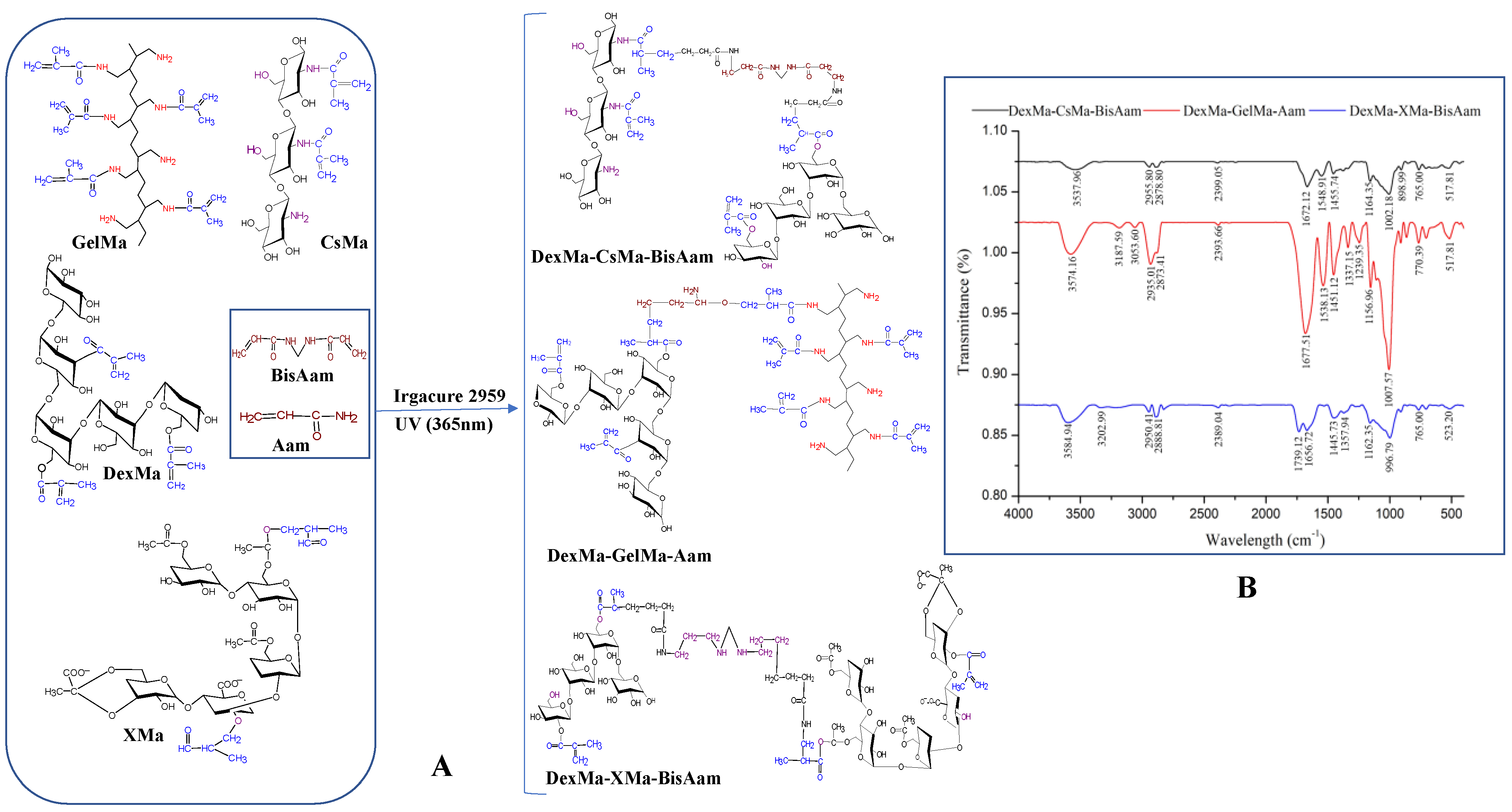
2.1. Synthesis of Hydrogels and FT-IR Data
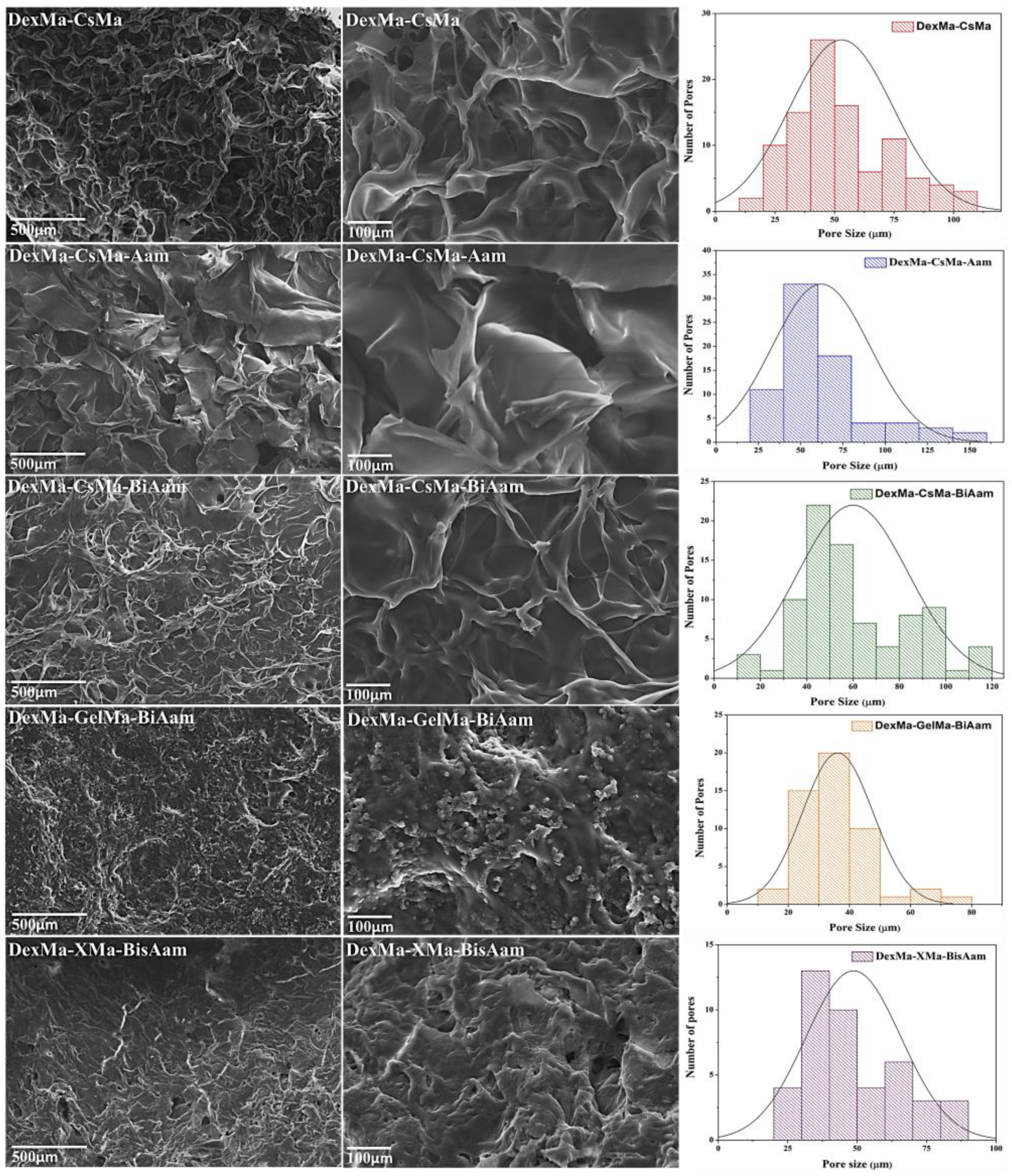
2.2. Hydrogel Morphology
2.3. Swelling Properties
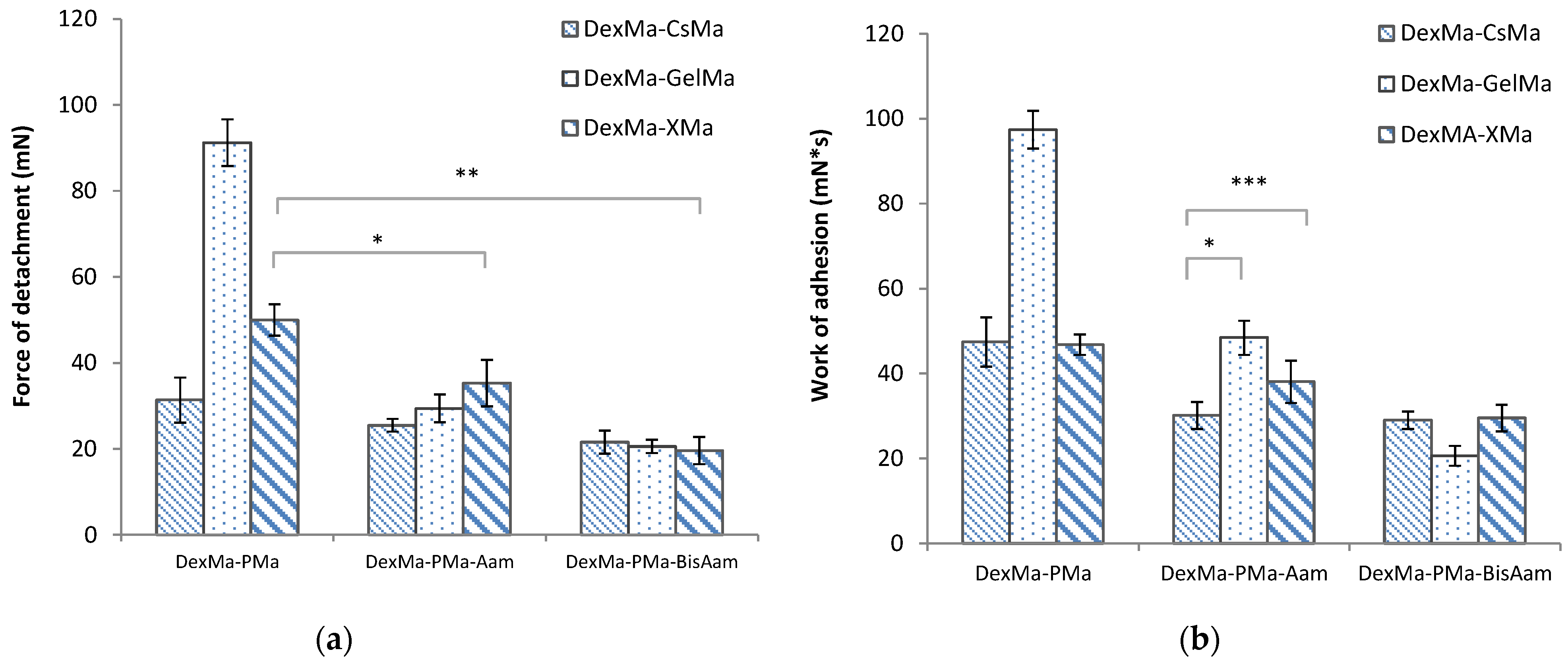
2.4. Bioadhesive Properties
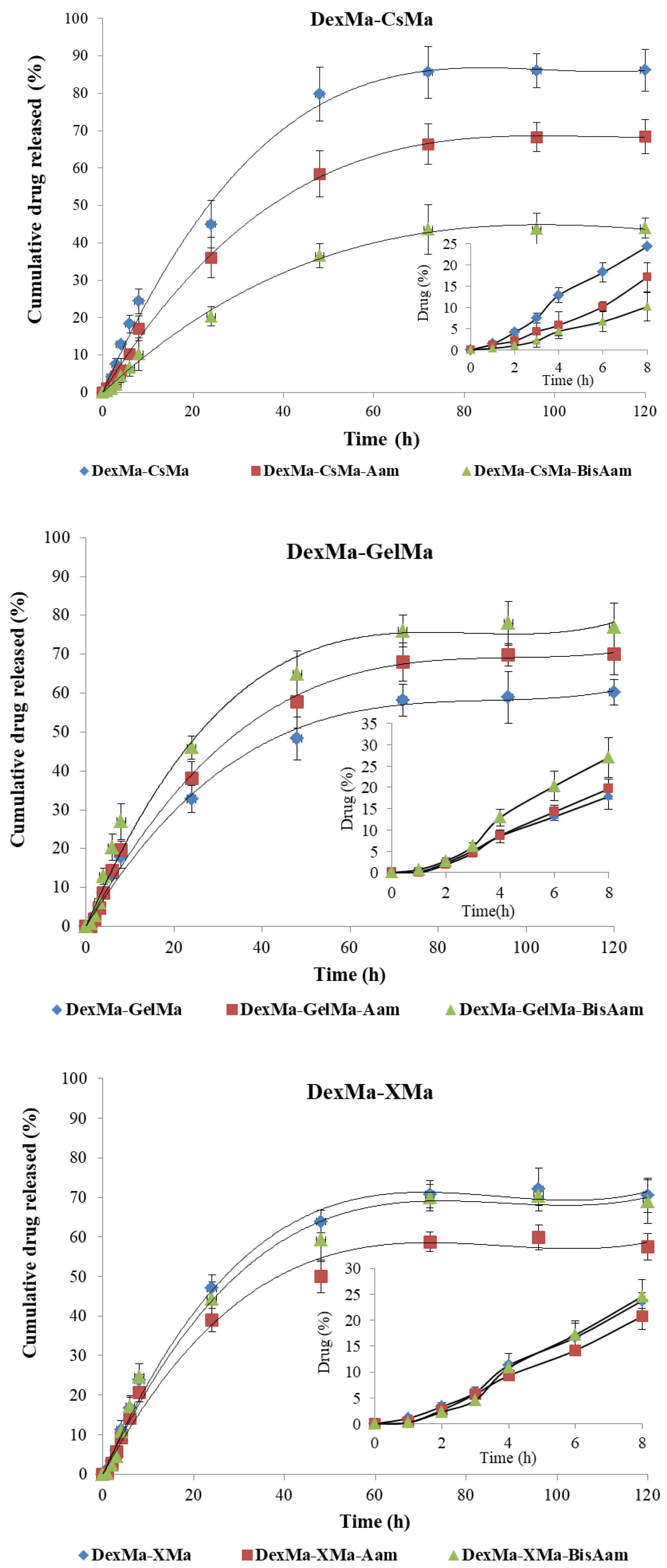
2.5. In Vitro Drug Release
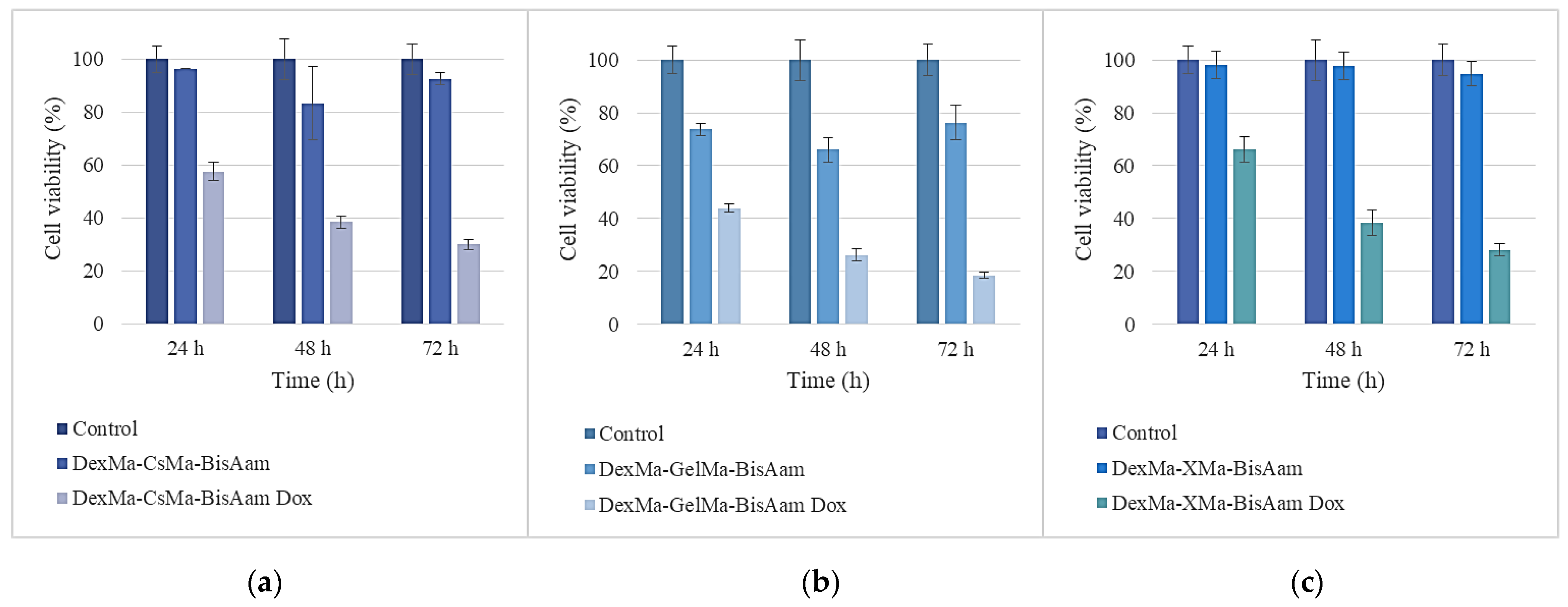
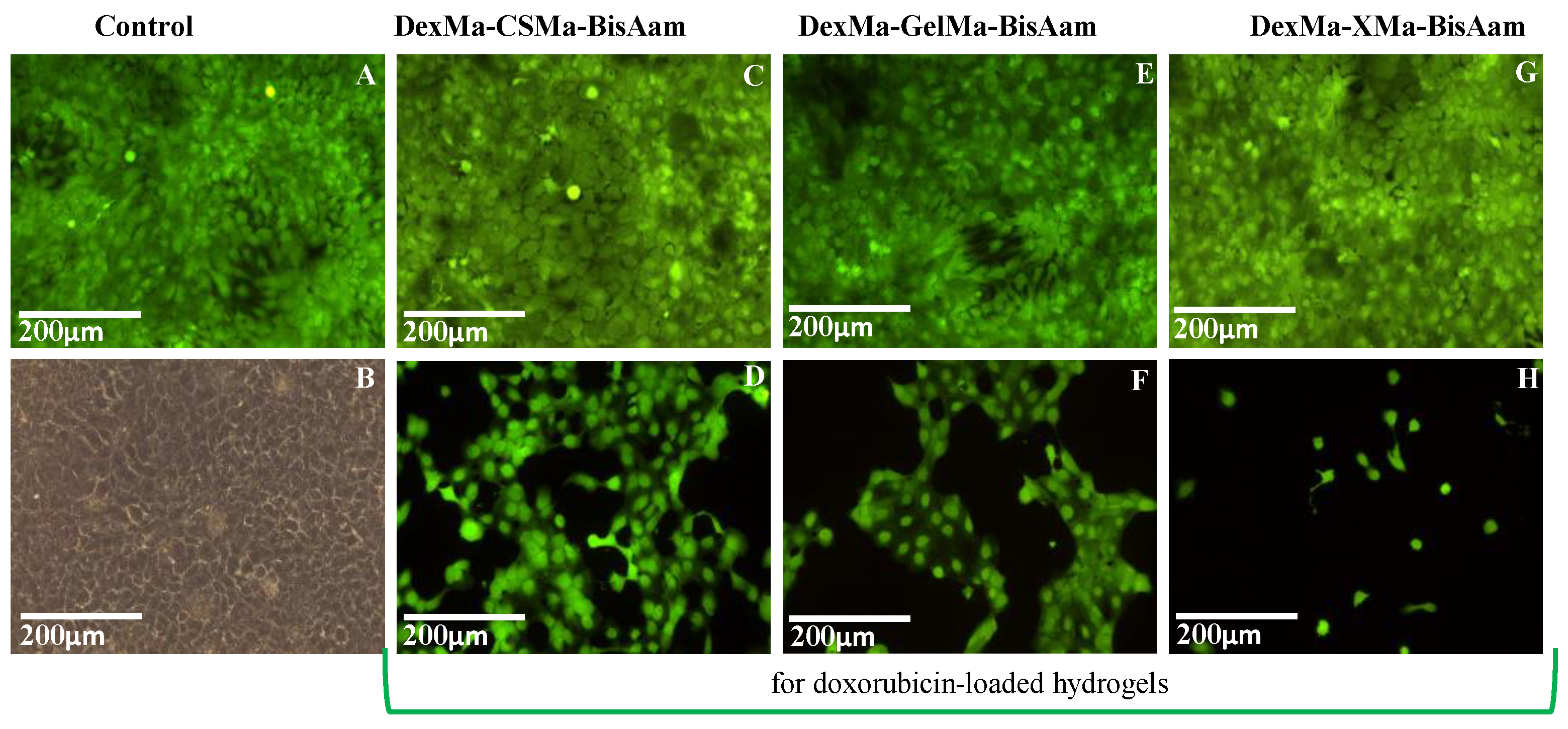
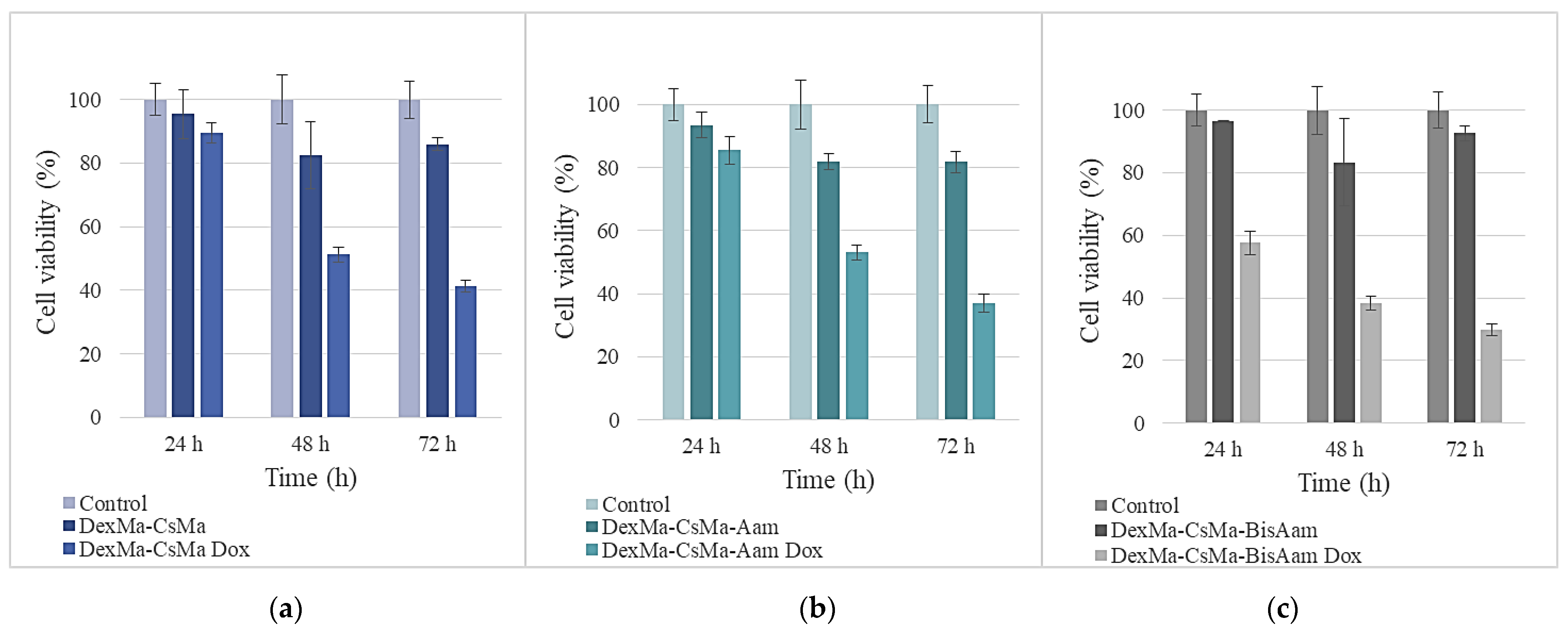
2.6. In Vitro Cytotoxicity Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogels
4.3. Hydrogel Characterisation
4.3.1. Fourier Transform Infrared Spectroscopy (FTIR) and Scanning Electron Microscopy (SEM)
4.3.2. Swelling Properties
4.3.3. Bioadhesive Characteristics
4.3.4. Drug-Loading and Drug-Release Studies
4.3.5. In vitro Cytotoxicity Studies
Hydrogel Preparation for Cytotoxicity Testing
Cell Morphology Evaluation by Fluorescence Microscopy
4.3.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altini, M.; Solinas, L.; Bucchi, L.; Gentili, N.; Gallegati, D.; Balzi, W.; Falcini, F.; Massa, I. Assessment of Cancer Care Costs in Disease-Specific Cancer Care Pathways. Int. J. Environ. Res. Public Health 2020, 17, 4765. [Google Scholar] [CrossRef] [PubMed]
- Iragorri, N.; De Oliveira, C.; Fitzgerald, N.; Essue, B. The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review. Curr. Oncol. 2021, 28, 1216–1248. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gowda, B.H.J.; Ahmed, M.G.; Abourehab, M.A.S.; Chen, Z.S.; Zhang, C.; Li, J.; Kesharwan, P. Advancements in nanoparticle-based treatment approaches for skin cancer therapy. Mol. Cancer 2023, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Mitragotri, S. Nanoparticles for topical drug delivery: Potential for skin cancer treatment. Adv. Drug Deliv. Rev. 2020, 153, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Jelonek, K.; Krzywon, A.; Jablonska, P.; Slominska, E.M.; Smolenski, R.T.; Polanska, J.; Rutkowski, T.; Mrochem-Kwarciak, J.; Skladowski, K.; Widlak, P. Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients-Comparison of Serum Metabolome Profiles. Metabolites 2020, 10, 60. [Google Scholar] [CrossRef]
- Jairam, V.; Lee, V.; Park, H.S.; Thomas, C.R., Jr.; Melnick, E.R.; Gross, C.P.; Presley, C.J.; Adelson, K.B.; Yu, J.B. Treatment-Related Complications of Systemic Therapy and Radiotherapy. JAMA Oncol. 2019, 5, 1028–1035. [Google Scholar] [CrossRef]
- Hu, J.K.; Suh, H.W.; Qureshi, M.; Lewis, J.M.; Yaqoob, S.; Moscato, Z.M.; Griff, S.; Lee, A.K.; Yin, E.S.; Saltzman, W.M.; et al. Nonsurgical treatment of skin cancer with local delivery of bioadhesive nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2020575118. [Google Scholar] [CrossRef]
- Skok, K.; Zidarič, T.; Orthaber, K.; Pristovnik, M.; Kostevšek, N.; Žužek Rožman, K.; Šturm, S.; Gradišnik, L.; Maver, U.; Maver, T. Novel Methacrylate-Based Multilayer Nanofilms with Incorporated FePt-Based Nanoparticles and the Anticancer Drug 5-Fluorouracil for Skin Cancer Treatment. Pharmaceutics 2022, 14, 689. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Carvalho, I.C.; Chagas, P.; de Oliveira, L.C.A.; Mansur, H.S. Bioengineered carboxymethyl cellulose-doxorubicin prodrug hydrogels for topical chemotherapy of melanoma skin cancer. Carbohydr. Polym. 2018, 195, 401–412. [Google Scholar] [CrossRef]
- Shukla, A.; Singh, A.P.; Maiti, P. Injectable hydrogels of newly designed brush biopolymers as sustained drug-delivery vehicle for melanoma treatment. Sig. Transduct. Target Ther. 2021, 6, 63. [Google Scholar] [CrossRef]
- Jung, H.; Kim, M.K.; Lee, J.Y.; Choi, S.W.; Kim, J. Adhesive Hydrogel Patch with Enhanced Strength and Adhesiveness to Skin for Transdermal Drug Delivery. Adv. Funct. Mater. 2020, 30, 2004407. [Google Scholar] [CrossRef]
- Fonseca, A.M.; Araújo, C.C.B.; Silva, J.H.; Honório, T.S.; Nasciutti, L.E.; Cabral, L.M.; Carmo, F.A.; Sousa, V.P. Development of transdermal based hydrogel formulations of vinorelbine with an evaluation of their in vitro profiles and activity against melanoma cells and in silico prediction of drug absorption. J. Drug Deliv. 2021, 63, 102449. [Google Scholar] [CrossRef]
- Nazir, S.; Khan, M.U.A.; Al-Arjan, W.S.; Abd Razak, S.I.; Javed, A.; Kadir, M.R.A. Nanocomposite Hydrogels for Melanoma Skin Cancer Care and Treatment: In-Vitro Drug Delivery, Drug Release Kinetics and Anti-Cancer Activities. Arab. J. Chem. 2021, 14, 103120. [Google Scholar] [CrossRef]
- Capanema, N.S.; Carvalho, I.C.; Mansur, A.A.; Carvalho, S.M.; Lage, A.P.; Mansur, H.S. Hybrid Hydrogel Composed of Carboxymethylcellulose–Silver Nanoparticles–Doxorubicin for Anticancer and Antibacterial Therapies against Melanoma Skin Cancer Cells. ACS Appl. Nano Mater. 2019, 2, 7393–7408. [Google Scholar] [CrossRef]
- Lalan, M.; Shah, P.; Barve, K.; Parekh, K.; Mehta, T.; Patel, P. Skin Cancer Therapeutics: Nano-Drug Delivery Vectors—Present and Beyond. Future J. Pharm. Sci. 2021, 7, 179. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Y.; Guo, S.; He, B.; Jiang, T. Topical Delivery of Chemotherapeutic Drugs Using Nano-Hybrid Hydrogels to Inhibit Post-Surgical Tumour Recurrence. Biomater. Sci. 2021, 9, 4356–4363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef]
- Abedian, Z.; Moghadamnia, A.A.; Zabihi, E.; Pourbagher, R.; Ghasemi, M.; Nouri, H.R.; Tashakorian, H.; Jenabian, N. Anticancer Properties of Chitosan Against Osteosarcoma, Breast Cancer and Cervical Cancer Cell Lines. Caspian J. Intern. Med. 2019, 10, 439–446. [Google Scholar] [CrossRef]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.A. Chitosan Exerts Anticancer Activity Through Induction of Apoptosis and Cell Cycle Arrest in Oral Cancer Cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, X.; Su, C.; Zhao, L.; Shi, Y. Chitosan Nanoparticles Induced the Antitumor Effect in Hepatocellular Carcinoma Cells by Regulating ROS-Mediated Mitochondrial Damage and Endoplasmic Reticulum Stress. Artif. Cells Nanomed. Biotechnol. 2019, 47, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, O.; Brandl, M.; Cirillo, G.; Kimpton, K.; Hinde, E.; Gaus, K.; Yee, E.; Kumar, N.; Duong, H.; Fleming, C.; et al. Dextran-Catechin: An Anticancer Chemically-Modified Natural Compound Targeting Copper that Attenuates Neuroblastoma Growth. Oncotarget 2016, 7, 47479–47493. [Google Scholar] [CrossRef]
- Bevacqua, E.; Curcio, M.; Saletta, F.; Vittorio, O.; Cirillo, G.; Tucci, P. Dextran-Curcumin Nanosystems Inhibit Cell Growth and Migration Regulating the Epithelial to Mesenchymal Transition in Prostate Cancer Cells. Int. J. Mol. Sci. 2021, 22, 7013. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, H.; Huang, S.; Fu, T.; Xue, W.; Guo, R. Dextran Methacrylate Hydrogel Microneedles Loaded with Doxorubicin and Trametinib for Continuous Transdermal Administration of Melanoma. Carbohydr. Polym. 2020, 246, 116650. [Google Scholar] [CrossRef]
- Huang, S.; Huang, G. Preparation and Drug Delivery of Dextran-Drug Complex. Drug Deliv. 2019, 26, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.; Sari, M.H.M.; Azambuja, J.H.; Da Silveira, E.F.; Cervi, V.F.; Marchiori, M.C.L.; Maria-Engler, S.S.; Wink, M.R.; Azevedo, J.G.; Nogueira, C.W.; et al. Xanthan Gum-Based Hydrogel Containing Nanocapsules for Cutaneous Diphenyl Diselenide Delivery in Melanoma Therapy. Investig. New Drugs 2020, 38, 662–674. [Google Scholar] [CrossRef]
- Tariq, A.; Bhawani, S.A.; Alotaibi, K.M. Xanthan gum-based nanocomposites for tissue engineering. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Bhawani, S.A., Karim, Z., Mohammad, J., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 191–206. ISBN 9780128212301. [Google Scholar]
- Feng, Z.; Xu, J.; Ni, C. Preparation of Redox Responsive Modified Xanthan Gum Nanoparticles and the Drug Controlled Release. Int. J. Polym. Mater. 2021, 70, 994–1001. [Google Scholar] [CrossRef]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Pahoff, S.; Bock, N.; Hutmacher, D.W. Gelatin Methacryloyl Hydrogels Control the Localized Delivery of Albumin-Bound Paclitaxel. Polymers 2020, 12, 501. [Google Scholar] [CrossRef]
- Bordini, E.A.F.; Ferreira, J.A.; Dubey, N.; Ribeiro, J.S.; De Souza Costa, C.A.; Soares, D.G.; Bottino, M.C. Injectable Multifunctional Drug Delivery System for Hard Tissue Regeneration under Inflammatory Microenvironments. ACS Appl. Bio Mater. 2021, 4, 6993–7006. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; You, H.; Xu, T.; Bei, H.P.; Piwko, I.Z.; Kwan, Y.Y.; Zhao, X. Biomedical Applications of Gelatin Methacryloyl Hydrogels. Eng. Regen. 2021, 2, 47–56. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, U.; Jagannathan, N.R.; Ray, R.; Rajeswari, M.R. Effect of Doxorubicin on Squamous Cell Carcinoma of Skin: Assessment by MRI Relaxometry at 4.7T. Cancer Investig. 2019, 37, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; He, J.; Xu, J.; Zhang, M.; Zhao, L.; Duan, G.; Cao, Y.; Zhou, R.; Ni, P. Polymeric Prodrugs Conjugated with Reduction-Sensitive Dextran–Camptothecin and pH-Responsive Dextran–Doxorubicin: An Effective Combinatorial Drug Delivery Platform for Cancer Therapy. Polym. Chem. 2016, 7, 4198–4212. [Google Scholar] [CrossRef]
- Said, M.M.T.; Bashirul, H.; Al Shehri, D.; Rahman, M.M.; Muhammed, N.S.; Mahmoud, M. Modification of Xanthan Gum for a High-Temperature and High-Salinity Reservoir. Polymers 2021, 13, 4212. [Google Scholar] [CrossRef]
- Özbaş, Z.; Torkay, G.; Bal-Öztürk, A.; Özkahraman, B. Preparation of Quercetin Incorporated Photocrosslinkable Methacrylated Gelatin/Methacrylated Kappa-Carrageenan Antioxidant Hydrogel Wound Dressings. Chem. Pap. 2022, 76, 7597–7606. [Google Scholar] [CrossRef]
- Szafulera, K.; Wach, R.A.; Olejnik, A.K.; Rosiak, J.M.; Ulański, P. Radiation Synthesis of Biocompatible Hydrogels of Dextran Methacrylate. Radiat. Phys. Chem. 2018, 142, 115–120. [Google Scholar] [CrossRef]
- Tulegenovna, K.P.; Negim, E.-S.; Al Azzam, K.M.; Bustam, M.A. Modification of Xanthan Gum with Methyl Methacrylate and Investigation of Its Rheological Properties. Int. J. Technol. 2022, 13, 389–397. [Google Scholar] [CrossRef]
- Maslov, M.Y.; Edelman, E.R.; Wei, A.E.; Pezone, M.J.; Lovich, M.A. High Concentrations of Drug in Target Tissues Following Local Controlled Release are Utilized for Both Drug Distribution and Biologic Effect: An Example with Epicardial Inotropic Drug Delivery. J Control Release 2013, 171, 201–207. [Google Scholar] [CrossRef]
- O’Donnell, K.; Boyd, A.; Meenan, B.J. Controlling Fluid Diffusion and Release through Mixed-Molecular-Weight Poly(ethylene) Glycol Diacrylate (PEGDA) Hydrogels. Materials 2019, 12, 3381. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G. Functional Polymers for Controlled Drug Release. Pharmaceutics 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Luo, Z.; Chen, T.; Ouyang, Y.; Xiao, L.; Liang, S.; Peng, Z.; Liu, Y.; Deng, Y. Bioadhesive Nanoparticles for Local Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2370. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Ahmady, A.; Samah, N.H.A. A Review: Gelatine as a Bioadhesive Material for Medical and Pharmaceutical Applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef]
- Matsumura, K.; Nakajima, N.; Sugai, H.; Hyon, S.H. Self-Degradation of Tissue Adhesive based on Oxidized Dextran and Poly-L-Lysine. Carbohydr. Polym. 2014, 113, 32–38. [Google Scholar] [CrossRef]
- Pathak, K.; Malviya, R. Introduction, theories and mechanisms of bioadhesion. In Bioadhesives in Drug Delivery; Mittal, K.L., Bakshi, I.S., Narang, J.K., Eds.; Scrivener Publishing: Beverly, MA, USA, 2020; pp. 1–27. [Google Scholar]
- Mashabela, L.T.; Maboa, M.M.; Miya, N.F.; Ajayi, T.O.; Chasara, R.S.; Milne, M.; Mokhele, S.; Demana, P.H.; Witika, B.A.; Siwe-Noundou, X.; et al. A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders. Gels 2022, 8, 563. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological Overviews from Bench to Bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control Release 2020, 328, 171–191. [Google Scholar] [CrossRef]
- Varela-López, A.; Battino, M.; Navarro-Hortal, M.D.; Giampieri, F.; Forbes-Hernández, T.Y.; Romero-Márquez, J.M.; Collado, R.; Quiles, J.L. An Update on the Mechanisms Related to Cell Death and Toxicity of Doxorubicin and the Protective Role of Nutrients. Food Chem. Toxicol. 2019, 134, 110834. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Adrover, A.; Venditti, C.; Giona, M. Swelling and Drug Release in Polymers through the Theory of Poisson-Kac Stochastic Processes. Gels 2021, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Concha, L.; Resende Pires, A.L.; Moraes, A.M.; Mas-Hernández, E.; Berres, S.; Hernandez-Montelongo, J. Cost Function Analysis Applied to Different Kinetic Release Models of Arrabidaea chica Verlot Extract from Chitosan/Alginate Membranes. Polymers 2022, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: A Review on the Most Used Mathematical Models for Drug Release. Processes 2022, 10, 1094. [Google Scholar] [CrossRef]
- Nowak, A.; Zakłos-Szyda, M.; Żyżelewicz, D.; Koszucka, A.; Motyl, I. Acrylamide Decreases Cell Viability, and Provides Oxidative Stress, DNA Damage, and Apoptosis in Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 368. [Google Scholar] [CrossRef] [PubMed]
- Baxter-Holland, M.; Dass, C.R. Doxorubicin, Mesenchymal Stem Cell Toxicity and Antitumour Activity: Implications for Clinical Use. J. Pharm. Pharmacol. 2018, 70, 320–327. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B. Doxorubicin Induces Cardiotoxicity through Upregulation of Death Receptors Mediated Apoptosis in Cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef]
- Yadav, N.; Francis, A.P.; Priya, V.V.; Patil, S.; Mustaq, S.; Khan, S.S.; Alzahrani, K.J.; Banjer, H.J.; Mohan, S.K.; Mony, U.; et al. Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy. Polymers 2022, 14, 950. [Google Scholar] [CrossRef]
- Cheah, I.K.; Tang, R.M.Y.; Wang, X.; Sachaphibulkij, K.; Chong, S.Y.; Lim, L.H.K.; Wang, J.-W.; Halliwell, B. Protection against Doxorubicin-Induced Cardiotoxicity by Ergothioneine. Antioxidants 2023, 12, 320. [Google Scholar] [CrossRef]
- Druzhkova, I.; Nikonova, E.; Ignatova, N.; Koryakina, I.; Zyuzin, M.; Mozherov, A.; Kozlov, D.; Krylov, D.; Kuznetsova, D.; Lisitsa, U.; et al. Effect of Collagen Matrix on Doxorubicin Distribution and Cancer Cells’ Response to Treatment in 3D Tumor Model. Cancers 2022, 14, 5487. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and Characterization of Hybrid Hyaluronic Acid-Gelatin Hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Goyal, A.K. Biomaterial-based Scaffolds–Current Status and Future Directions. Expert Opin. Drug. Deliv. 2014, 11, 767–789. [Google Scholar] [CrossRef]
- Brako, F.; Thorogate, R.; Mahalingam, S.; Abraham, B.R.; Craig, D.Q.M.; Edirisinghe, M. Mucoadhesion of Progesterone-Loaded Drug Delivery Nanofiber Constructs. ACS Appl. Mater. Interfaces 2018, 10, 13381–13389. [Google Scholar] [CrossRef]
- Khdair, A.; Hamad, I.; Al-Hussaini, M.; Albayati, D.; Alkhatib, H.; Alkhalidi, B. In vitro artificial membrane-natural mucosa correlation of carvedilol buccal delivery. J. Drug Del. Sci. Technol. 2013, 23, 603–609. [Google Scholar] [CrossRef]



| DexMa-CsMa (µm) | DexMa-CsMa-Aam (µm) | DexMa-CsMa-BisAam (µm) | DexMa-GelMa-BisAam (µm) | DexMa-XMa-BisAam (µm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max |
| 27.75 ± 4.17 | 80.95 ± 6.43 | 47.15 ± 3.49 | 143.10 ± 9.12 | 52.07 ± 6.88 | 131.46 ± 2.09 | 30.68 ± 5.04 | 46.15 ± 4.66 | 41.95 ± 11.17 | 88.96 ± 6.09 |
| Hydrogel | Correlation Coefficient (r2) | Release Rate Constant, k (h−n) | Release Exponent, n | |
|---|---|---|---|---|
| Higuchi | Korsmeyer–Peppas | |||
| DexMa-CsMa | 0.9812 | 0.9955 | 0.1403 | 0.5629 |
| DexMa-CsMa-Aam | 0.9799 | 0.9962 | 0.1612 | 0.5719 |
| DexMa-CsMa-BisAam | 0.9843 | 0.9987 | 0.1822 | 0.5685 |
| DexMa-GelMa | 0.9892 | 0.9904 | 0.1074 | 0.5270 |
| DexMa-GelMa-Aam | 0.9792 | 0.9973 | 0.0934 | 0.5374 |
| DexMa-GelMa-BisAam | 0.9765 | 0.9965 | 0.0962 | 0.5907 |
| DexMa-XMa | 0.9803 | 0.9944 | 0.0962 | 0.5507 |
| DexMa-XMa-Aam | 0.9789 | 0.9952 | 0.0943 | 0.5531 |
| DexMa-XMa- BisAam | 0.9806 | 0.9986 | 0.1019 | 0.5496 |
| Hydrogels | DexMa (%) | CsMa (%) | GelMa (%) | XMa (%) | Aam (%) | BisAam (%) |
|---|---|---|---|---|---|---|
| DexMa-CsMa | 50 | 50 | - | - | - | - |
| DexMa-CsMa-Aam | 45 | 45 | - | - | 10 | |
| DexMa-CsMa-BisAam | 45 | 45 | - | - | 8 | 2 |
| DexMa-GelMa | 50 | - | 50 | - | - | - |
| DexMa-GelMa-Aam | 45 | - | 45 | - | 10 | |
| DexMa-GelMa-BisAam | 45 | - | 45 | - | 8 | 2 |
| DexMa-XMa | 50 | - | - | 50 | - | - |
| DexMa-XMa-Aam | 45 | - | - | 45 | 10 | |
| DexMa-XMa-BisAam | 45 | - | - | 45 | 8 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, A.; Nacu, I.; Tanasache, S.; Peptu, C.A.; Butnaru, M.; Verestiuc, L. New Methacrylated Biopolymer-Based Hydrogels as Localized Drug Delivery Systems in Skin Cancer Therapy. Gels 2023, 9, 371. https://doi.org/10.3390/gels9050371
Luca A, Nacu I, Tanasache S, Peptu CA, Butnaru M, Verestiuc L. New Methacrylated Biopolymer-Based Hydrogels as Localized Drug Delivery Systems in Skin Cancer Therapy. Gels. 2023; 9(5):371. https://doi.org/10.3390/gels9050371
Chicago/Turabian StyleLuca, Andreea, Isabella Nacu, Sabina Tanasache, Cătălina Anişoara Peptu, Maria Butnaru, and Liliana Verestiuc. 2023. "New Methacrylated Biopolymer-Based Hydrogels as Localized Drug Delivery Systems in Skin Cancer Therapy" Gels 9, no. 5: 371. https://doi.org/10.3390/gels9050371