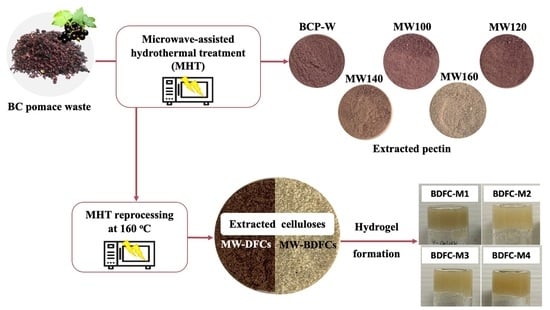
Production of Hydrogels from Microwave-Assisted Hydrothermal Fractionation of Blackcurrant Pomace
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pectin Yield and Characterization
2.1.1. Pectin Yield and Degree of Esterification
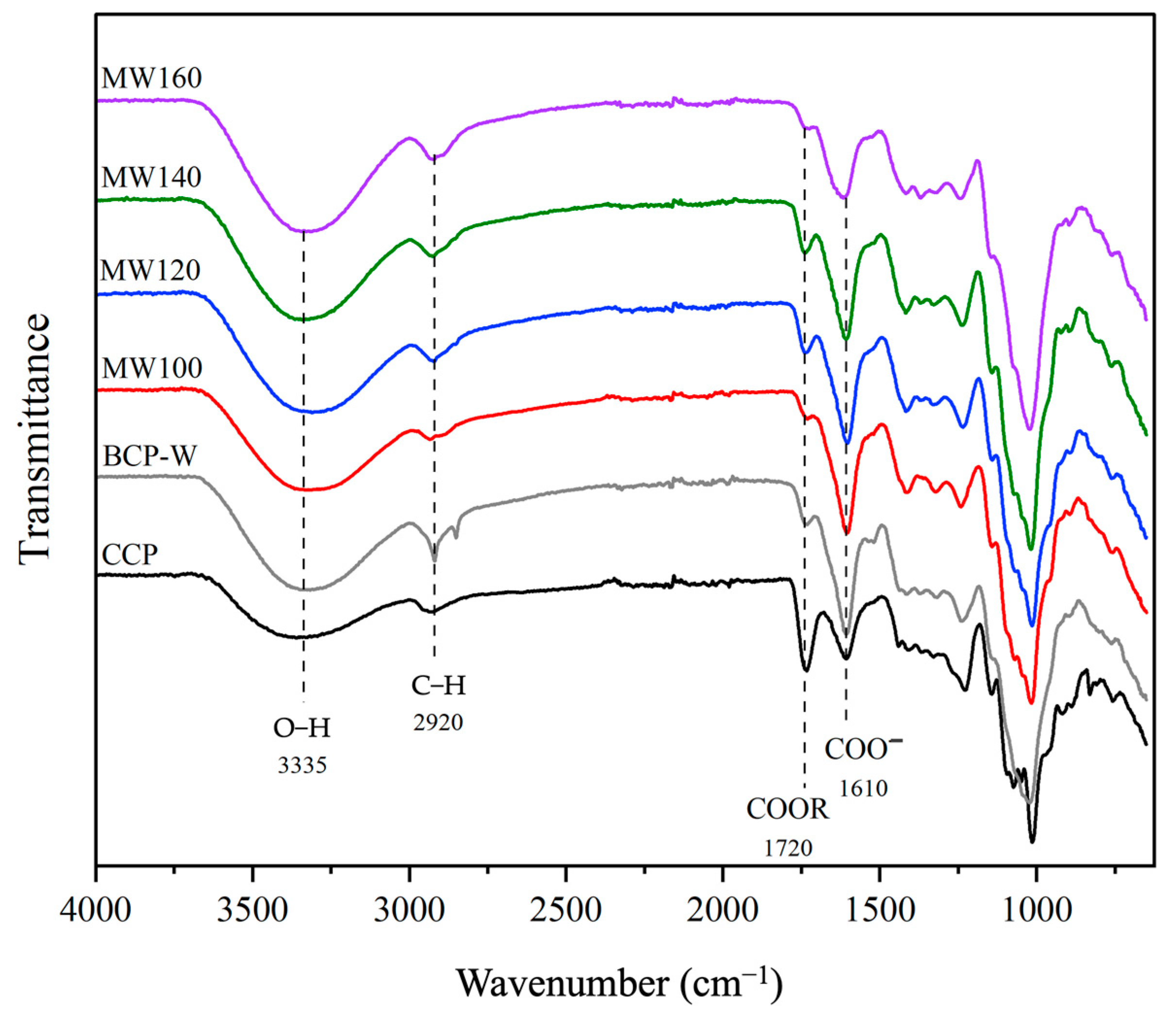
2.1.2. Pectin Attenuated Total Reflection Infrared Spectroscopy (ATR-IR)
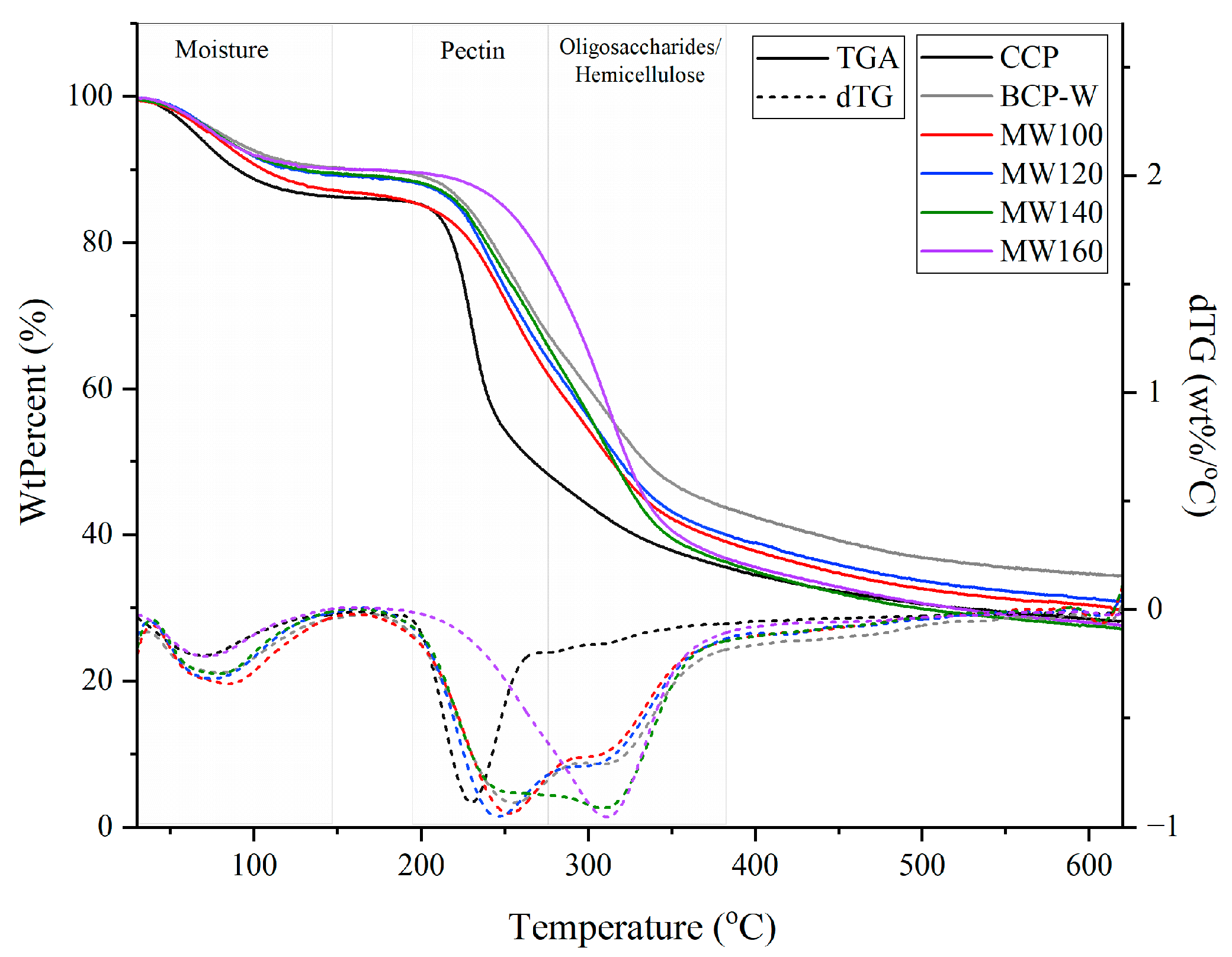
2.1.3. Pectin Thermogravimetric Analysis (TGA)
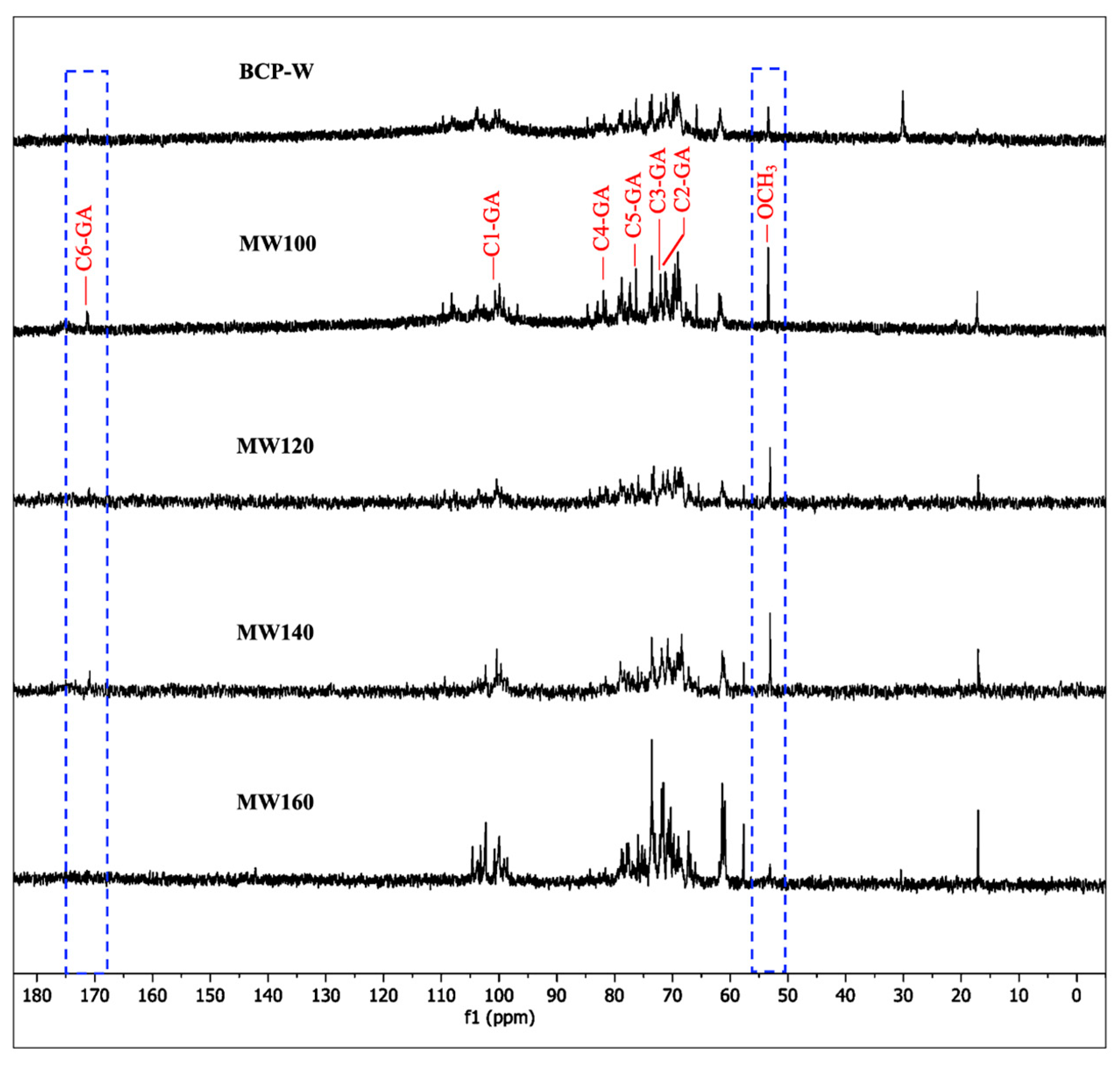
2.1.4. Pectin Carbon Nuclear Magnetic Resonance Spectroscopy (13C NMR)
2.2. Hydrogels Formation and Defibrillated Cellulose Characterization
2.2.1. Hydrogel Formation of Defibrillated Cellulose
2.2.2. Klason Lignin and Water-Holding Capacity (WHC)
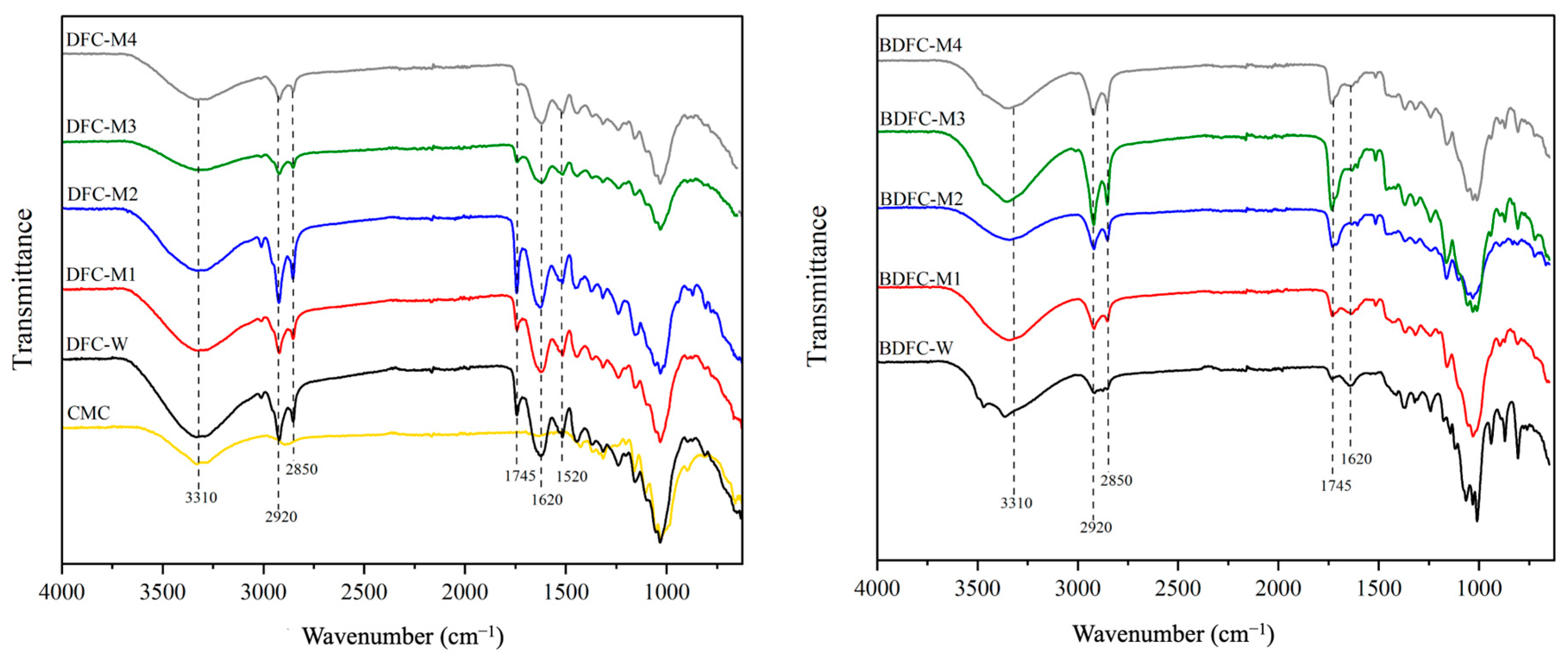
2.2.3. Attenuated Total Reflection Infrared Spectroscopy (ATR-IR) of Defibrillated Celluloses
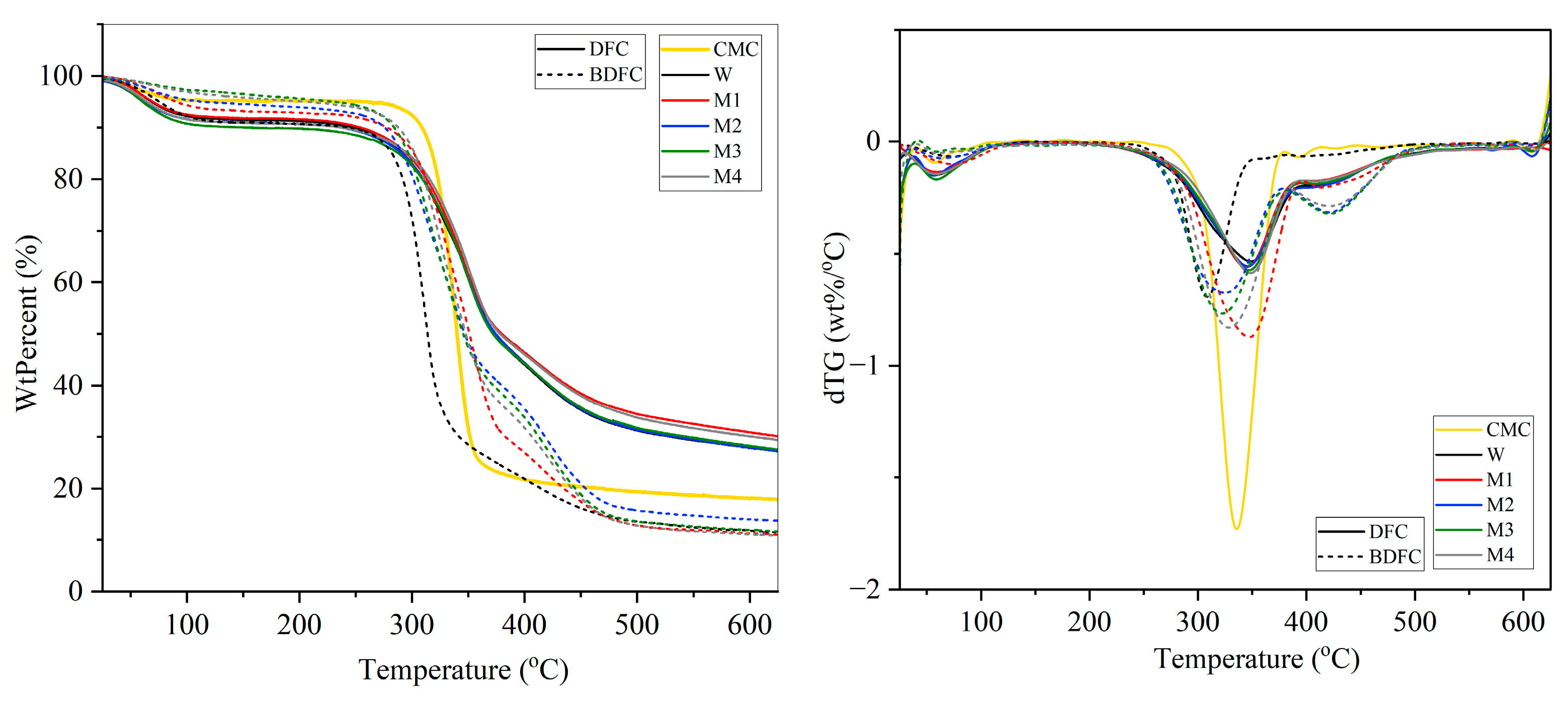
2.2.4. Thermogravimetric Analysis (TGA) of Defibrillated Cellulose Samples
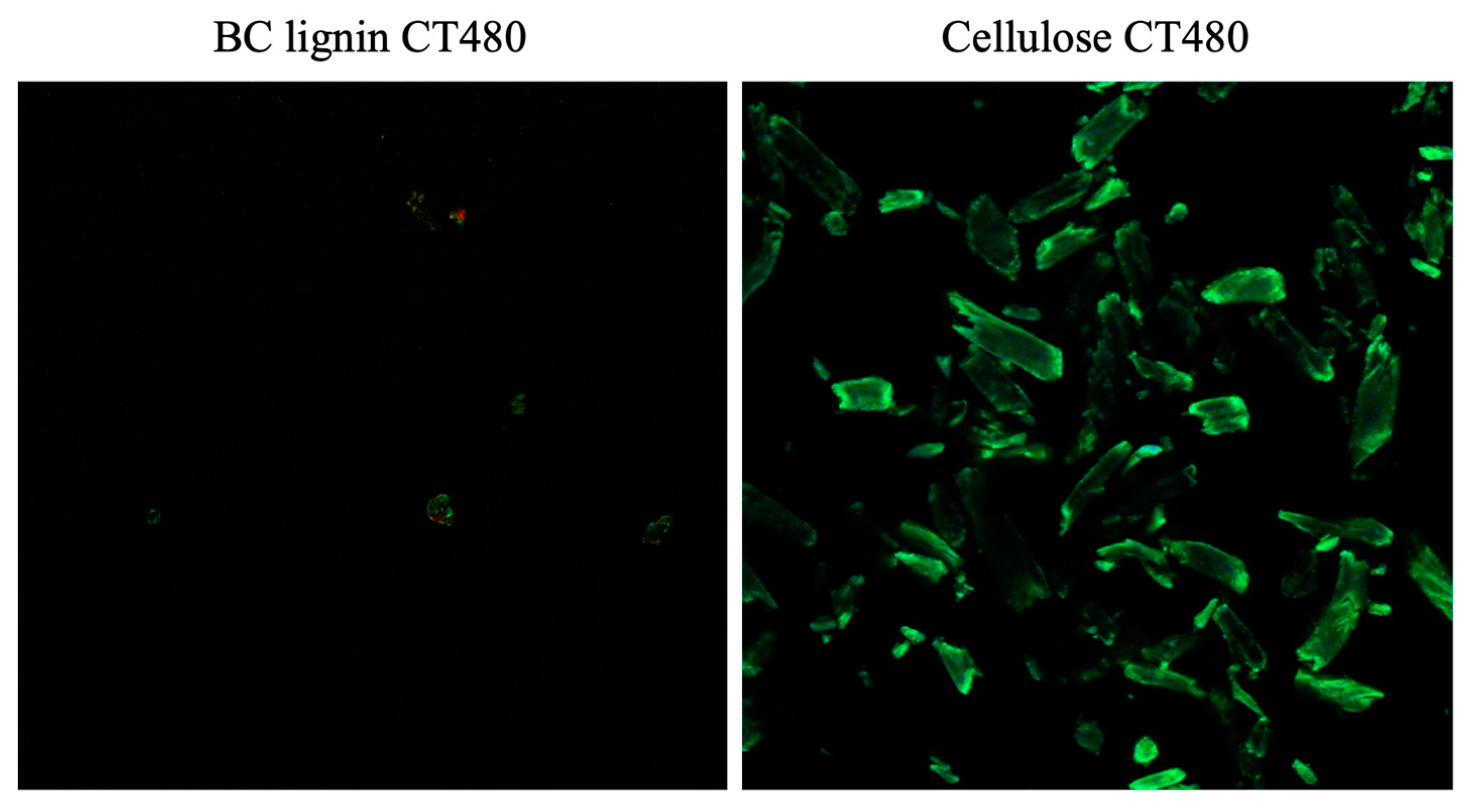
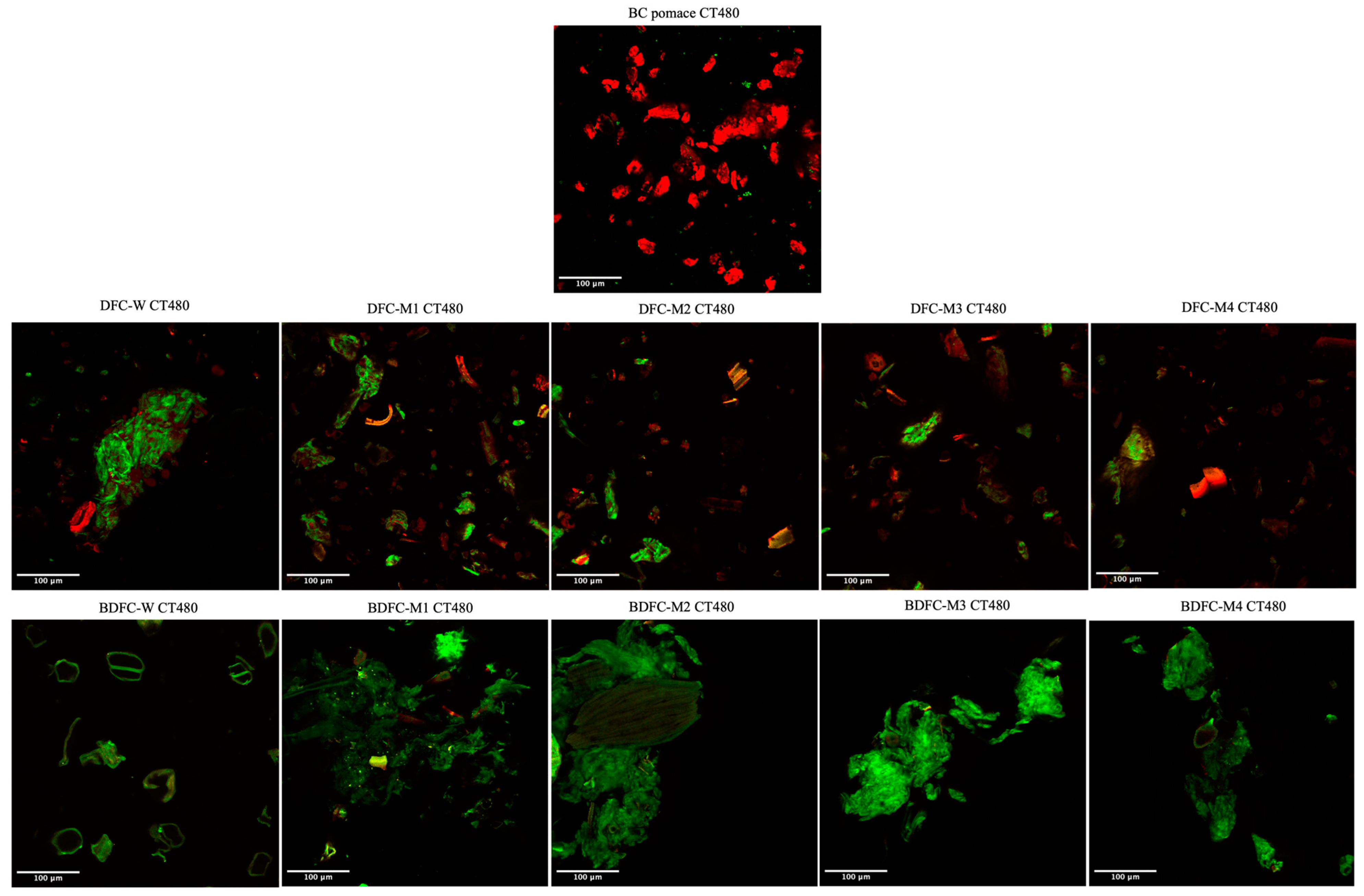
2.2.5. Confocal Laser Scanning Microscopy (CLSM) Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
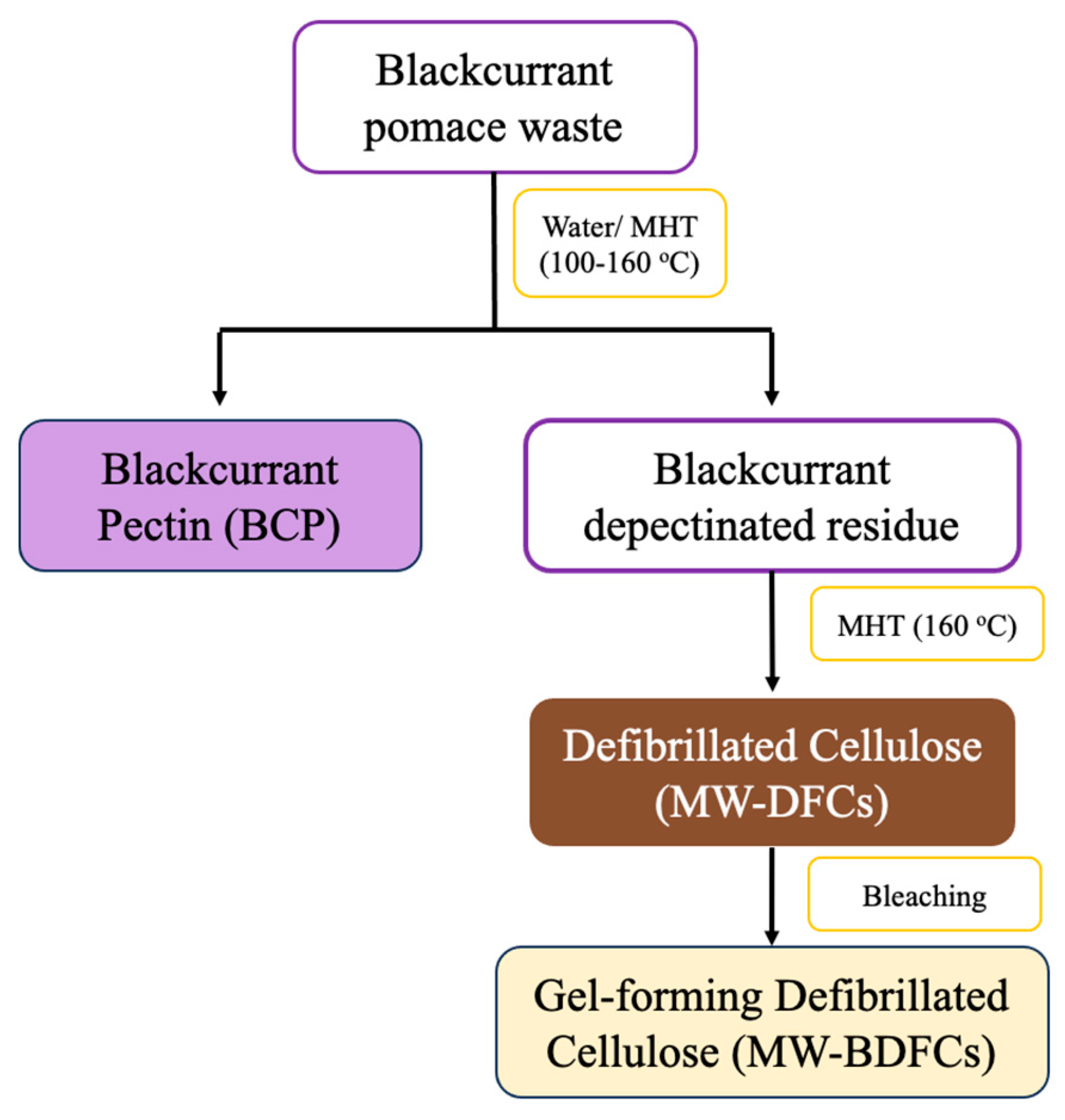
4.2. Preparation of Depectinated BC Residues
4.2.1. Water Pre-Treatment
4.2.2. Microwave Pre-Treatment
4.3. Isolation of Defibrillated Cellulose
4.4. Characterization of BC Pectin, MW-DFCs, and MW-BDFCs
4.4.1. Carbon Nuclear Magnetic Resonance (13C NMR) Analysis
4.4.2. Attenuated Total Reflection Infrared (ATR-IR) Spectroscopy Analysis
4.4.3. Thermogravimetric Analysis (TGA)
4.4.4. Klason Lignin Analysis
4.4.5. Water-Holding Capacity (WHC)
4.4.6. Confocal Laser Scanning Microscopy (CLSM) Analysis
4.4.7. Hydrogel Formation
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.G.; Vance, T.M.; Nam, T.-G.; Kim, D.-O.; Koo, S.I.; Chun, O.K. Contribution of Anthocyanin Composition to Total Antioxidant Capacity of Berries. Plant Foods Hum. Nutr. 2015, 70, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins Profile, Total Phenolics and Antioxidant Activity of Black Currant Ethanolic Extracts as Influenced by Genotype and Ethanol Concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef]
- Cortez, R.E.; Gonzalez de Mejia, E. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Farooque, S.; Rose, P.M.; Benohoud, M.; Blackburn, R.S.; Rayner, C.M. Enhancing the Potential Exploitation of Food Waste: Extraction, Purification, and Characterization of Renewable Specialty Chemicals from Blackcurrants (Ribes nigrum L.). J. Agric. Food Chem. 2018, 66, 12265–12273. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of Anthocyanins from Blackcurrant (Ribes nigrum L.) Fruit Waste as Renewable Hair Dyes. J. Agric. Food Chem. 2018, 66, 6790–6798. [Google Scholar] [CrossRef]
- Archaina, D.; Leiva, G.; Salvatori, D.; Schebor, C. Physical and Functional Properties of Spray-Dried Powders from Blackcurrant Juice and Extracts Obtained from the Waste of Juice Processing. Food Sci. Technol. Int. 2018, 24, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Gagneten, M.; Leiva, G.; Salvatori, D.; Schebor, C.; Olaiz, N. Optimization of Pulsed Electric Field Treatment for the Extraction of Bioactive Compounds from Blackcurrant. Food Bioprocess Technol. 2019, 12, 1102–1109. [Google Scholar] [CrossRef]
- Salleh, N.; Goh, K.K.T.; Sims, I.M.; Bell, T.J.; Huffman, L.M.; Weeks, M.; Matia-Merino, L. Characterization of Anthocyanin-Bound Pectin-Rich Fraction Extracted from New Zealand Blackcurrant (Ribes nigrum) Juice. ACS Food Sci. Technol. 2021, 1, 1130–1142. [Google Scholar] [CrossRef]
- Alba, K.; MacNaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and Characterisation of Dietary Fibre from Blackcurrant Pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- de Melo, E.M.; Clark, J.H.; Matharu, A.S. The Hy-MASS Concept: Hydrothermal Microwave Assisted Selective Scissoring of Cellulose for in Situ Production of (Meso)Porous Nanocellulose Fibrils and Crystals. Green Chem. 2017, 19, 3408–3417. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-Assisted Hydrothermal Treatments for Biomass Valorisation: A Critical Review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, H.; Sulaeman, A.P.; de Melo, E.M.; Dugmore, T.I.J.; Matharu, A.S. Defibrillated Celluloses via Dual Twin-Screw Extrusion and Microwave Hydrothermal Treatment of Spent Pea Biomass. ACS Sustain. Chem. Eng. 2019, 7, 11861–11871. [Google Scholar] [CrossRef]
- Sulaeman, A.P.; Gao, Y.; Dugmore, T.; Remón, J.; Matharu, A.S. From Unavoidable Food Waste to Advanced Biomaterials: Microfibrilated Lignocellulose Production by Microwave-Assisted Hydrothermal Treatment of Cassava Peel and Almond Hull. Cellulose 2021, 28, 7687–7705. [Google Scholar] [CrossRef]
- Zitzmann, F.L.; Ward, E.; Meng, X.; Matharu, A.S. Microwave-Assisted Defibrillation of Microalgae. Molecules 2021, 26, 4972. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, F.L.; Ward, E.; Matharu, A.S. Use of Carbotrace 480 as a Probe for Cellulose and Hydrogel Formation from Defibrillated Microalgae. Gels 2022, 8, 383. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose Nanocrystals and Cellulose Nanofibrils Based Hydrogels for Biomedical Applications. Carbohydr. Polym. 2019, 209, 130. [Google Scholar] [CrossRef]
- Owoyokun, T.; Pérez Berumen, C.M.; Luévanos, A.M.; Cantú, L.; Lara Ceniceros, A.C. Cellulose Nanocrystals: Obtaining and Sources of a Promising Bionanomaterial for Advanced Applications. Biointerface Res. Appl. Chem. 2021, 11, 11797–11816. [Google Scholar] [CrossRef]
- Matharu, A.S.; Houghton, J.A.; Lucas-Torres, C.; Moreno, A. Acid-Free Microwave-Assisted Hydrothermal Extraction of Pectin and Porous Cellulose from Mango Peel Waste-towards a Zero Waste Mango Biorefinery. Green Chem. 2016, 18, 5280–5287. [Google Scholar] [CrossRef]
- Demir, D.; Ceylan, S.; Göktürk, D.; Bölgen, N. Extraction of Pectin from Albedo of Lemon Peels for Preparation of Tissue Engineering Scaffolds. Polym. Bull. 2021, 78, 2211–2226. [Google Scholar] [CrossRef]
- Ursu, M.S.; Aprodu, I.; Milea, Ș.A.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Thermal Degradation Kinetics of Anthocyanins Extracted from Purple Maize Flour Extract and the Effect of Heating on Selected Biological Functionality. Foods 2020, 9, 1593. [Google Scholar] [CrossRef]
- Taboada, E.; Fisher, P.; Jara, R.; Zúñiga, E.; Gidekel, M.; Cabrera, J.C.; Pereira, E.; Gutiérrez-Moraga, A.; Villalonga, R.; Cabrera, G. Isolation and Characterisation of Pectic Substances from Murta (Ugni molinae Turcz) Fruits. Food Chem. 2010, 123, 669–678. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, L.; Liu, X.; Wang, Y.; Song, C.; Pak, U.H.; Mayo, K.H.; Sun, L.; Zhou, Y. Determining Methyl-Esterification Patterns in Plant-Derived Homogalacturonan Pectins. Front. Nutr. 2022, 9, 925050. [Google Scholar] [CrossRef] [PubMed]
- Cherian, R.M.; Tharayil, A.; Varghese, R.T.; Antony, T.; Kargarzadeh, H.; Chirayil, C.J.; Thomas, S. A Review on the Emerging Applications of Nano-Cellulose as Advanced Coatings. Carbohydr. Polym. 2022, 282, 119123. [Google Scholar] [CrossRef]
- Gao, Y.; Ozel, M.Z.; Dugmore, T.; Sulaeman, A.; Matharu, A.S. A Biorefinery Strategy for Spent Industrial Ginger Waste. J. Hazard. Mater. 2021, 401, 123400. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.; Cordeiro, R.; Dias, P.A.N.; Moura, C.; Henriques, M.; Seabra, I.J.; Malça, C.M.; Morouço, P. Recovery and Evaluation of Cellulose from Agroindustrial Residues of Corn, Grape, Pomegranate, Strawberry-Tree Fruit and Fava. Bioresour. Bioprocess. 2021, 8, 25. [Google Scholar] [CrossRef]
- Rashid, T.; Fai, C.; Murugesan, T. A “Fourier Transformed Infrared” Compound Study of Lignin Recovered from a Formic Acid Process. Procedia Eng. 2016, 148, 1312–1319. [Google Scholar] [CrossRef]
- Kalita, R.D.; Nath, Y.; Ochubiojo, M.E.; Buragohain, A.K. Extraction and Characterization of Microcrystalline Cellulose from Fodder Grass; Setaria Glauca (L) P. Beauv, and Its Potential as a Drug Delivery Vehicle for Isoniazid, a First Line Antituberculosis Drug. Colloids Surf. B Biointerfaces 2013, 108, 85–89. [Google Scholar] [CrossRef]
- Sonia, A.; Priya Dasan, K. Chemical, Morphology and Thermal Evaluation of Cellulose Microfibers Obtained from Hibiscus Sabdariffa. Carbohydr. Polym. 2013, 92, 668–674. [Google Scholar] [CrossRef]
- Díaz, M.J.; Ruiz-Montoya, M.; Palma, A.; de-Paz, M.-V. Thermogravimetry Applicability in Compost and Composting Research: A Review. Appl. Sci. 2021, 11, 1692. [Google Scholar] [CrossRef]
- Werner, K.; Pommer, L.; Broström, M. Thermal Decomposition of Hemicelluloses. J. Anal. Appl. Pyrolysis 2014, 110, 130–137. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, H.W.; Kim, S.; Watanabe, C.; Park, Y.-K. Non-Isothermal Pyrolysis of Citrus Unshiu Peel. BioEnergy Res. 2015, 8, 431–439. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The Effect of the Biomass Components Lignin, Cellulose and Hemicellulose on TGA and Fixed Bed Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Hussin, M.H.; Pohan, N.A.; Garba, Z.N.; Kassim, M.J.; Rahim, A.A.; Brosse, N.; Yemloul, M.; Fazita, M.R.N.; Haafiz, M.K.M. Physicochemical of Microcrystalline Cellulose from Oil Palm Fronds as Potential Methylene Blue Adsorbents. Int. J. Biol. Macromol. 2016, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Romruen, O.; Kaewprachu, P.; Karbowiak, T.; Rawdkuen, S. Isolation and Characterization Cellulose Nanosphere from Different Agricultural By-Products. Polymers 2022, 14, 2534. [Google Scholar] [CrossRef] [PubMed]
- Choong, F.X.; Bäck, M.; Schulz, A.; Nilsson, K.P.R.; Edlund, U.; Richter-Dahlfors, A. Stereochemical Identification of Glucans by Oligothiophenes Enables Cellulose Anatomical Mapping in Plant Tissues. Sci. Rep. 2018, 8, 3108. [Google Scholar] [CrossRef]
- Choong, F.X.; Lantz, L.; Shirani, H.; Schulz, A.; Nilsson, K.P.R.; Edlund, U.; Richter-Dahlfors, A. Stereochemical Identification of Glucans by a Donor–Acceptor–Donor Conjugated Pentamer Enables Multi-Carbohydrate Anatomical Mapping in Plant Tissues. Cellulose 2019, 26, 4253–4264. [Google Scholar] [CrossRef]
- Choong, F.X.; Bäck, M.; Steiner, S.E.; Melican, K.; Nilsson, K.P.R.; Edlund, U.; Richter-Dahlfors, A. Nondestructive, Real-Time Determination and Visualization of Cellulose, Hemicellulose and Lignin by Luminescent Oligothiophenes. Sci. Rep. 2016, 6, 35578. [Google Scholar] [CrossRef]
- Alba, K.; Campbell, G.M.; Kontogiorgos, V. Dietary Fibre from Berry-Processing Waste and Its Impact on Bread Structure: A Review. J. Sci. Food Agric. 2019, 99, 4189–4199. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Zain, N.F.; Yusop, S.M.; Ahmad, I. Preparation and Characterization of Cellulose and Nanocellulose from Pomelo (Citrus Grandis) Albedo. J. Nutr. Food Sci. 2014, 5, 334. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inthalaeng, N.; Dugmore, T.I.J.; Matharu, A.S. Production of Hydrogels from Microwave-Assisted Hydrothermal Fractionation of Blackcurrant Pomace. Gels 2023, 9, 674. https://doi.org/10.3390/gels9090674
Inthalaeng N, Dugmore TIJ, Matharu AS. Production of Hydrogels from Microwave-Assisted Hydrothermal Fractionation of Blackcurrant Pomace. Gels. 2023; 9(9):674. https://doi.org/10.3390/gels9090674
Chicago/Turabian StyleInthalaeng, Natthamon, Tom I. J. Dugmore, and Avtar S. Matharu. 2023. "Production of Hydrogels from Microwave-Assisted Hydrothermal Fractionation of Blackcurrant Pomace" Gels 9, no. 9: 674. https://doi.org/10.3390/gels9090674