Temperature-Sensitive Hydrogels as Carriers for Modulated Delivery of Acetaminophen
Abstract
:1. Introduction
2. Results and Discussion
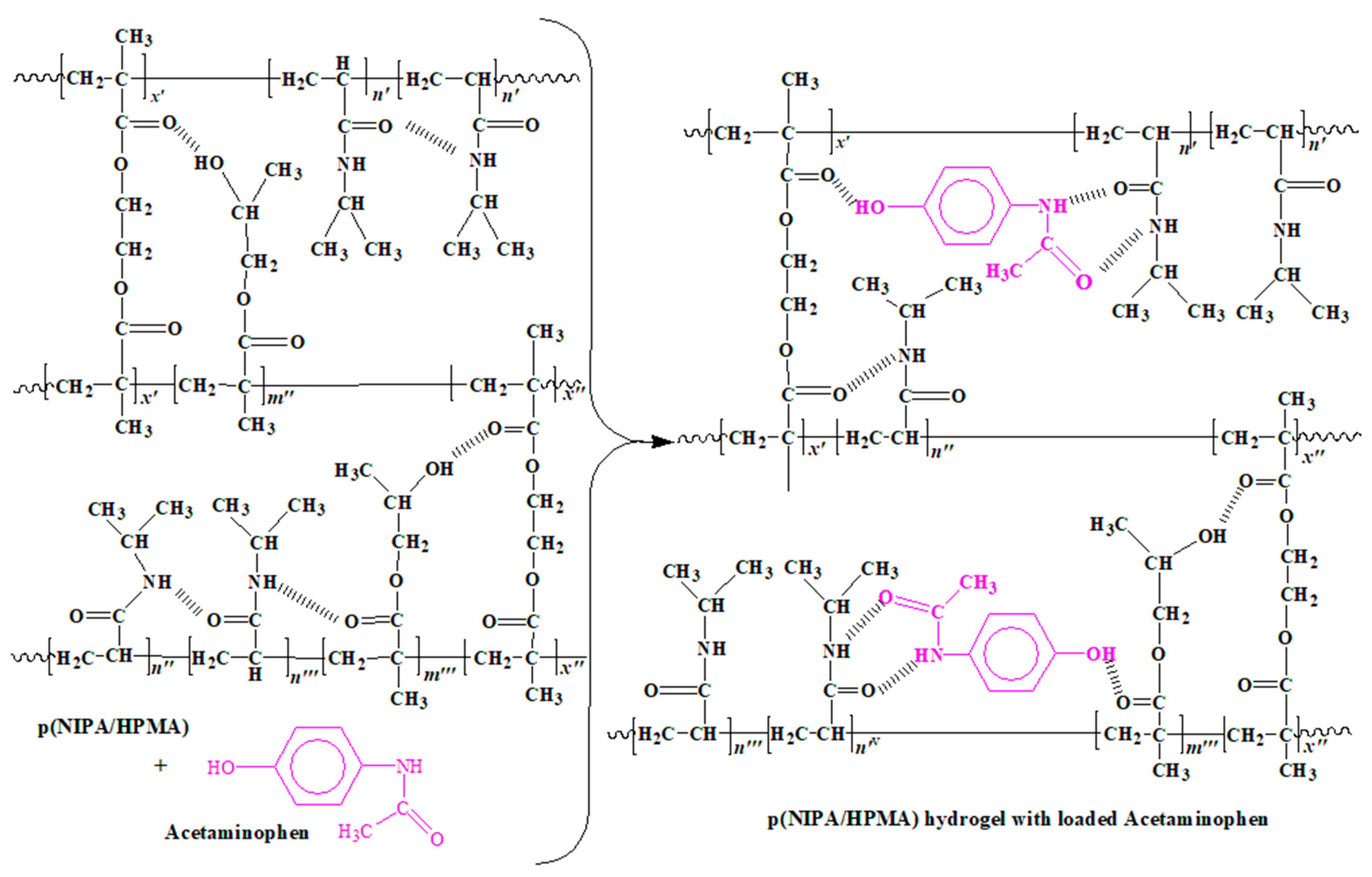
2.1. Synthesis of p(NIPA/HPMA) Hydrogel
2.2. Differential Scanning Calorimetry
2.3. Swelling Behavior
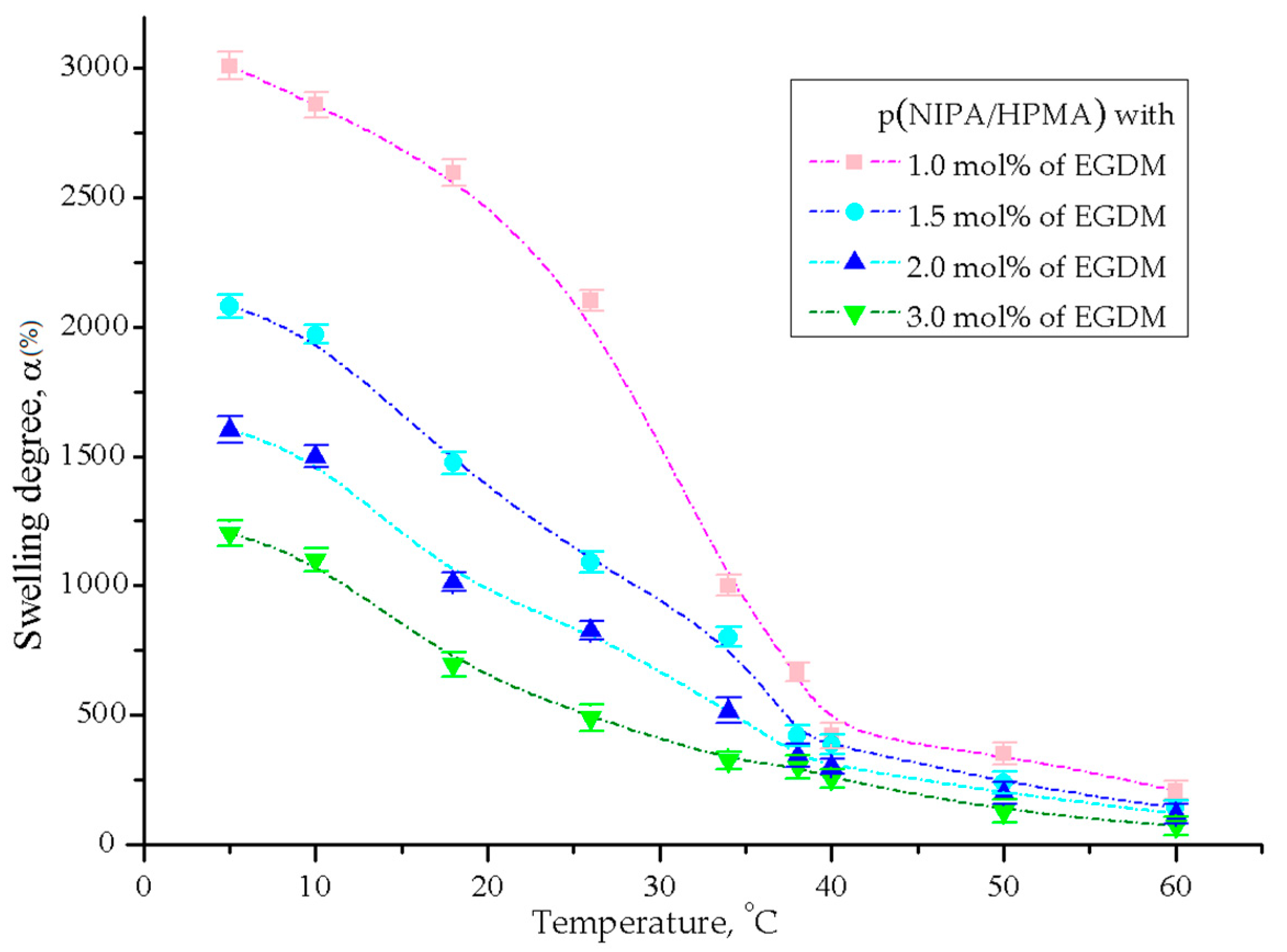
2.3.1. Temperature Sensitivity Analysis
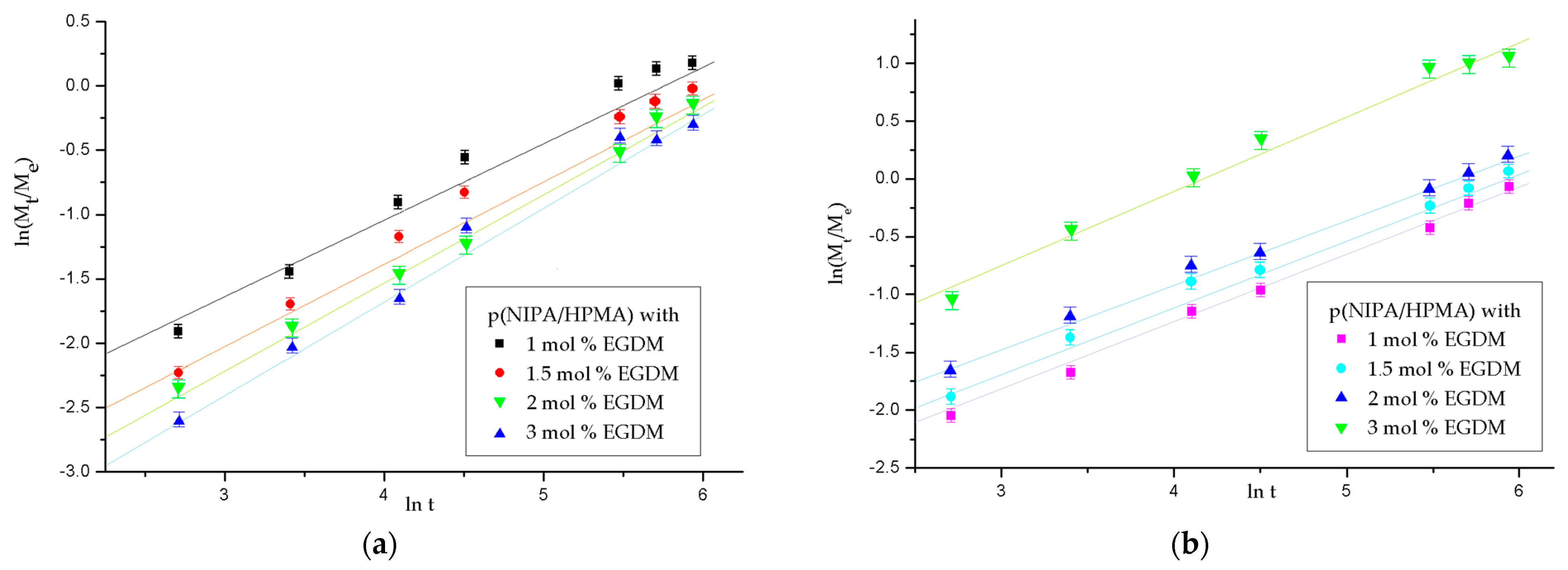
2.3.2. Analysis of the p(NIPA/HPMA) Hydrogel Swelling Kinetics
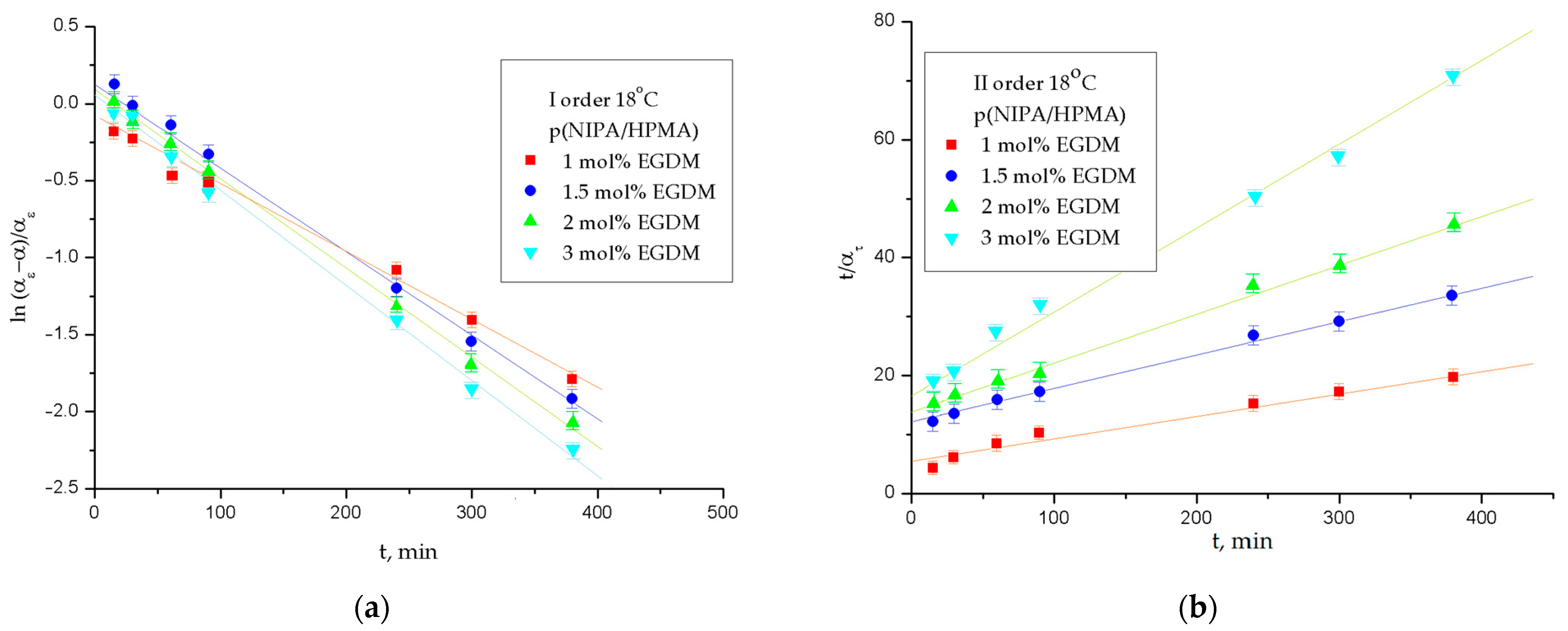
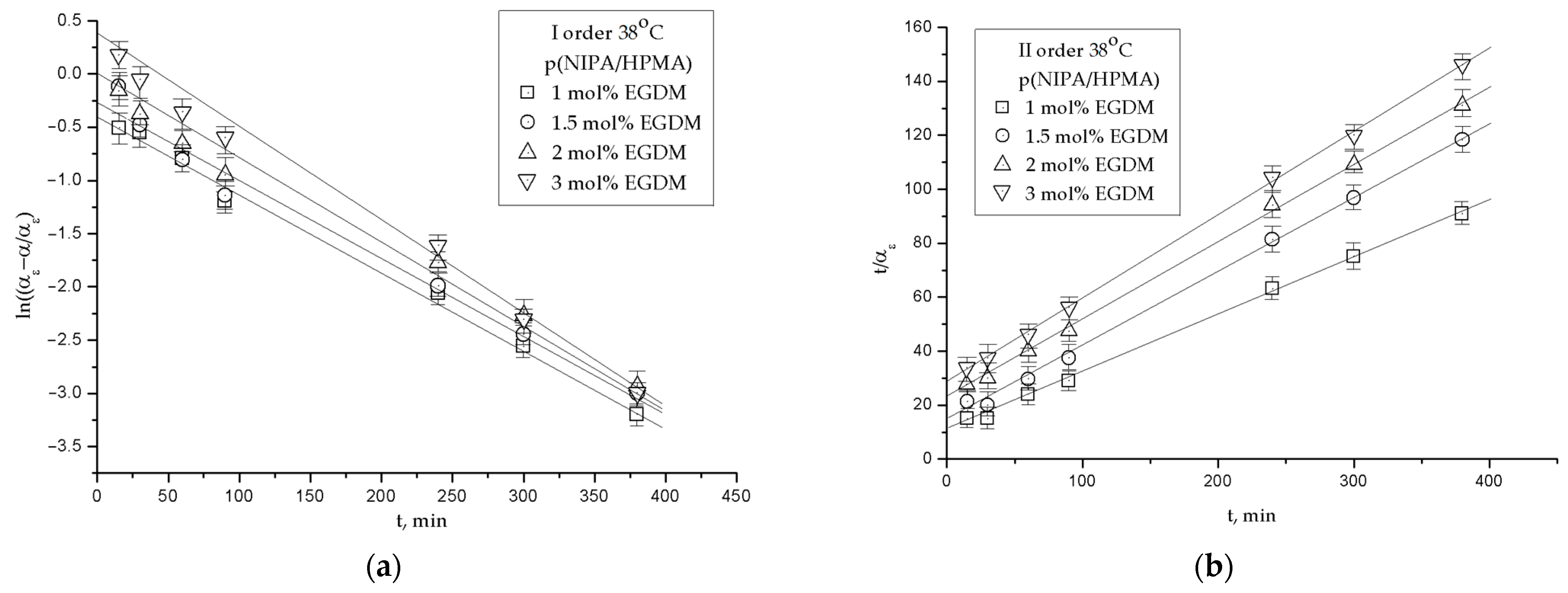
2.3.3. The Order of the Swelling Reaction
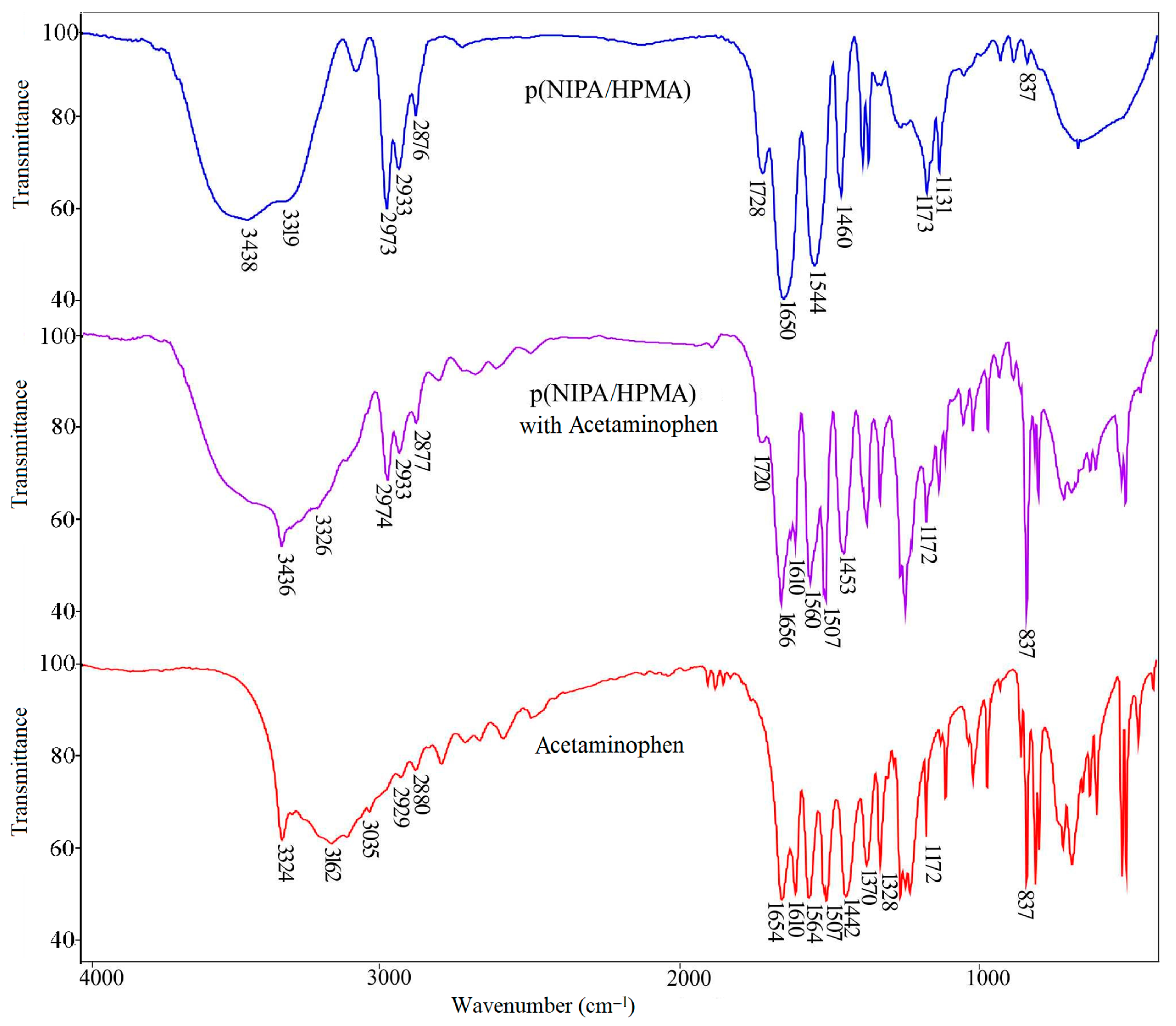
2.4. Fourier Transform Infrared Spectra
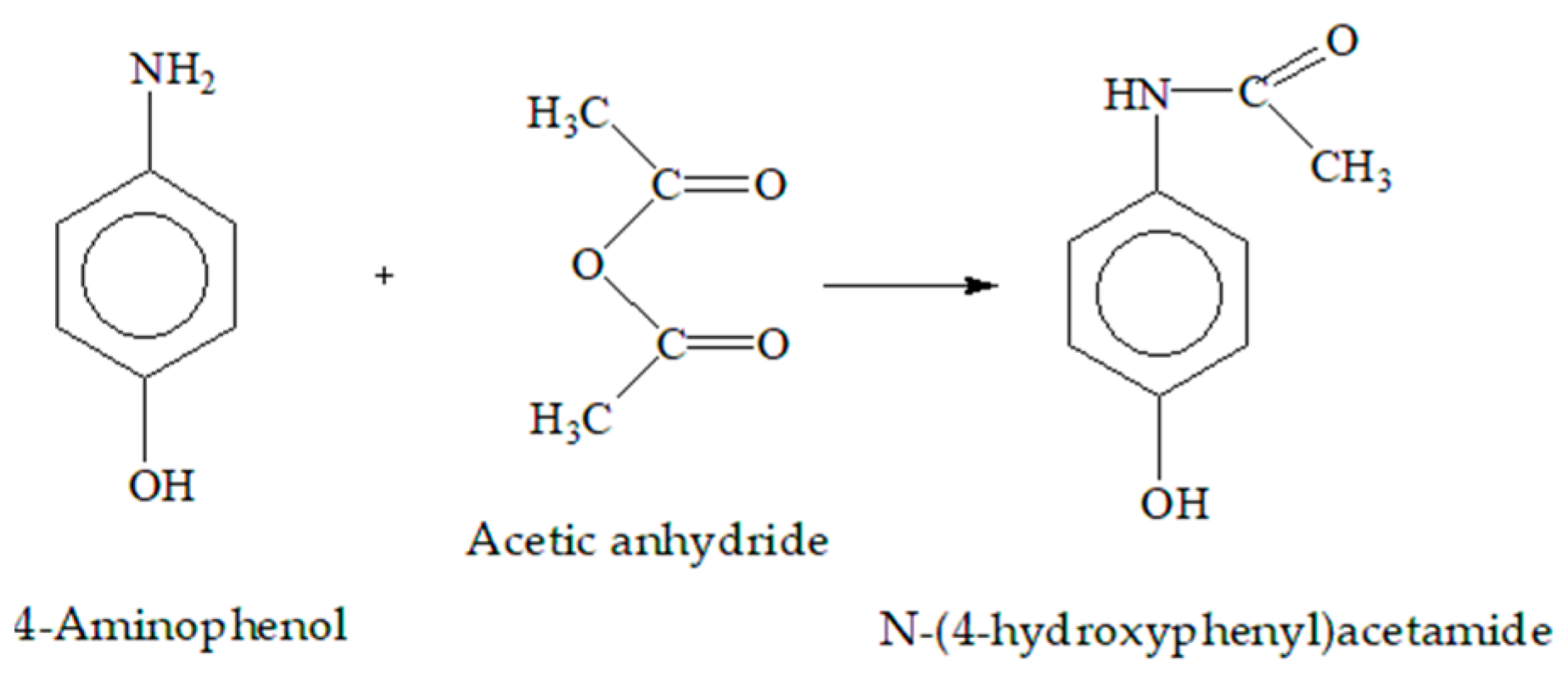
2.4.1. FTIR Spectrum Analysis of Acetaminophen
2.4.2. FTIR Spectrum Analysis of p(NIPA/HPMA) Hydrogel with Loaded Acetaminophen
2.5. Acetaminophen Loading Efficiency into p(NIPA/HPMA) Hydrogels
2.6. Morphology Characterization
2.7. In Vitro Acetaminophen Delivery from p(NIPA/HPMA) Copolymers
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Hydroges Synthesis
4.3. DSC Method
4.4. Swelling Behavior
4.4.1. Temperature Sensitivity
4.4.2. Kinetic Analysis
4.4.3. The Order of the Swelling Reaction
4.5. Acetaminophen Loading into the p(NIPA/HPMA)
4.6. In Vitro Acetaminophen Release Study
4.7. Characterization
4.7.1. FTIR Method
4.7.2. Freeze-Drying of Hydrogels
4.7.3. Scanning Electron Microscopy
4.8. Statistical Analysis
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Vatankhah-Varnoosfaderani, M.; Gutiérrez, T.J.; Saeb, M.R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]
- Vanparijs, N.; Nuhn, L.; De Geest, B.G. Transiently thermoresponsive polymers and their applications in biomedicine. Chem. Soc. Rev. 2017, 46, 1193–1239. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Dušek, K.; Dušková-Smrčková, M. Volume phase transition in gels: Its discovery and development. Gels 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.A. Responsive Gels: Volume Transitions I.; Dusek, K., Ed.; Advanced in Polymer Science; Springer-Verlag: Berlin/Heidelberg, Germany, 1993; Volume 109, pp. 233–267. [Google Scholar]
- Barrett, C.J.; Mamiya, J.I.; Yager, K.G.; Ikeda, T. Photo-mechanical effects in azobenzene-containing soft materials. Soft Matter 2007, 3, 1249–1261. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S. Synthesis and Characterization of Negatively Thermosensitive Hydrogels; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2015; ISBN 978-3-659-47484-2. [Google Scholar]
- Lizundia, E.; Meaurio, E.; Laza, J.M.; Vilas, J.L.; Isidro, L.L. Study of the chain microstructure effects on the resulting thermal properties of poly (L-lactide)/poly (N-isopropylacrylamide) biomedical materials. Mater. Sci. Eng. C 2015, 50, 97–106. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci.—Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar] [CrossRef]
- Coughlan, D.C.; Quilty, F.P.; Corrigan, O.I. Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly(N-isopropyl acrylamide) hydrogels. J. Control. Release 2004, 98, 97–114. [Google Scholar] [CrossRef]
- Urošević, M.Z.; Nikolić, L.B.; Ilić-Stojanović, S.; Zdravković, A.; Nikolić, V.D. Synthesis and characterization of poly(N-isopropylmethacrylamide-co-N-isopropyl acrylamide) copolymers. Hem. Ind. 2020, 74, 103–117. [Google Scholar] [CrossRef]
- Urošević, M.Z.; Nikolić, L.B.; Ilić-Stojanović, S.S.; Nikolić, V.D.; Petrović, S.M.; Zdravković, A.S. Hydrogels based on N-isopropylmethacrylamide and N-isopropyl acrylamide. Adv. Technol. 2018, 7, 79–91. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive nanocomposite hydrogels based on Gelatin/poly (N–isopropylacrylamide)(PNIPAM) for controlled drug delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Yamato, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly (butylmethacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.C.; Mirica, A.C.I.; Hudita, A.; Tanasa, E.; Iovu, H.; Zaharia, C.; Galateanu, B. Thermosensitive Behavior Defines the Features of Poly(N-isopropylacrylamide)/Magnetite Nanoparticles for Cancer Management. Appl. Sci. 2023, 13, 4870. [Google Scholar] [CrossRef]
- Huang, Y.C.; Zeng, Y.J.; Lin, Y.W.; Tai, H.C.; Don, T.M. In Situ Encapsulation of Camptothecin by Self-Assembly of Poly (acrylic acid)-b-Poly (N-Isopropylacrylamide) and Chitosan for Controlled Drug Delivery. Polymers 2023, 15, 2463. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Okano, T. Thermoresponsive-polymer-based materials for temperature-modulated bioanalysis and bioseparations. J. Mater. Chem. B 2016, 4, 6381–6397. [Google Scholar] [CrossRef]
- Cai, W.; Anderson, E.C.; Gupta, R.B. Separation of lignin from aqueous mixtures by ionic and nonionic temperature-sensitive hydrogels. Ind. Eng. Chem. Res. 2001, 40, 2283–2288. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Toto, E.; Santonicola, M.G. Conductive and Thermo-Responsive Composite Hydrogels with Poly (N-isopropylacrylamide) and Carbon Nanotubes Fabricated by Two-Step Photopolymerization. Polymers 2023, 15, 1022. [Google Scholar] [CrossRef]
- 4-Acetamidophenol, 98% Material Safety Data Sheet. Available online: https://fscimage.fishersci.com/msds/00229.htm (accessed on 12 March 2023).
- USP. U.S. Pharmacopeia National Formulary 2018: USP 41 NF 36; United States Pharmacopeial Convention: Rockville, MD, USA, 2018. [Google Scholar]
- Čeković, Ž. Organske Sinteze: Reakcije i Metode; Zavod za Udžbenike i Nastavna Sredstva: Belgrade, Serbia, 2006. (In Serbian) [Google Scholar]
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. Paracetamol: New vistas of an old drug. CNS Drug Rev. 2006, 12, 250–275. [Google Scholar] [CrossRef]
- Knights, K.; Rowland, A.; Darroch, S.; Bushell, M. Pharmacology for Health Professionals, 6th ed.; Elsevier: Melbourne, Australia, 2023. [Google Scholar]
- Byrant, B.; Knights, K.; Salerno, E. Pharmacology for Health Professionals; Elsevier: Melbourne, Australia, 2003; p. 270. [Google Scholar]
- Diener, H.C.; Tfelt-Hansen, P.; Dahlöf, C.; Láinez, M.J.; Sandrini, G.; Wang, S.J.; Neto, W.; Vijapurkar, U.; Doyle, A.; Jacobs, D. MIGR-003 Study Group. Topiramate in migraine prophylaxis: Results from a placebo–controlled trial with propranolol as an active control. J. Neurol. 2004, 251, 943–950. [Google Scholar] [PubMed]
- Qin, N.; Zhang, S.P.; Reitz, T.L.; Mei, J.M.; Flores, C.M. Cloning, expression, and functional characterization of human cyclooxygenase-1 splicing variants: Evidence for intron 1 retention. J. Pharmacol. Exp. Ther. 2005, 315, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Grammas, P. Acetaminophen protects brain endothelial cells against oxidative stress. Microvasc. Res. 2009, 77, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Shayani-Jam, H.; Nematollahi, D. Electrochemical evidences in oxidation of acetaminophen in the presence of glutathione and N-acetylcysteine. Chem. Comm. 2010, 46, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Folkers, G.; Waterbeemd, H.; Lennernäs, H.; Artursson, P.; Mannhold, R.; Kubinyi, H. Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability (Methods and Principles in Medicinal Chemistry); Wiley-VCH, Verlag GmbH & Co. KgaA: Weinheim, Germany, 2003. [Google Scholar]
- Abdelbary, G.; Prinderre, P.; Eouani, C.; Joachim, J.; Reynier, J.P.; Piccerelle, P.H. The preparation of orally disintegrating tablets using a hydrophilic waxy binder. Int. J. Pharm. 2004, 278, 423–433. [Google Scholar] [CrossRef]
- Li, G.; Chu, J.; Song, E.S.; Row, K.H.; Lee, K.H.; Lee, Y.W. Crystallization of acetaminophen micro-particle using supercritical carbon dioxide. Korean J. Chem. Eng. 2006, 23, 482–487. [Google Scholar] [CrossRef]
- Devine, D.M.; Devery, S.M.; Lyons, J.G.; Geever, L.M.; Kennedy, J.E.; Higginbotham, C.L. Multifunctional polyvinylpyrrolidinone-polyacrylic acid copolymer hydrogels for biomedical applications. Int. J. Pharm. 2006, 326, 50–59. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Oprea, A.M.; Vasile, C. A drug delivery system based on stimuli-responsive alginate/N-isopropylacryl amide hydrogel. Cellul. Chem. Technol. 2009, 43, 251–262. [Google Scholar]
- Stanojević, M.; Kalagasidis-Krušić, M.; Filipović, J.; Parojčić, J.; Stupar, M. An investigation into the influence of hydrogel composition on swelling behavior and drug release from poly(acrylamide-co-itaconic acid) hydrogels in various media. Drug Deliv. 2006, 13, 1–7. [Google Scholar] [CrossRef]
- Gong, C.; Wong, K.L.; Lam, M.H. Photoresponsive molecularly imprinted hydrogels for the photoregulated release and uptake of pharmaceuticals in the aqueous media. Chem. Mater. 2008, 20, 1353–1358. [Google Scholar] [CrossRef]
- Subramanian, K.G.; Vijayakumar, V. Characterization of crosslinked poly(2-hydroxyethyl methacrylate-co-n-vinyl-2-pyrrolidone) as a carrier for controlled drug delivery. J. Pharm. Res. 2011, 4, 743–747. [Google Scholar]
- Díaz-Guerrero, A.M.; Castillo-Miranda, C.A.; Castro-Guerrero, C.F.; Peraza-Vázquez, H.; Morales-Cepeda, A.B.; Peña-Delgado, A.F. Mathematical modelling of acetaminophen release in HPC/PAAm hydrogel: Synthesis and application. Int. J. Polym. Sci. 2019, 2019, 9306459. [Google Scholar] [CrossRef]
- Miotke, M.; Strankowska, J.; Kwela, J.; Strankowski, M.; Józefowicz, M. Transport of paracetamol in swellable and relaxing polyurethane nanocomposite hydrogels. Polym. Bull. 2020, 77, 483–499. [Google Scholar] [CrossRef]
- Elham, K.; Omid, R.; Farshad, F.; Afshin, J.; Farnaz Sadat Mirzazadeh, T. Preparation and investigation of poly [N-isopropylacrylamide-acrylamide] membranes in temperature responsive drug delivery. Iran. J. Basic Med. Sci. 2010, 13, 102–110. [Google Scholar]
- O’Donnell, K.L.; Oporto-Velásquez, G.S.; Comolli, N. Evaluation of Acetaminophen Release from Biodegradable Poly(Vinyl Alcohol) (PVA) and Nanocellulose Films Using a Multiphase Release Mechanism. Nanomaterials 2020, 10, 301. [Google Scholar] [CrossRef]
- Nizam El-Din, H.M. Characterization and caffeine release properties of N-isopropyl acrylamide/hydroxypropyl methacrylate, copolymer hydrogel synthesized by gamma radiation. J. Appl. Polym. Sci. 2011, 119, 577–585. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Ristić, I.; Cakić, S.; Petrović, S.D. Biomedical applications of thermosensitive hydrogels for controlled/modulated piroxicam delivery. Gels 2023, 9, 70. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Petrović, S.; Oro, V.; Mitić, Ž.; Najman, S. Semi-Crystalline copolymer hydrogels as smart drug carriers: In vitro thermo-responsive naproxen release study. Pharmaceutics 2021, 13, 158. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Milić, J.; Stamenković, J.; Nikolić, G.M.; Petrović, S.D.; Kapor, A. Potential application of thermosensitive hydrogels for controlled release of phenacetin. Chem. Ind. 2012, 66, 831–839. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.S.; Nikolić, L.B.; Nikolić, V.D.; Milić, J.R.; Stamenković, J.; Nikolić, G.M.; Petrović, S.D. Synthesis and characterization of thermosensitive hydrogels and the investigation of modified release of ibuprofen. Hem. Ind. 2013, 67, 901–912. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.B.; Nikolić, V.; Stanković, M.; Stamenković, J.; Mladenović-Ranisavljević, I.; Petrović, S. Influence of monomer and crosslinker molar ratio on the swelling behaviour of thermosensitive hydrogels. Chem. Ind. Chem. Eng. 2012, 18, 1–9. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Ristić, I.; Budinski-Simendić, J.; Kapor, A.; Nikolić, G.M. The structure characterization of thermosensitive poly(N-isopropyl acrylamide-co-2-hydroxypropylmethacrylate) hydrogel. Polym. Int. 2013, 63, 973–981. [Google Scholar] [CrossRef]
- Khare, A.R.; Peppas, N.A. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T.H. Thermal and structural studies of poly(vinyl alcohol) and hydroxypropyl cellulose blends. Nat. Sci. 2012, 4, 57–67. [Google Scholar] [CrossRef]
- De Kee, D.; Liu, Q.; Hinestroza, J. Viscoelastic (non-Fickian) diffusion. Can. J. Chem. Eng. 2005, 83, 913–929. [Google Scholar] [CrossRef]
- Habiba, U.; Alam, A.; Rahman, S.; Shamim, S.U.D.; Piya, A.A. IR spectra of paracetamol. Bangladesh J. Sci. Ind. Res. 2021, 56, 255–262. [Google Scholar] [CrossRef]
- Boldyreva, E.V. High-pressure studies of the anisotropy of structural distortion of molecular crystals. J. Mol. Struct. 2003, 647, 159–179. [Google Scholar] [CrossRef]
- Milosavljević, S.M. Strukturne Instrumentalne Metode; Univerzitet u Beogradu, Hemijski Fakultet: Beograd, Serbia, 1994. (In Serbian) [Google Scholar]
- What Is Extended-Release Medication? | PainScale. Available online: www.painscale.com/article/what-is-extended-release-medication, (accessed on 20 April 2023).
- Acetaminophen Dosage. Available online: www.drugs.com/dosage/acetaminophen.html (accessed on 20 April 2023).
- Diez-Pena, E.; Quijada-Garrido, I.; Barrales-Rienda, J.M. Hydrogen-bonding effects on the dynamic swelling of P(N-iPAAm-co-MAA) copolymers. A case of autocatalytic swelling kinetics. Macromolecules 2002, 35, 8882–8888. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Bajpai, S.K. Swelling–deswelling behavior of poly(acrylamide-co-maleic acid) hydrogels. J. Appl. Polym. Sci. 2001, 80, 2782–2789. [Google Scholar] [CrossRef]
- Schott, H.J. Swelling kinetics of polymers. Macromol. Chem. Phys. 1992, B31, 1–9. [Google Scholar] [CrossRef]
- Schott, H. Kinetics of swelling of polymers and their gels. J. Pharm Sci. 1992, 81, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Krušić, M.K.; Filipović, J. Copolymer hydrogels based on N-isopropylacrylamide and itaconic acid. Polymer 2006, 47, 148–155. [Google Scholar] [CrossRef]




| Poly(N-isopropyl acrylamide-co-2-hydroxypropylmethacrylate) | Melting Transition Temperature, °C | Melting Enthalpy, J∙g−1 | Glass Transition Temperature, Tg, °C | |||
|---|---|---|---|---|---|---|
| p(NIPA/HPMA) with | Tm1 | Tm2 | ΔHm | Tg1 | Tg2 | Tg3 |
| 1 mol % of EGDM | 153.90 | 159.07 | 5.99 | 63.41 | 77.53 | 131.76 |
| 2 mol % of EGDM | 156.49 | 160.73 | 9.85 | 64.79 | 85.17 | 134.16 |
| 3 mol % of EGDM | 154.16 | 156.98 | 2.79 | 64.23 | 86.47 | - |
| p(NIPA/HPMA) * with | Equilibrium Swelling Degree, αe | Diffusion Exponent, n | Kinetic Constant, k × 102, min−1/2 | Linear Correlation Coefficient, R2 | Diffusion Coefficient, D, cm2·min−1 |
|---|---|---|---|---|---|
| 18 °C | |||||
| 1 mol % of EGDM | 25.960 | 0.822 | 1.998 | 0.981 | 8.998 × 10−6 |
| 1.5 mol % of EGDM | 14.748 | 0.761 | 1.105 | 0.991 | 2.703 × 10−6 |
| 2 mol % of EGDM | 10.143 | 0.681 | 0.989 | 0.993 | 0.379 × 10−6 |
| 3 mol % of EGDM | 6.943 | 0.584 | 0.971 | 0.996 | 0.691 × 10−6 |
| 38 °C | |||||
| 1 mol % of EGDM | 6.662 | 0.984 | 3.323 | 0.996 | 0.784 × 10−5 |
| 1.5 mol % of EGDM | 4.196 | 0.701 | 2.601 | 0.964 | 0.676 × 10−5 |
| 2 mol % of EGDM | 3.429 | 0.692 | 2.251 | 0.981 | 1.399 × 10−5 |
| 3 mol % of EGDM | 2.986 | 0.611 | 2.292 | 0.936 | 3.102 × 10−5 |
| p(NIPA/HPMA) * with | Equilibrium Swelling Ratio, αe (exp) | Equilibrium Swelling Ratio, αe (I-order) | Rate Constant (I-order), K·103, min−1 | Linear Correlation Coefficient (I-order), R2 | Equilibrium Swelling Ratio, αe (II-order) | Rate Constant (II-order), K·103, min−1 | Linear Correlation Coefficient (II-order), R2 |
|---|---|---|---|---|---|---|---|
| 18 °C | |||||||
| 1 mol % of EGDM | 25.960 | 27.214 | 2.35 | 0.982 | 26.113 | 26.286 | 0.999 |
| 1.5 mol % of EGDM | 14.748 | 15.972 | 3.96 | 0.971 | 14.986 | 5.691 | 0.999 |
| 2 mol % of EGDM | 10.143 | 11.266 | 3.23 | 0.934 | 10.534 | 13.118 | 0.999 |
| 3 mol % of EGDM | 6.943 | 8.32 | 3.98 | 0.961 | 7.112 | 4.882 | 0.999 |
| 38 °C | |||||||
| 1 mol % of EGDM | 6.662 | 8.012 | 9.36 | 0.989 | 6.892 | 6.141 | 0.999 |
| 1.5 mol % of EGDM | 4.196 | 4.949 | 7.33 | 0.991 | 4.464 | 6.841 | 0.999 |
| 2 mol % of EGDM | 3.429 | 3.833 | 7.18 | 0.996 | 3.678 | 5.654 | 0.999 |
| 3 mol % of EGDM | 2.986 | 3.524 | 8.59 | 0.991 | 3.274 | 4.991 | 0.998 |
| Wavenumber of Functional Group, cm−1 | Functional Group | Shifts in Relation to the FTIR Spectra, cm−1 | |||
|---|---|---|---|---|---|
| p(NIPA/HPMA) * | Acetaminophen | p(NIPA/HPMA) with Acetaminophen | p(NIPA/HPMA) | Acetaminophen | |
| 3438 | 3436 | ν(OH) | −2 | ||
| 3319 | 3324 | 3326 | ν(NH) | +7 | +2 |
| 3162 | - | ν(Ar-OH) | - | ||
| 3035 | - | ν(C-H) Ar | - | ||
| 2973 | 2929 | 2974 | νas(CH3) | +1 | |
| 2933 | 2934 | νas(CH2) | +1 | ||
| 2876 | 2880 | 2877 | νs(CH3) | +1 | −3 |
| 1728 | 1720 | ν(C=O) ester | −8 | ||
| 1650 | 1654 | 1656 | ν(C=O) amide I | +6 | +2 |
| 1610 | 1610 | ν(C=C) Ar | 0 | ||
| 1544 | 1564 | 1560 | δ(N-H) amide II | +16 | −4 |
| 1507 | 1507 | ν(C=C) Ar | 0 | ||
| 1460, 1387 | 1442 | 1453, 1369 | δ(OH) | −7, −9 | +11 |
| 1367 | 1369 | δ(CH)-isopropyl | +2 | ||
| 1306 | - | νs(C-N) amide III | |||
| 1370, 1328 | 1327 | δs(CH3) | −1 | ||
| 1260 | 1260 | ν(C-O) | |||
| 1227 | 1243 | ν(NCH) | +16 | ||
| 1173 | 1172 | 1172 | ν(CN) | −1 | 0 |
| 1131 | 1131 | νs(C-O) | 0 | ||
| 837 | 837 | 837 | γ(CH) | 0 | 0 |
| 808 | 797 | γ(CH) | −11 | ||
| 674 | 686 | 688 | γ(OH) | +14 | +2 |
| p(NIPA/HPMA) * with | Amount of Loaded Acetaminophen, Lg, mg/gxerogel | Acetaminophen Loading Efficiency, ηacetaminophen, % |
|---|---|---|
| 1 mol % of EGDM | 480.34 | 96.06 |
| 1.5 mol % of EGDM | 460.84 | 92.16 |
| 2 mol % of EGDM | 467.63 | 93.52 |
| 3 mol % of EGDM | 480.81 | 96.14 |
| Poly(N-isopropyl acrylamide-co-2-hydroxypropylmethacrylate) with | Quantity of Released Acetaminophen, mg/gxerogel% | Diffusion Exponent, n | Kinetic Constant, K, min−1/2 | Linear Correlation Coefficient, R2 | Diffusion Coefficient, D, cm2/min | |
|---|---|---|---|---|---|---|
| pH 7.40 | ||||||
| 1 mol % of EGDM | 492.77 | 98.56 | 0.297 | 0.641 | 0.972 | 3.79 × 10−3 |
| 1.5 mol % of EGDM | 484.11 | 96.82 | 0.325 | 0.694 | 0.997 | 3.74 × 10−3 |
| 2 mol % of EGDM | 471.59 | 94.32 | 0.297 | 0.756 | 0.972 | 4.48 × 10−3 |
| 3 mol % of EGDM | 455.58 | 91.12 | 0.375 | 0.639 | 0.944 | 3.21 × 10−3 |
| pH 2.2 | ||||||
| 1 mol % of EGDM | 480.16 | 96.03 | 0.486 | 0.747 | 0.881 | 4.38 × 10−3 |
| 1.5 mol % of EGDM | 474.78 | 94.96 | 0.541 | 0.737 | 0.846 | 4.26 × 10−3 |
| 2 mol % of EGDM | 465.66 | 93.12 | 0.533 | 0.722 | 0.961 | 4.10 × 10−3 |
| 3 mol % of EGDM | 451.43 | 90.32 | 0.662 | 0.666 | 0.961 | 3.48 × 10−3 |
| Polymeric Carrier | Experimental Conditions and Released Acetaminophen | Mechanism of Acetaminophen Diffusion | Reference |
|---|---|---|---|
| Poly(N-isopropyl acrylamide-co-2-hydroxypropylmethacrylate) with ethyleneglycoldimethacrylate | 98.56% at pH = 7.4, and 96.03% at pH = 2.2 during 24 h at 38 °C. | At pH = 7.4—Fickian diffusion. At pH = 2.2—non-Fickian diffusion (gels with 1.5, 2 and 3 mol % of EGDM). | [current study] |
| Poly(N-vinylpyrrolidinone-co-acrylic acid) (with 30 wt % AA) and polyethylene glycol 600 dimethacrylate | At pH 2 approx. 24 h pH 6.8 approx. 5 h pH 9 approx. 5 h at 37 °C. | - | [36] |
| Sodium alginate and N-isopropyl acrylamide crosslinked with N,N’methylenebisacrylamide | At 37 °C in pH 2.2 for 9 days ~90%. | The first-order kinetic. Non-Fickian (anomalous) diffusion. | [37] |
| Poly(acrylamide-co-itaconic acid) crosslinked with N,N-methylenebisacrylamide | At 37 °C during 8 h: at pH = 2.2 ~20–55% pH = 4.5 ~90–99% pH = 6.8 ~90–99%. | Slow drug release under acidic conditions and rapid release at higher pH value. Fickian diffusion. | [38] |
| Polyacrylamide with 4-[(4-methacryloyloxy) phenylazo] benzenesulfonic acid and N,N′-hexylenebismethacrylamide | The photoregulated release at 353 nm for 120 min of irradiation, a total of 83.6% was released in aqueous HEPES buffer pH 7.16. | - | [39] |
| Poly(2-hydroxyethyl methacrylate-co-N-vinyl-2-pyrrolidone) with N,N’-methylenebisacrylamide | at 37 °C and SGF pH = 1.2, SIF pH = 7.2 ~95% during 240 min (4 h). | The tablet from the polymer NVP3 crumbled within an hour of immersion, resulting in burst release. Non-Fickian (anomalous) diffusion. | [40] |
| Hydroxypropyl cellulose with polyacrylamide, 25/75 wt % | In deionized water, pH 7 in phosphate buffer, pH 7.38 at 35 °C, 37 °C, 39 °C, during 6 h. | Drug was crystallized on the gel surface. Fickian diffusion. | [41] |
| Polyurethane nanocomposite hydrogels PU/PEG 4000 with 1% of organofillized montmorillonite (Cloisite® 30B) | In water for 24 h at 23 °C and 37 °C. | Easier release from nanocomposites than from a pure hydrogel matrix. Non-Fickian (anomalous) diffusion. | [42] |
| Poly(N-isopropylacrylamide-acrylamide) with N,N-methylenbisacrylamide, and N,N,N,N-tetra-methylethylenediamine | pH= 7.4 at 27 °C, 32 °C, 41 °C, 44 °C ± 0.1 °C | The pore mechanism of drug transport. | [43] |
| Poly(vinyl alcohol) with nanofibrillated cellulose, (NFC)/PVA, and 2,2,6,6-tetramethylpiperidine-N-oxyl-oxidized nanofibrillated cellulose (TNFC)/PVA without any chemical linkers | At 37 °C and phosphate buffer, 14% from (NFC)/PVA and about 28% from (TNFC)/PVA over 144 h (6 days). | Diffusion-controlled and burst release, with small fractions of relaxation-induced and prolonged-diffusional release. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Ristić, I.; Cakić, S.; Petrović, S.D. Temperature-Sensitive Hydrogels as Carriers for Modulated Delivery of Acetaminophen. Gels 2023, 9, 684. https://doi.org/10.3390/gels9090684
Ilić-Stojanović S, Nikolić L, Nikolić V, Ristić I, Cakić S, Petrović SD. Temperature-Sensitive Hydrogels as Carriers for Modulated Delivery of Acetaminophen. Gels. 2023; 9(9):684. https://doi.org/10.3390/gels9090684
Chicago/Turabian StyleIlić-Stojanović, Snežana, Ljubiša Nikolić, Vesna Nikolić, Ivan Ristić, Suzana Cakić, and Slobodan D. Petrović. 2023. "Temperature-Sensitive Hydrogels as Carriers for Modulated Delivery of Acetaminophen" Gels 9, no. 9: 684. https://doi.org/10.3390/gels9090684





