Electrosprayed Stearic-Acid-Coated Ethylcellulose Microparticles for an Improved Sustained Release of Anticancer Drug
Abstract
:1. Introduction
2. Results and Discussion
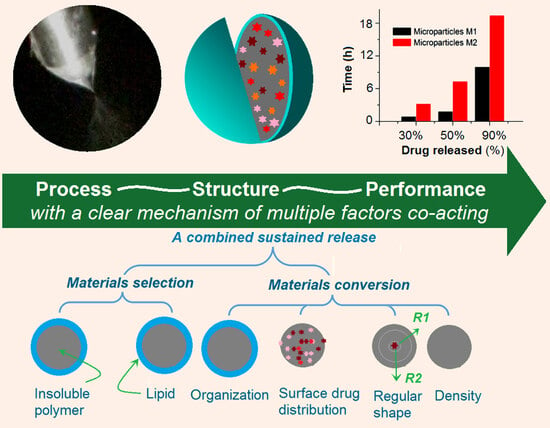
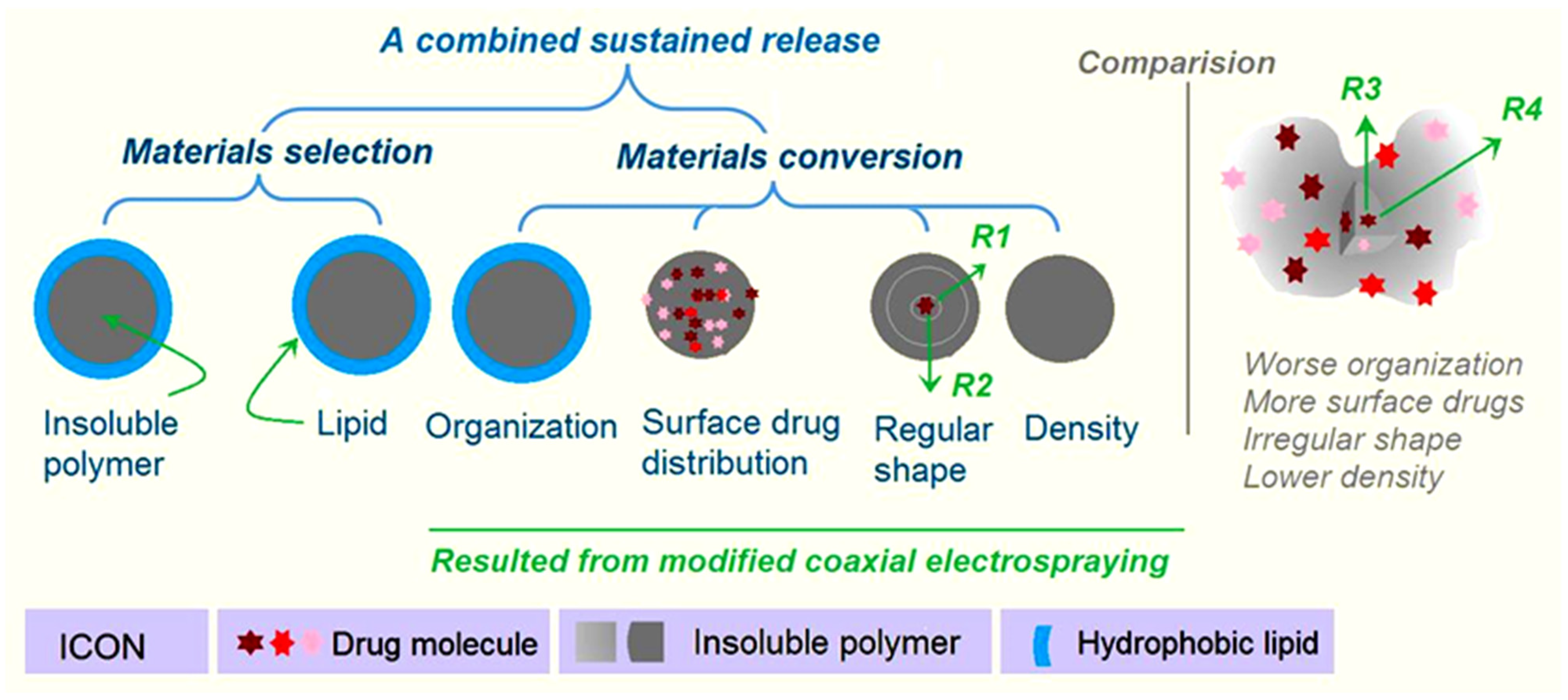
2.1. The Coaxial Electrospraying and the Core–Shell Structures in Drug Delivery
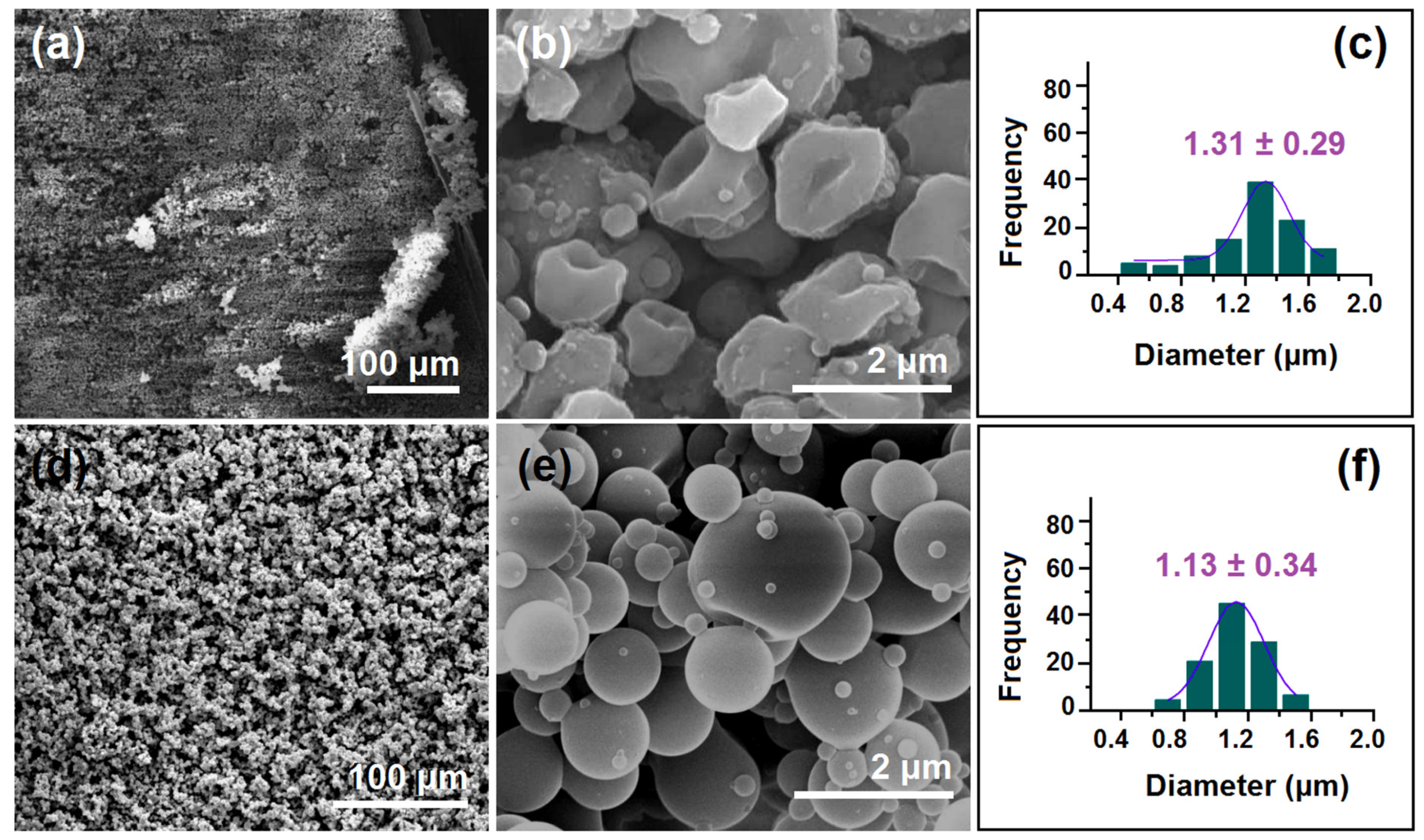
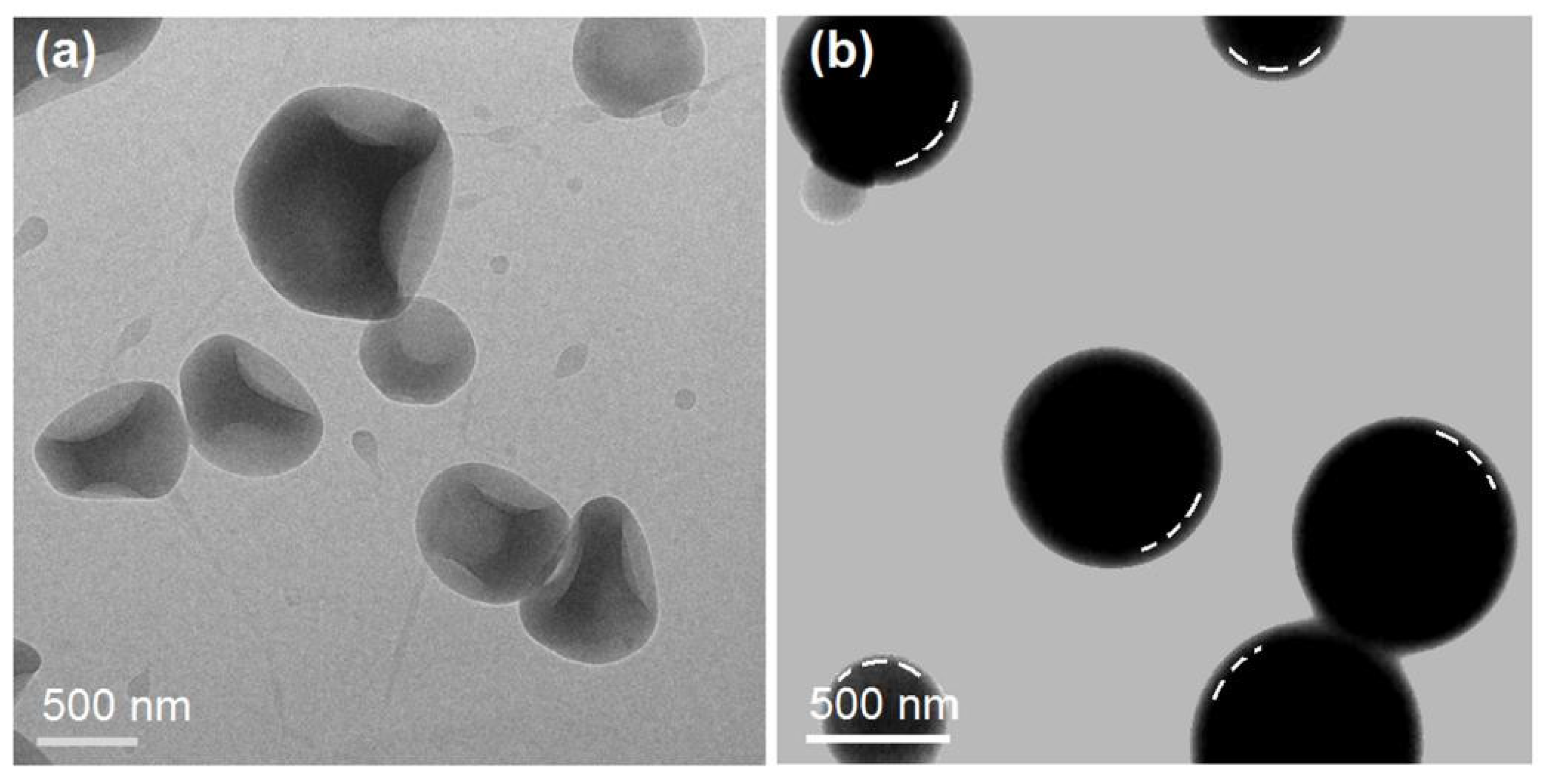
2.2. The Morphology and Structure of the Microparticles
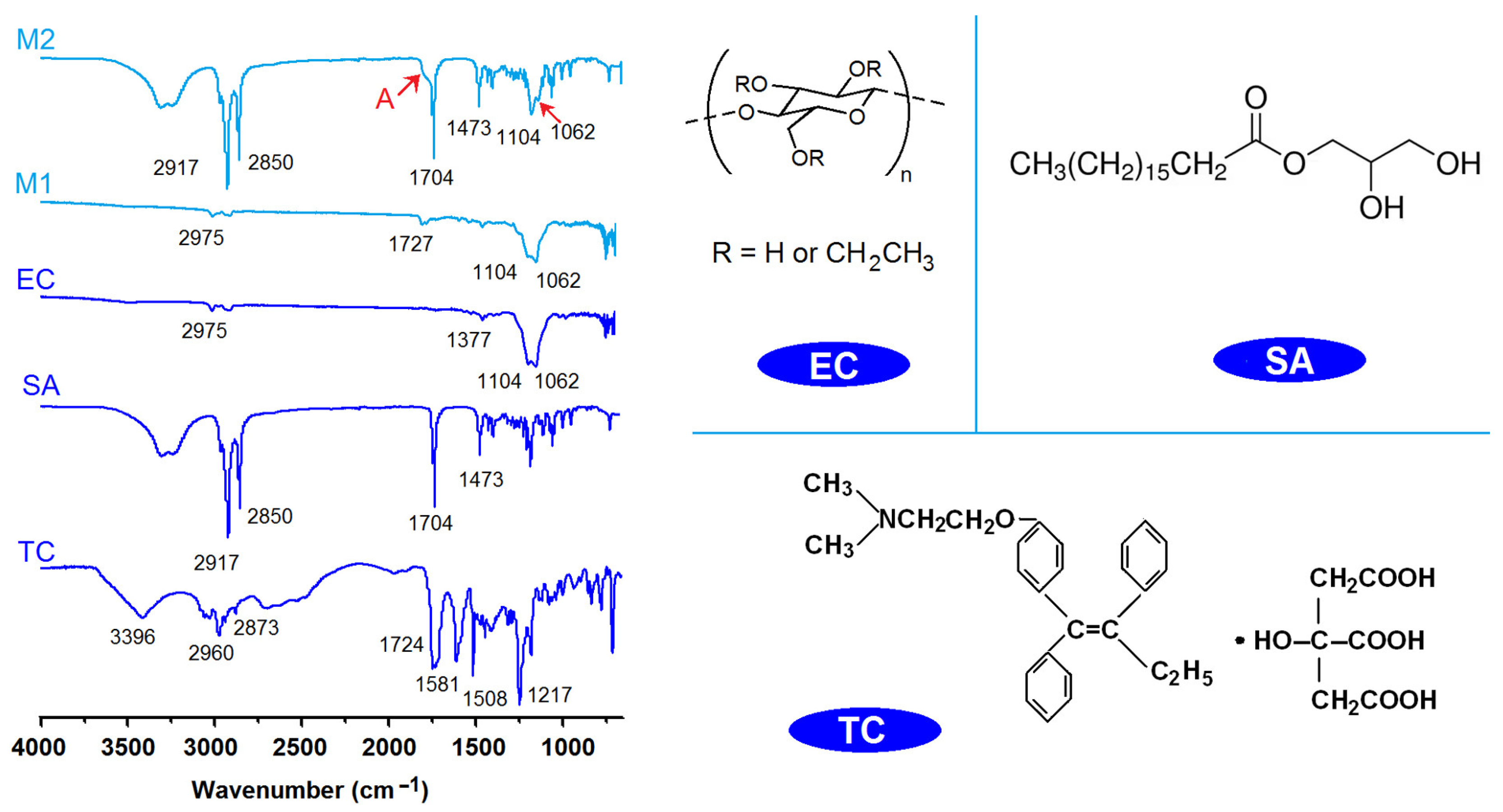
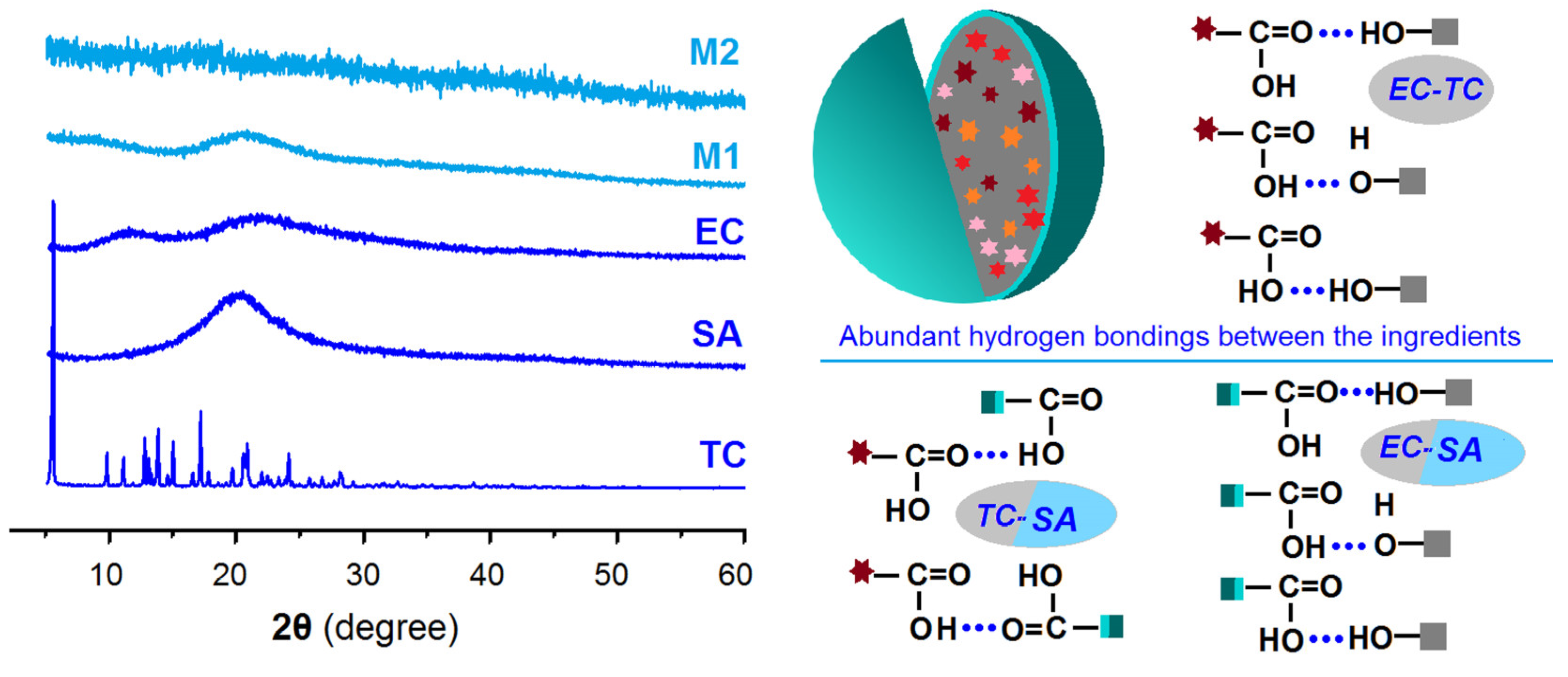
2.3. The Physical State and Compatibility
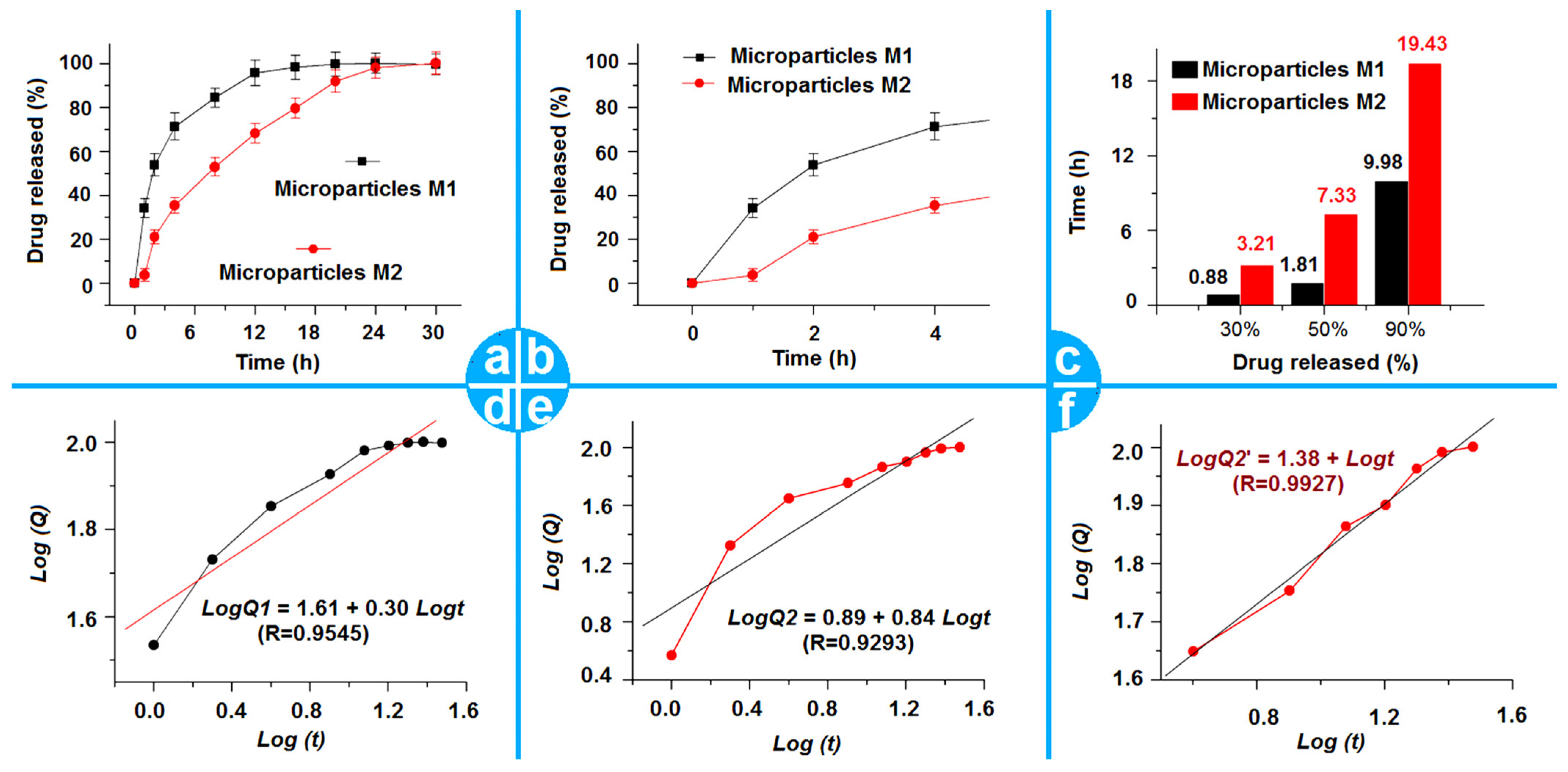
2.4. The Drug Encapsulation Ratio of the Dual-Stage Drug Controlled-Release Profile
2.5. The Proposed Drug Controlled-Release Mechanism Based on the Insoluble Gel Forming
3. Conclusions
4. Materials and Methods
4.1. Materials
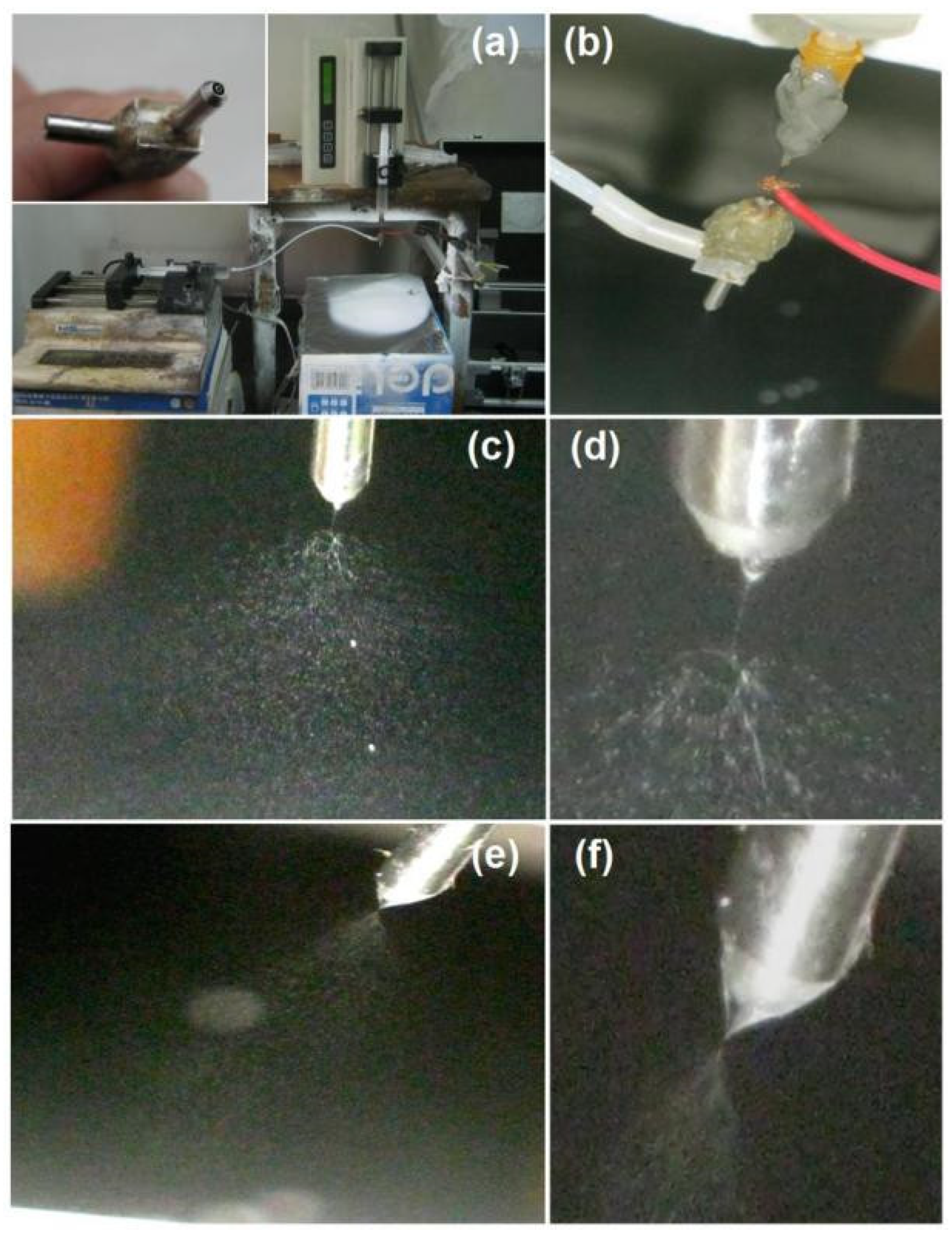
4.2. Electrospraying
4.3. Characterization
4.3.1. Morphology and Inner Structure
4.3.2. Physical State and Compatibility
4.4. Functional Performances
4.4.1. Entrapment Efficiency
4.4.2. In Vitro Dissolution Tests
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zahra, N.; Seyed, A.S.; Sepideh, H. A Novel Self-Assembled Micelles Based on Stearic Acid Modified Schizophyllan for Efficient Delivery of Paclitaxel. Colloids Surf. B Biointerfaces 2021, 199, 111524. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, V.; Patel, R. A Brief Review of the Essential Role of Nanovehicles for Improving the Therapeutic Efficacy of Pharmacological Agents Against Tumours. Curr. Drug Deliv. 2022, 19, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Raman, S. Recent Trends in Carbon Nanotubes Based Prostate Cancer Therapy: A Biomedical Hybrid for Diagnosis and Treatment. Curr. Drug Deliv. 2022, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Naik, I.; Menon, M.; Ambre, P.; Coutinho, E. A Review on Exploring the Opportunities of Polymer Drug Conjugated Systems for Targeted Cancer Treatment. Curr. Drug Deliv. 2023, 20, 8–30. [Google Scholar] [CrossRef]
- Dalia, S.S.; Mohamed, A.S.; Mohmoud, S.H. Cellular Uptake, Cytotoxicity and in-Vivo Evaluation of Tamoxifen Citrate Loaded Niosomes. Int. J. Pharm. 2015, 493, 285–294. [Google Scholar] [CrossRef]
- Fahima, M.H.; Mohamed, N.; Ahmed, K. In Vitro Cytotoxicity and Bioavailability of Solid Lipid Nanoparticles Containing Tamoxifen Citrate. Pharm. Dev. Technol. 2014, 19, 824–832. [Google Scholar] [CrossRef]
- Assi, S.; El Hajj, H.; Hayar, B.; Pisano, C.; Saad, W.; Darwiche, N. Development and Challenges of Synthetic Retinoid Formulations in Cancer. Curr. Drug Deliv. 2022, 20, 1314–1326. [Google Scholar] [CrossRef]
- Ajalli, N.; Pourmadadi, M.; Yazdian, F.; Abdouss, M.; Rashedi, H.; Rahdar, A. PVA Based Nanofiber Containing GO Modified with Cu Nanoparticles and Loaded Curcumin; High Antibacterial Activity with Acceleration Wound Healing. Curr. Drug Deliv. 2023, 20, 1569–1583. [Google Scholar] [CrossRef]
- Kolisnyk, O.; Vashchenko, O.; Ruban, O.; Fil, N.; Slipchenko, G. Assessing Compatibility of Excipients Selected for A Sustained Release Formulation of Bilberry Leaf Extract. Braz. J. Pharm. Sci. 2022, 58, e19753. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Geng, C.; Shen, H.; Zhang, Q.; Miao, Y.; Wu, J.; Ouyang, R.; Zhou, S. Two Hawks with One Arrow: A Review on Bifunctional Scaffolds for Photothermal Therapy and Bone Regeneration. Nanomaterials 2023, 13, 551. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, Q.; Li, Y.; Silva, D.Z.; Ruiz, M.E.L.; Ouyang, R.; Liu, B.; Miao, Y. Recent Development of Rhenium-Based Materials in the Application of Diagnosis and Tumor Therapy. Molecules 2023, 28, 2733. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhou, X.; Xiao, B.; Duan, L.; Zhu, Z. Mucus-Penetrating Silk Fibroin-Based Nanotherapeutics for Efficient Treatment of Ulcerative Colitis. Biomolecules 2022, 12, 1263. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Z.; Zhou, Z.; Xu, Q.; Yan, J.; Li, Q. Study on Sustained-Release Pesticides Blended with Fosthiazate-Stearic Acid/Expanded Perlite. J. Renew Mater. 2023, 11, 257–272. [Google Scholar] [CrossRef]
- Blinov, A.V.; Kachanov, M.D.; Gvozdenko, A.A.; Nagdalian, A.A.; Blinova, A.A.; Rekhman, Z.A.; Golik, A.B.; Vakalov, D.S.; Maglakelidze, D.G.; Nagapetova, A.G.; et al. Synthesis and Characterization of Zinc Oxide Nanoparticles Stabilized with Biopolymers for Application in Wound-Healing Mixed Gels. Gels 2023, 9, 57. [Google Scholar] [CrossRef]
- Zhu, L.F.; Zheng, Y.; Fan, J.; Yao, Y.; Ahmad, Z.; Chang, M.W. A Novel Core-Shell Nanofiber Drug Delivery System Intended for the Synergistic Treatment of Melanoma. Eur. J. Pharm. Sci. 2019, 137, 105002. [Google Scholar] [CrossRef]
- Bita, B.; Stancu, E.; Stroe, D.; Dumitrache, M.; Ciobanu, S.C.; Iconaru, S.L.; Predoi, D.; Groza, A. The Effects of Electron Beam Irradiation on the Morphological and Physicochemical Properties of Magnesium-Doped Hydroxyapatite/Chitosan Composite Coatings. Polymers 2022, 14, 582. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Korlos, A.; Kechagias, J.D.; Tsikritzis, D.; Mountakis, N. Medical-Grade Polyamide 12 Nanocomposite Materials for Enhanced Mechanical and Antibacterial Performance in 3D Printing Applications. Polymers 2022, 14, 440. [Google Scholar] [CrossRef]
- Du, X.-Y.; Li, Q.; Wu, G.; Chen, S. Multifunctional Micro/Nanoscale Fibers Based on Microfluidic Spinning Technology. Adv. Mater. 2019, 31, 1903733. [Google Scholar] [CrossRef]
- Windbergs, M.; Zhao, Y.; Heyman, J.; Weitz, D.A. Biodegradable Core-Shell Carriers for Simultaneous Encapsulation of Synergistic Actives. J. Am. Chem. Soc. 2013, 135, 7933–7937. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, X.; Tian, C.; Zhong, Y.; Yan, M.; Miao, C.; Wu, T.; Zhou, X. Preparation and Characterization of Porous Cellulose Acetate Nanofiber Hydrogels. Gels 2023, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Z.; Huang, X.; Wang, J.; Xiao, Y.; He, J.; Feng, J.; Xiong, S.; Li, Z. Hydrophobic Cellulose Acetate Aerogels for Thermal Insulation. Gels 2022, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Riva, L.; Lotito, A.D.; Punta, C.; Sacchetti, A. Zinc- and Copper-Loaded Nanosponges from Cellulose Nanofibers Hydrogels: New Heterogeneous Catalysts for the Synthesis of Aromatic Acetals. Gels 2022, 8, 54. [Google Scholar] [CrossRef]
- Huang, H.; Song, Y.; Zhang, Y.; Li, Y.; Li, J.; Lu, X.; Wang, C. Electrospun Nanofibers: Current Progress and Applications in Food Systems. J. Agric. Food. Chem. 2022, 70, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Bhaskar, R.; Sudhakar, K.; Nam, D.H.; Han, S.S. Polydopamine-Functionalized Bacterial Cellulose as Hydrogel Scaffolds for Skin Tissue Engineering. Gels 2023, 9, 656. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kolbuk, D.; Sajkiewicz, P. Multifluid Electrospinning for Multi-drug Delivery Systems: Pros and Cons, Challenges, and Future Directions. Biomater. Sci. 2022, 11, 37–61. [Google Scholar] [CrossRef]
- Weon, S.H.; Han, J.; Choi, Y.-K.; Park, S.; Lee, S.H. Development of Blended Biopolymer-Based Photocatalytic Hydrogel Beads for Adsorption and Photodegradation of Dyes. Gels 2023, 9, 630. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-layer Nanodepots for Sustained Release of Acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Yang, H.; Yu, D.-G.; Lu, X. The Influence of the Ultrasonic Treatment of Working Fluids on Electrospun Amorphous Solid Dispersions. Front. Mol. Biosci. 2023, 10, 1184767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dai, Y.; Fu, J.; Yan, C.; Yu, D.-G.; Yi, T. Dual-Step Controlled Release of Berberine Hydrochloride from the Trans-Scale Hybrids of Nanofibers and Microparticles. Biomolecules 2023, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.G. Engineered Spindles of Little Molecules Around Electrospun Nanofibers for Biphasic Drug Release. Adv. Fiber Mater. 2022, 4, 305–317. [Google Scholar] [CrossRef]
- Vanheusden, C.; Vanminsel, J.; Reddy, N.; Samyn, P.; D’Haen, J.; Peeters, R.; Ethirajan, A.; Buntinx, M. Fabrication of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Fibers Using Centrifugal Fiber Spinning: Structure, Properties and Application Potential. Polymers 2023, 15, 1181. [Google Scholar] [CrossRef]
- Mouro, C.; Martins, R.; Gomes, A.P.; Gouveia, I.C. Upcycling Wool Waste into Keratin Gel-Based Nanofibers Using Deep Eutectic Solvents. Gels 2023, 9, 661. [Google Scholar] [CrossRef]
- Yu, D.-G.; Xu, L. Impact Evaluations of Articles in Current Drug Delivery Based on Web of Science. Curr. Drug Deliv. 2023, 20, 1–8. [Google Scholar] [CrossRef]
- Xia, M.; Ji, S.; Fu, Y.; Dai, J.; Zhang, J.; Ma, X.; Liu, R. Alumina Ceramic Nanofibers: An Overview of the Spinning Gel Preparation, Manufacturing Process, and Application. Gels 2023, 9, 599. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Chang, Y.-C.; Tsai, M.-T.; Yang, T.-W.; Huang, M.-T.; Wu, S.-H.; Wang, C. Electrospinning of Aqueous Solutions of Atactic Poly(N-isopropylacrylamide) with Physical Gelation. Gels 2022, 8, 716. [Google Scholar] [CrossRef]
- Liu, H.; Dai, Y.; Li, J.; Liu, P.; Zhou, W.; Yu, D.-G.; Ge, R. Fast and Convenient Delivery of Fluidextracts Liquorice Through Electrospun Core-Shell Nanohybrids. Front. Bioeng. Biotechnol. 2023, 11, 1172133. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zheng, Z.-B.; Yu, D.-G.; Liu, X.-K.; Qu, Y.-L.; Li, H.-L. Electrosprayed Sperical Ethylcellulose Nanoparticles for An Improved Sustained Release Profile of Anticancer Drug. Cellulose 2017, 24, 5551–5564. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Zhang, Y.-Y.; Yu, D.-G.; Wu, D.; Li, H.-L. Fabrication of Sustained-Release Zein Nanoparticles via Modified Coaxial Electrospraying. Chem. Eng. J. 2018, 334, 807–816. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Li, Z.; Ding, B. Self-Healing Fibrous Membranes. Angew. Chem. Int. Ed. 2022, 61, e202208949. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.; Zhao, P.; Bai, Y.; Cui, W.; Shen, S.; Liu, Y.; Wang, Z.; Yu, D.-G. Insight into the Superior Piezophotocatalytic Performance of BaTiO3//ZnO Janus Nanofibrous Heterostructures in the Treatment of Multi-pollutants from Water. Appl. Catal. B 2023, 330, 122623. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Z.; Kang, S.; Yu, D.-G.; Chen, X.; Shao, J. A Sequential Electrospinning of a Coaxial and Blending Process for Creating Double-Layer Hybrid Films to Sense Glucose. Sensors 2023, 23, 3685. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Y.; Bai, Y.; Shi, H.; Zhou, W.; Chen, Y.; Liu, Y.; Yu, D.-G. Recent Combinations of Electrospinning with Photocatalytic Technology for Treating Polluted Water. Catalysts 2023, 13, 758. [Google Scholar] [CrossRef]
- Du, Y.; Yu, D.-G.; Yi, T. Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors 2023, 11, 208. [Google Scholar] [CrossRef]
- Nguyen, D.-N.; Moon, W. Significant Electromechanical Characteristic Enhancement of Coaxial Electrospinning Core–Shell Fibers. Polymers 2022, 14, 1739. [Google Scholar] [CrossRef]
- Kan, Y.; Bondareva, J.V.; Statnik, E.S.; Cvjetinovic, J.; Lipovskikh, S.; Abdurashitov, A.S.; Kirsanova, M.A.; Sukhorukhov, G.B.; Evlashin, S.A.; Salimon, A.I.; et al. Effect of Graphene Oxide and Nanosilica Modifications on Electrospun Core-Shell PVA–PEG–SiO2@PVA–GO Fiber Mats. Nanomaterials 2022, 12, 998. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-decorated BixBiyOz/TiO2 Flexible Carbon Nanofibers and Their Applications on Degradating of Organic Pollutants under Solar Radiation. J. Mater. Sci. Technol. 2023, 150, 114–123. [Google Scholar] [CrossRef]
- Pankaj, K.K.; Nivedita, G.; Karthikeyan, K. Tamoxifen Citrate Loaded Chitosan-Gellan Nanocapsules for Breast Cancer Therapy: Development, Characterisan and in-Vitro Cell Viability Study. J. Microencapsul. 2018, 35, 292–300. [Google Scholar] [CrossRef]
- Hiremath, J.G.; Rudani, C.G.; Domb, A.J.; Suthar, R.V.; Khamar, N.S. Preparation and in Vitro Characterization of Poly(Sebacic Acid-co-Ricinoleic Acid)-Based Tamoxifen Citrate-Loaded Microparticles for Breast Cancer. J. Appl. Polym. Sci. 2011, 124, 4747–4754. [Google Scholar] [CrossRef]
- Qian, C.; Liu, Y.; Chen, S.; Zhang, C.; Chen, X.; Liu, Y.; Liu, P. Electrospun Core–Sheath PCL Nanofibers Loaded with nHA and Simvastatin and Their Potential Bone Regeneration Applications. Front. Bioeng. Biotechnol. 2023, 11, 1205252. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Ma, X.; Li, J.; Cheng, Y.; Cao, Y.; Wang, Z.; Shi, X.; Du, Y.; Deng, H.; Li, Z. Incorporating Platelet-Rich Plasma into Coaxial Electrospun Nanofibers for Bone Tissue Engineering. Int. J. Pharm. 2018, 547, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Brimo, N.; Serdaroglu, D.C.; Uysal, B. Comparing Antibiotic Pastes with Electrospun Nanofibers as Modern Drug Delivery Systems for Regenerative Endodontics. Curr. Drug Deliv. 2022, 19, 904–917. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Liu, P.; Zhang, M.; Wang, C.; Zhang, B. Multifunctional Chitosan/Polycaprolactone Nanofiber Scaffolds with Varied Dual-Drug Release for Wound-Healing Applications. ACS Biomater. Sci. Eng. 2020, 6, 4666–4676. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Du, Y.; Chen, J.; Song, W.; Zhou, T. A Correlation Analysis between Undergraduate Students’ Safety Behaviors in the Laboratory and Their Learning Efficiencies. Behav. Sci. 2023, 13, 127. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, R.G. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Huang, Y.-C.; Kuo, C.-Y.; Chuang, C.-C.; Chen, C.-C.; Chen, N.-Y.; Yip, P.K.; Chen, J.-P. Co-Delivery of Docosahexaenoic Acid and Brain-Derived Neurotropic Factor from Electrospun Aligned Core–Shell Fibrous Membranes in Treatment of Spinal Cord Injury. Pharmaceutics 2022, 14, 321. [Google Scholar] [CrossRef]
- Huang, J.; Feng, C. Aniline Dimers Serving as Stable and Efficient Transfer Units for Intermolecular Charge-Carrier Transmission. iScience 2023, 26, 105762. [Google Scholar] [CrossRef]
- Wu, W.; Li, T. Deepening the Understanding of the in Vivo and Cellular Fate of Nanocarriers. Adv. Drug Deliv. Rev. 2022, 189, 114529. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Chunta, S.; Wu, W.; Chen, Z.; Lu, Y. Enhanced Oral Bioavailability from Food Protein Nanoparticles: A Mini Review. J. Control. Release 2023, 354, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Yang, Y.; He, H.; Qi, J.; Wu, W.; Lu, Y. Establishment of In Vitro Dissolution Based on Similarity with In Vivo Dissolution: A Case Study on Aripiprazole. Mol. Pharm. 2023, 20, 2579–2588. [Google Scholar] [CrossRef]
- Cai, Y.; Qi, J.; Lu, Y.; He, H.; Wu, W. The in Vivo Fate of Polymeric Micelles. Adv. Drug Deliv. Rev. 2022, 188, 114463. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.K.; Kuppusamy, U.R.; Chua, K.H.; Arumugam, B. Emerging Strategies to Improve the Stability and Bioavailability of Insulin: An Update on Formulations and Delivery Approaches. Curr. Drug Deliv. 2022, 20, 1141–1162. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N. Analysis of Fickian and Non-Fickian Drug Release from Polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Bobokalonov, J.; Muhidinov, Z.; Nasriddinov, A.; Jomnurodov, A.; Khojaeva, F.; Komilova, G.; Yusufi, S.; Liu, L. Evaluation of Extended-Release of Piroxicam-Loaded Pectin-Zein Hydrogel Microspheres: In Vitro, Ex Vivo, and In Vivo Studies. Curr. Drug Deliv. 2022, 19, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Kim, E.J.; Tang, Y.; Xu, H.; Yu, D.-G.; Song, W.; Kim, B.J. Rational Design of Hyper-Crosslinked Polymers for Biomedical Applications. J. Polym. Sci. 2023. [Google Scholar] [CrossRef]
- Fan, W.; Peng, H.; Yu, Z.; Wang, L.; He, H.; Ma, Y.; Qi, J.; Lu, Y.; Wu, W. The Long-Circulating Effect of Pegylated Nanoparticles Revisited via Simultaneous Monitoring of Both the Drug Payloads and Nanocarriers. Acta Pharm. Sin. B 2022, 12, 2479–2493. [Google Scholar] [CrossRef]
- Yu, D.G.; Zhou, J. How Can Electrospinning Further Service Well for Pharmaceutical Researches? J. Pharm. Sci. 2023. [CrossRef]
- Guler, E.; Nur Hazar-Yavuz, A.; Tatar, E.; Morid Haidari, M.; Sinemcan Ozcan, G.; Duruksu, G.; Graça, M.P.F.; Kalaskar, D.M.; Gunduz, O.; Emin Cam, M. Oral Empagliflozin-Loaded Tri-layer Core-Sheath Fibers Fabricated Using Tri-axial Electrospinning: Enhanced in Vitro and in Vivo Antidiabetic Performance. Int. J. Pharm. 2023, 635, 122716. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, P.; Yu, D.-G.; Zhu, Y. Biphasic Drug Release from Electrospun Structures. Exp. Opin. Drug Deliv. 2023, 20, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Ilić-Stojanović, S.; Nikolić, L.; Cakić, S. A Review of Patents and Innovative Biopolymer-Based Hydrogels. Gels 2023, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, W.; Li, F. Polymeric DNA Hydrogels and Their Applications in Drug Delivery for Cancer Therapy. Gels 2023, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Gong, W.; Wang, B.; Yu, D.-G.; Zhu, Y. Integrating Chinese Herbs and Western Medicine for New Wound Dressings through Handheld Electrospinning. Biomedicines 2023, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.G.; Guo, Z. Electrospun structural nanohybrids combining three composites for fast helicide delivery. Adv. Compos.Hybrid Mater. 2022, 5, 1017–1029. [Google Scholar] [CrossRef]
- Yu, D.G.; Huang, C. Electrospun Biomolecule-Based Drug Delivery Systems. Biomolecules 2023, 13, 1152. [Google Scholar] [CrossRef]
- Pooja, C.; Bright, B.; Siew, H.N.; Aaron, W.; Lynn, P.W.; Heather, L.W. Solidified Saturated Fats Coating Subunit Vaccines Greatly Extended Vaccine Booster Release and Contributed to a Th1/Th2 Mixed Immune Response in Mice. Vaccine 2023, 41, 3989–4001. [Google Scholar] [CrossRef]
- Agrawal, A.; Joshi, A.; Bhattacharya, S. Recent Excavation of Nanoethosomes in Current Drug Delivery. Curr. Drug Deliv. 2022, 21, 168–183. [Google Scholar] [CrossRef]
- Sare, F.; Mohammad, R.A.; Seyyedeh, E.M.; Seyyed, M.R.S.; Mandana, K.; Shirzad, A.; Solmaz, G. Diazepam Loaded Solid Lipid Nanoparticles: In Vitro and In Vivo Evaluations. Adv. Pharm. Bull. 2022, 12, 86–92. [Google Scholar] [CrossRef]
- Alam, M.; Rizwanullah, M.; Mir, S.R.; Amin, S. Statistically Optimized Tacrolimus and Thymoquinone Co-Loaded Nanostructured Lipid Carriers Gel for Improved Topical Treatment of Psoriasis. Gels 2023, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Rincón, M.; Silva-Abreu, M.; Sosa, L.; Pesantez-Narvaez, J.; Calpena, A.C.; Rodríguez-Lagunas, M.J.; Mallandrich, M. Semi-Solid Dosage Forms Containing Pranoprofen-Loaded NLC as Topical Therapy for Local Inflammation: In Vitro, Ex Vivo and In Vivo Evaluation. Gels 2023, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Tang, Y.; Qian, C.; Kim, B.J.; Liao, Y.; Yu, D.-G. Electrospinning Spinneret: A Bridge between the Visible World and the Invisible Nanostructures. Innovation 2023, 4, 100381. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, E.K.; Schindler, M.; Lukas, D. AC Electrospinning: Impact of High Voltage and Solvent on the Electrospinnability and Productivity of Polycaprolactone Electrospun Nanofibrous Scaffolds. Mater. Today Chem. 2022, 26, 101025. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, M.; Song, N.; Wang, C.; Lu, X. General Synthesis of Pt and Ni Co-Doped Porous Carbon Nanofibers to Boost HER Performance in Both Acidic and Alkaline Solutions. Chin. Chem. Lett. 2023, 34, 107359. [Google Scholar] [CrossRef]
- Eslami, H.; Gharibi, A.; Muller-Plathe, F. Mechanisms of Nucleation and Solid−Solid-Phase Transitions in Triblock Janus Assemblies. J. Chem. Theory Comput. 2021, 17, 1742–1754. [Google Scholar] [CrossRef]
- Bahri, K.; Eslami, H.; Muller-Plathe, F. Self-Assembly of Model Triblock Janus Colloidal Particles in Two Dimensions. J. Chem. Theory Comput. 2022, 18, 1870–1882. [Google Scholar] [CrossRef]
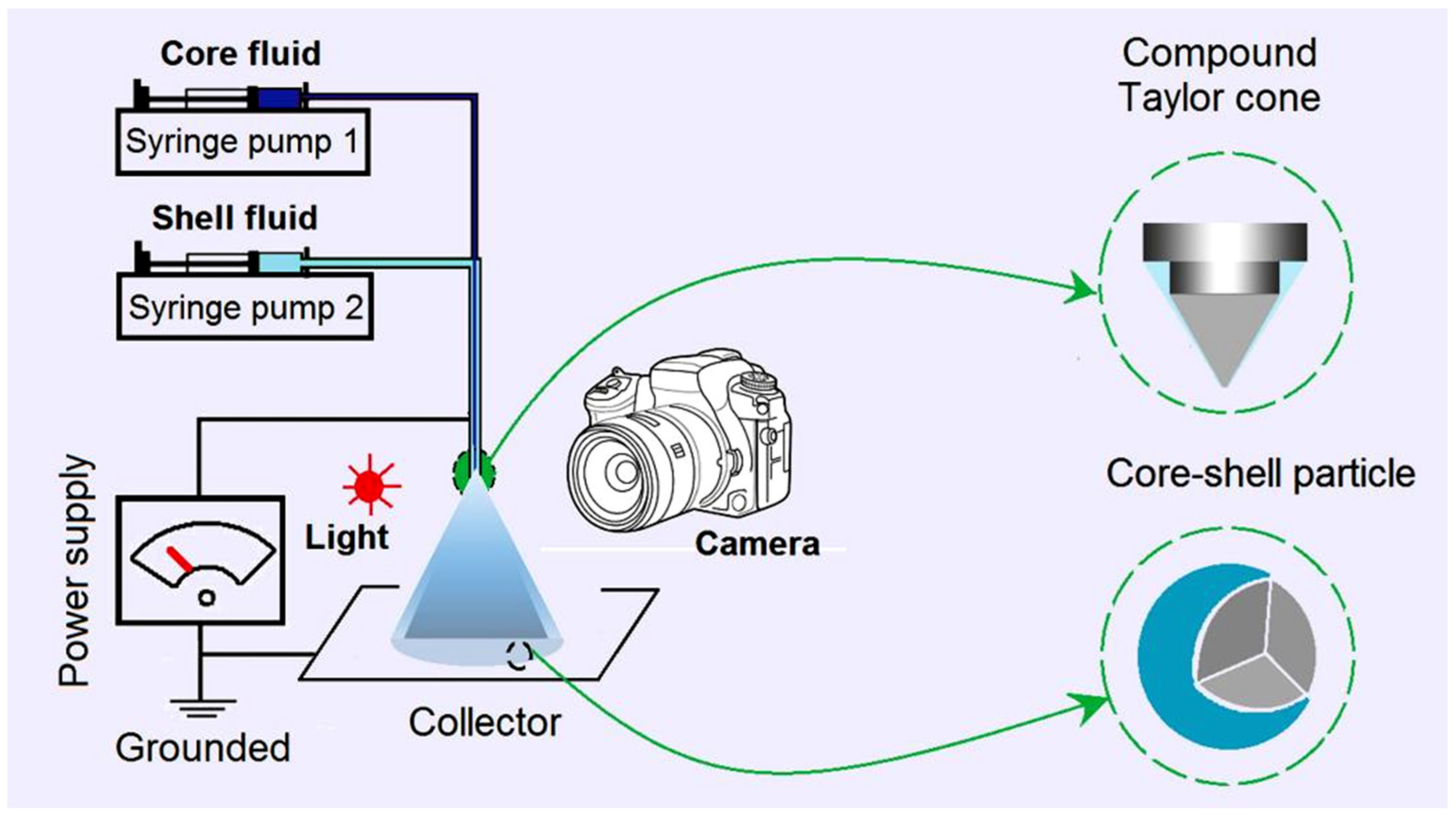

| No. | Electrospraying | Applied Voltage (kV) | Fluid Flow Rate (mL/h) | Products | |
|---|---|---|---|---|---|
| Core a | Shell b | ||||
| M1 | Single fluid | 21 | -- | 1.0 | Monolithic microparticles |
| M2 | Coaxial | 18 | 0.4 | 1.0 | Core–shell microparticles |
| M3 | Single fluid | 12 | 0.4 | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Zhao, H.; Liu, H.; Zhao, P.; Yu, D.-G. Electrosprayed Stearic-Acid-Coated Ethylcellulose Microparticles for an Improved Sustained Release of Anticancer Drug. Gels 2023, 9, 700. https://doi.org/10.3390/gels9090700
Ji Y, Zhao H, Liu H, Zhao P, Yu D-G. Electrosprayed Stearic-Acid-Coated Ethylcellulose Microparticles for an Improved Sustained Release of Anticancer Drug. Gels. 2023; 9(9):700. https://doi.org/10.3390/gels9090700
Chicago/Turabian StyleJi, Yuexin, Hua Zhao, Hui Liu, Ping Zhao, and Deng-Guang Yu. 2023. "Electrosprayed Stearic-Acid-Coated Ethylcellulose Microparticles for an Improved Sustained Release of Anticancer Drug" Gels 9, no. 9: 700. https://doi.org/10.3390/gels9090700