Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications
Abstract
:1. Introduction
2. Pectin Extraction and Characterization
2.1. Extraction of Pectin from Various Sources
2.2. Pectin Structure and Characterization
3. Gelling Mechanism of Pectin
3.1. High-Ester Pectin
3.2. Low-Ester Pectin
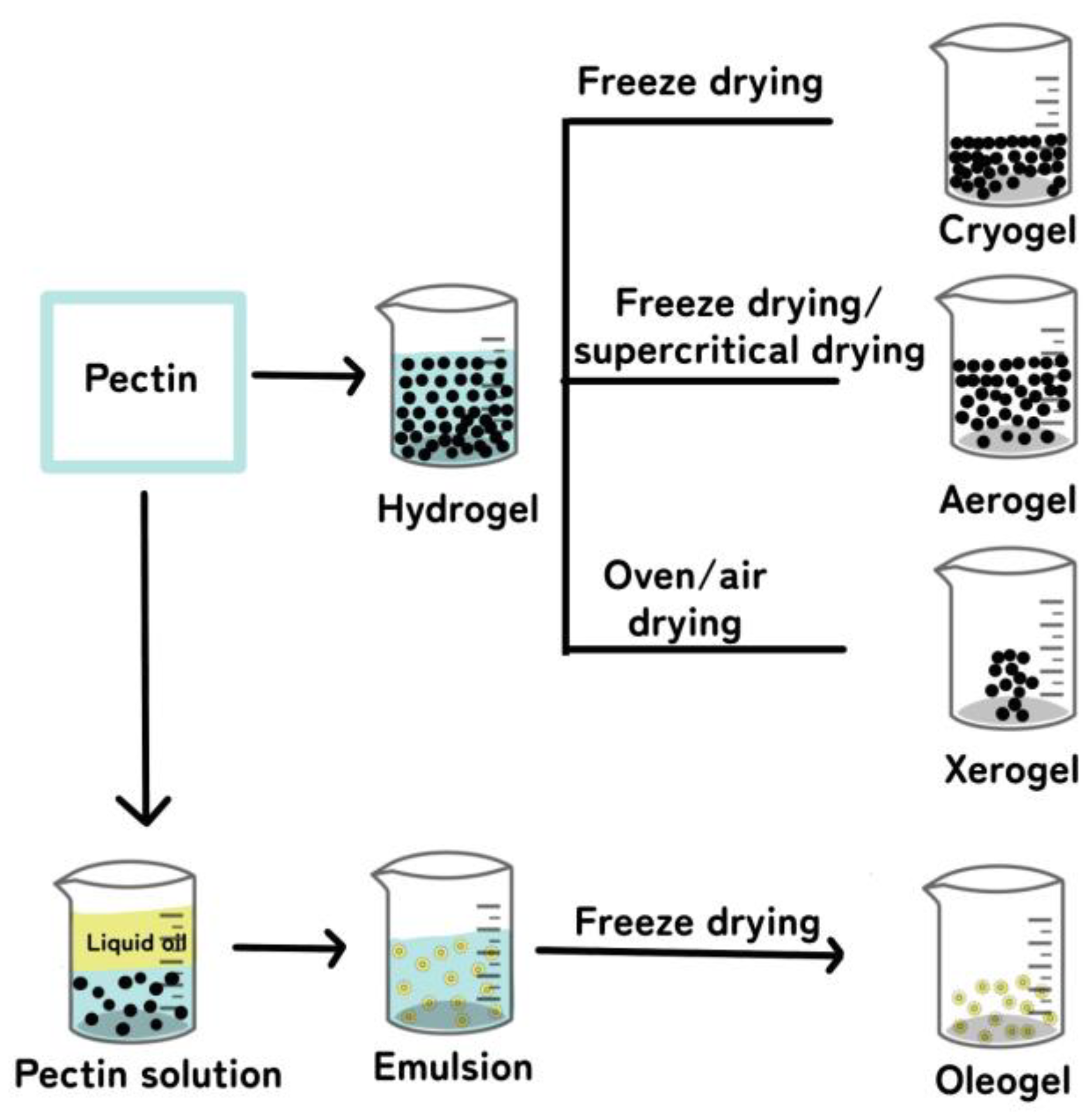
4. Types of Pectin Gels
4.1. Hydrogels
Hydrogel Preparation
4.2. Cryogels
4.3. Aerogels
4.4. Xerogels
4.5. Oleogels
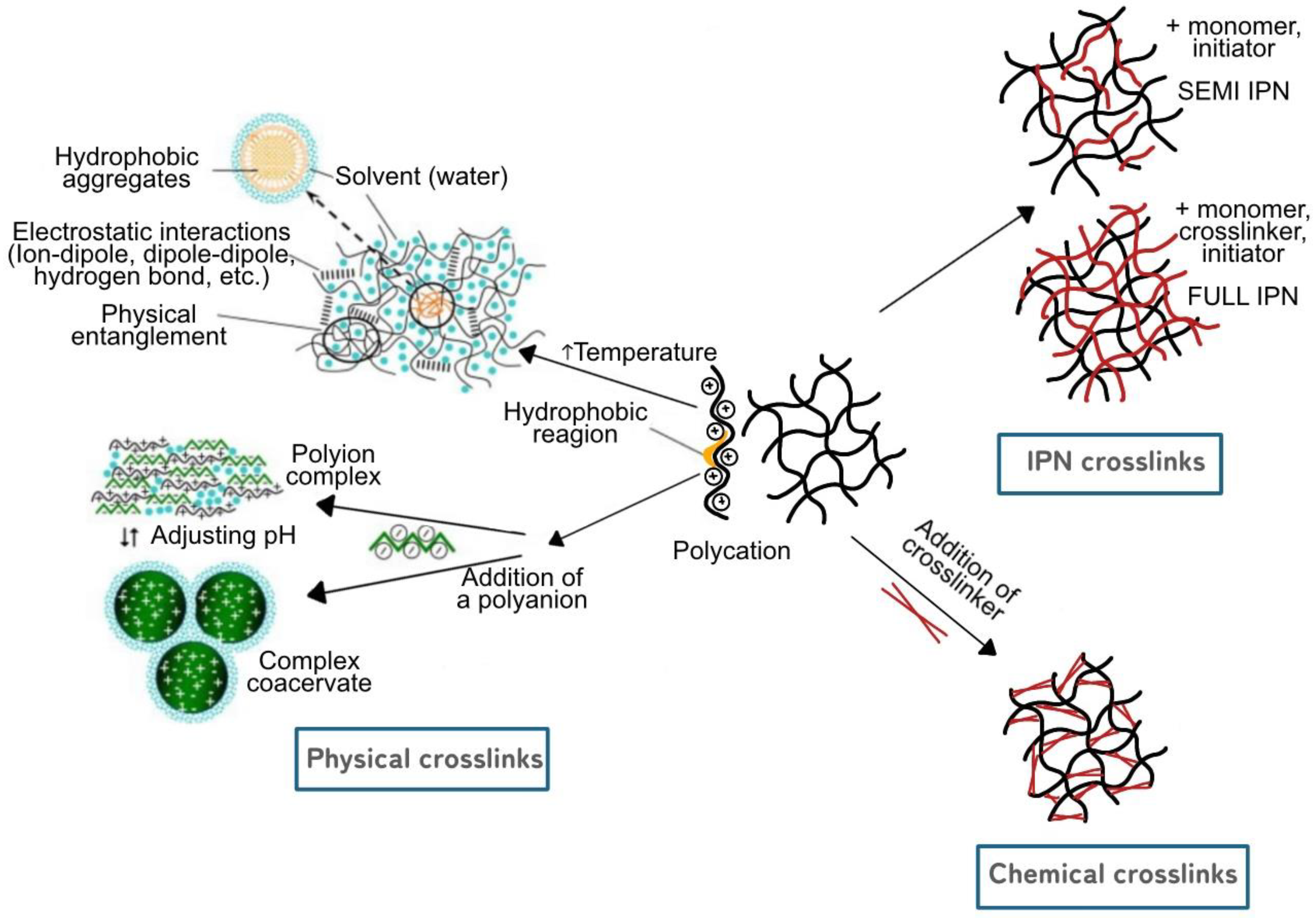
5. Crosslinking in Hydrogel
5.1. Physical Crosslink Hydrogels
5.2. Chemical Crosslink Hydrogels
5.3. IPN (Interpreting Polymer Network) Crosslink Hydrogels
6. Potential Application of Pectin Hydrogel in Food Industry
6.1. Carrier for Active Compound
6.2. Fat Replacement and Emulsifiers
6.3. Three-Dimensionally-Printed Food
6.4. Food Packaging
6.4.1. Film-Based Applications
6.4.2. Coating Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho, A.J. Starch: Major sources, properties and applications as thermoplastic materials. Monomers Polym. Compos. Renew. Resour. 2008, 321–342. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Gholizadeh Vazvani, M.; Hassanisaadi, M.; Skorik, Y.A. Micro-/nano-carboxymethyl cellulose as a promising biopolymer with prospects in the agriculture sector: A review. Polymers 2023, 15, 440. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kumar, P.; Alavi, S.; Sandeep, K. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Wang, X.; Fang, J.; Cheng, L.; Gu, Z.; Hong, Y. Interaction of starch and non-starch polysaccharides in raw potato flour and their effects on thickening stability. Int. J. Biol. Macromol. 2023, 242, 124702. [Google Scholar] [CrossRef] [PubMed]
- Fangwei, L.; Junyi, Y.; Shaoping, N. Classification, properties and development trend of polysaccharides thickeners. China Food Addit. 2023, 34, 37–45. [Google Scholar]
- Chen, Z.; Aziz, T.; Sun, H.; Ullah, A.; Ali, A.; Cheng, L.; Ullah, R.; Khan, F.U. Advances and applications of cellulose bio-composites in biodegradable materials. J. Polym. Environ. 2023, 31, 2273–2284. [Google Scholar] [CrossRef]
- Casalini, S.; Giacinti Baschetti, M. The use of essential oils in chitosan or cellulose-based materials for the production of active food packaging solutions: A review. J. Sci. Food Agric. 2023, 103, 1021–1041. [Google Scholar] [CrossRef]
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Application of cellulose-and chitosan-based edible coatings for quality and safety of deep-fried foods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1418–1437. [Google Scholar] [CrossRef]
- Markov, P.A.; Krachkovsky, N.S.; Durnev, E.A.; Martinson, E.A.; Litvinets, S.G.; Popov, S.V. Mechanical properties, structure, bioadhesion, and biocompatibility of pectin hydrogels. J. Biomed. Mater. Res. Part A 2017, 105, 2572–2581. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Dhyani, A.; Juyal, D. Hydrogel: Preparation, characterization and applications. Pharma Innov. 2017, 6, 25. [Google Scholar]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Intech Open: London, UK, 2011; Volume 117150. [Google Scholar]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.; Mujtaba, M.; Alghamdi, N.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels 2022, 9, 1. [Google Scholar]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Harholt, J.; Suttangkakul, A.; Vibe Scheller, H. Biosynthesis of pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, S.-J. Pectin modifications: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, characterization, and applications of pectins from plant by-products. Appl. Sci. 2021, 11, 6596. [Google Scholar]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar]
- Humerez-Flores, J.N.; Verkempinck, S.H.; De Bie, M.; Kyomugasho, C.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Understanding the impact of diverse structural properties of homogalacturonan rich citrus pectin-derived compounds on their emulsifying and emulsion stabilizing potential. Food Hydrocoll. 2022, 125, 107343. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Drusch, S.; Acevedo, F.; Castro-Alvarez, A.; Benie, A.; Poncelet, D.; Dragosavac, M.M.; Tesoriero, M.V.D.; Löwenstein, P.; Yonaha, V. Structure, controlled release mechanisms and health benefits of pectins as an encapsulation material for bioactive food components. Food Funct. 2022, 13, 10870–10881. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, M.; Søndergaard, K.M.; Wichmann, J.; Vidal-Melgosa, S.; Willats, W.G.; Meyer, A.S.; Mikkelsen, J.D. Application of enzymes for efficient extraction, modification, and development of functional properties of lime pectin. Food Hydrocoll. 2014, 40, 273–282. [Google Scholar]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Grabacka, M. Multicatalytic enzyme preparations as effective alternative to acid in pectin extraction. Food Hydrocoll. 2015, 44, 156–161. [Google Scholar] [CrossRef]
- Kurita, O.; Fujiwara, T.; Yamazaki, E. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydr. Polym. 2008, 74, 725–730. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Green extraction of pectin from Citrus limetta peels using organic acid and its characterization. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Ultrasound-assisted extraction of pectin from Citrus limetta peels: Optimization, characterization, and its comparison with commercial pectin. Food Biosci. 2023, 51, 102231. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Preparation and characterization of pectin fraction from pineapple peel as a natural plasticizer and material for biopolymer film. Food Bioprod. Process. 2019, 118, 198–206. [Google Scholar] [CrossRef]
- Cho, E.-H.; Jung, H.-T.; Lee, B.-H.; Kim, H.-S.; Rhee, J.-K.; Yoo, S.-H. Green process development for apple-peel pectin production by organic acid extraction. Carbohydr. Polym. 2019, 204, 97–103. [Google Scholar] [PubMed]
- Xie, J.; Zhang, Y.; Klomklao, S.; Simpson, B.K. Pectin from plantain peels: Green recovery for transformation into reinforced packaging films. Waste Manag. 2023, 161, 225–233. [Google Scholar] [CrossRef]
- Ptichkina, N.; Markina, O.; Rumyantseva, G. Pectin extraction from pumpkin with the aid of microbial enzymes. Food Hydrocoll. 2008, 22, 192–195. [Google Scholar] [CrossRef]
- Cui, S.W.; Chang, Y.H. Emulsifying and structural properties of pectin enzymatically extracted from pumpkin. LWT-Food Sci. Technol. 2014, 58, 396–403. [Google Scholar] [CrossRef]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef]
- Ribeiro, A.C.B.; Cunha, A.P.; da Silva, L.M.R.; Mattos, A.L.A.; de Brito, E.S.; de Souza, M.d.S.M.; de Azeredo, H.M.C.; Ricardo, N.M.P.S. From mango by-product to food packaging: Pectin-phenolic antioxidant films from mango peels. Int. J. Biol. Macromol. 2021, 193, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.N.; Shang, J.J.; He, L.B.; Dan, J.M. Comparisons of microwave-assisted and conventional heating extraction of pectin from seed watermelon peel. Adv. Mater. Res. 2012, 550, 1801–1806. [Google Scholar] [CrossRef]
- Petkowicz, C.; Vriesmann, L.; Williams, P. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydr. Polym. 2014, 101, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Hartati, I.; Subekti, E. Microwave assisted extraction of watermelon rind pectin. Int. J. ChemTech Res. 2015, 8, 163–170. [Google Scholar]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Process optimization and analysis of microwave assisted extraction of pectin from dragon fruit peel. Carbohydr. Polym. 2014, 112, 622–626. [Google Scholar] [CrossRef]
- Mollea, C.; Chiampo, F.; Conti, R. Extraction and characterization of pectins from cocoa husks: A preliminary study. Food Chem. 2008, 107, 1353–1356. [Google Scholar] [CrossRef]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Vasco-Correa, J.; Paz Astudillo, I.C. Enzymatic extraction and characterization of pectin from cocoa pod husks (Theobroma cacao L.) using Celluclast® 1.5 L. Molecules 2021, 26, 1473. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chem. 2001, 73, 393–396. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Ruiz-Torralba, A.; Méndez-Albiñana, P.; Guerra-Hernández, E.; García-Villanova, B.; Moreno, R.; Villamiel, M.; Montilla, A. Berry fruits as source of pectin: Conventional and non-conventional extraction techniques. Int. J. Biol. Macromol. 2021, 186, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High hydrostatic pressure increased stability and activity of immobilized lipase in hexane. Enzym. Microb. Technol. 2009, 45, 118–125. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The influence of extraction conditions on the yield and physico-chemical parameters of pectin from grape pomace. Polymers 2022, 14, 1378. [Google Scholar] [CrossRef]
- Ropartz, D.; Ralet, M.-C. Pectin structure. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer: Cham, Switzerland, 2020; Volume 1, pp. 17–36. [Google Scholar]
- Li, D.-Q.; Li, J.; Dong, H.-L.; Li, X.; Zhang, J.-Q.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef]
- Niu, H.; Chen, X.; Luo, T.; Chen, H.; Fu, X. Relationships between the behavior of three different sources of pectin at the oil-water interface and the stability of the emulsion. Food Hydrocoll. 2022, 128, 107566. [Google Scholar] [CrossRef]
- Shahin, L.; Zhang, L.; Mohnen, D.; Urbanowicz, B.R. Insights into Pectin O-acetylation in plant cell wall: Structure, synthesis, and modification. Cell Surf. 2023, 9, 100099. [Google Scholar] [CrossRef]
- Celus, M.; Kyomugasho, C.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Influence of pectin structural properties on interactions with divalent cations and its associated functionalities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1576–1594. [Google Scholar] [CrossRef]
- Flutto, L. Pectin: Properties and determination. In Encyclopedia of food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: San Diego, CA, USA, 2003; pp. 4440–4449. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Cacao pod husks as a source of low-methoxyl, highly acetylated pectins able to gel in acidic media. Int. J. Biol. Macromol. 2017, 101, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Filippov, M.; Kohn, R. Determination of the esterification degree of carboxyl groups of pectin with methanol by means of infrared spectroscopy. Chem. Zvesti 1975, 29, 88–91. [Google Scholar]
- Bociek, S.M.; Welti, D. The quantitative analysis of uronic acid polymers by infrared spectroscopy. Carbohydr. Res. 1975, 42, 217–226. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Liang, D.; Hou, Y.; Sun, L.; Guo, Y. Low methoxyl pectin gelation under alkaline conditions and its rheological properties: Using NaOH as a pH regulator. Food Hydrocoll. 2018, 79, 560–571. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Zhang, H.; Wu, D.; Ye, X.; Linardt, R.J.; Chen, S. Gelling mechanism of RG-I enriched citrus pectin: Role of arabinose side-chains in cation-and acid-induced gelation. Food Hydrocoll. 2020, 101, 105536. [Google Scholar] [CrossRef]
- Sousa, A.G.; Nielsen, H.L.; Armagan, I.; Larsen, J.; Sørensen, S.O. The impact of rhamnogalacturonan-I side chain monosaccharides on the rheological properties of citrus pectin. Food Hydrocoll. 2015, 47, 130–139. [Google Scholar]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Trends in “green” and novel methods of pectin modification-A review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar]
- Zhang, L.; Zheng, J.; Wang, Y.; Ye, X.; Chen, S.; Pan, H.; Chen, J. Fabrication of rhamnogalacturonan-I enriched pectin-based emulsion gels for protection and sustained release of curcumin. Food Hydrocoll. 2022, 128, 107592. [Google Scholar]
- Bu, K.; Wu, S.; Zhu, C.; Wei, M. Comparative study of HG-type low-ester hawthorn pectin as a promising material for the preparation of hydrogel. Carbohydr. Polym. 2022, 296, 119941. [Google Scholar] [PubMed]
- Ochoa-Villarreal, M.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Martínez-Téllez, M.Á. Plant cell wall polymers: Function, structure and biological activity of their derivatives. Polymerization 2012, 4, 63–86. [Google Scholar]
- Schols, H.A.; Voragen, A. The chemical structure of pectins. In Pectins and Their Manipulation; Blackwell: Oxford, UK, 2003; pp. 1–29. [Google Scholar]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Oakenfull, D.; Scott, A. Hydrophobic interaction in the gelation of high methoxyl pectins. J. Food Sci. 1984, 49, 1093–1098. [Google Scholar] [CrossRef]
- Löfgren, C.; Hermansson, A.-M. Synergistic rheological behaviour of mixed HM/LM pectin gels. Food Hydrocoll. 2007, 21, 480–486. [Google Scholar] [CrossRef]
- Kastner, H.; Einhorn-Stoll, U.; Drusch, S. Structure formation in sugar containing pectin gels-Influence of gel composition and cooling rate on the gelation of non-amidated and amidated low-methoxylated pectin. Food Hydrocoll. 2017, 73, 13–20. [Google Scholar] [CrossRef]
- BeMiller, J.N. An introduction to pectins: Structure and properties. In Chemistry and Function of Pectins; Fishman, M.L., Jen, J.J., Eds.; American Chemical Society: Washington, DC, USA, 1986; pp. 2–21. [Google Scholar]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Williamson, G.; Faulds, C.B.; Matthew, J.A.; Archer, D.B.; Morris, V.J.; Brownsey, G.J.; Ridout, M.J. Gelation of sugarbeet and citrus pectins using enzymes extracted from orange peel. Carbohydr. Polym. 1990, 13, 387–397. [Google Scholar] [CrossRef]
- Tibbits, C.W.; MacDougall, A.J.; Ring, S.G. Calcium binding and swelling behaviour of a high methoxyl pectin gel. Carbohydr. Res. 1998, 310, 101–107. [Google Scholar] [CrossRef]
- Genovese, D.B.; Ye, A.; Singh, H. High methoxyl pectin/apple particles composite gels: Effect of particle size and particle concentration on mechanical properties and gel structure. J. Texture Stud. 2010, 41, 171–189. [Google Scholar] [CrossRef]
- Liners, F.; Thibault, J.-F.; Van Cutsem, P. Influence of the degree of polymerization of oligogalacturonates and of esterification pattern of pectin on their recognition by monoclonal antibodies. Plant Physiol. 1992, 99, 1099–1104. [Google Scholar] [CrossRef]
- Luzio, G.A.; Cameron, R.G. Demethylation of a model homogalacturonan with the salt-independent pectin methylesterase from citrus: Part II. Structure–function analysis. Carbohydr. Polym. 2008, 71, 300–309. [Google Scholar] [CrossRef]
- Powell, D.; Morris, E.; Gidley, M.; Rees, D. Conformations and interactions of pectins: II. Influence of residue sequence on chain association in calcium pectate gels. J. Mol. Biol. 1982, 155, 517–531. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Fraeye, I.; Colle, I.; Vandevenne, E.; Duvetter, T.; Van Buggenhout, S.; Moldenaers, P.; Van Loey, A.; Hendrickx, M. Influence of pectin structure on texture of pectin–calcium gels. Innov. Food Sci. Emerg. Technol. 2010, 11, 401–409. [Google Scholar] [CrossRef]
- Ngouémazong, D.E.; Tengweh, F.F.; Fraeye, I.; Duvetter, T.; Cardinaels, R.; Van Loey, A.; Moldenaers, P.; Hendrickx, M. Effect of de-methylesterification on network development and nature of Ca2+-pectin gels: Towards understanding structure–function relations of pectin. Food Hydrocoll. 2012, 26, 89–98. [Google Scholar] [CrossRef]
- Sharma, B.; Naresh, L.; Dhuldhoya, N.; Merchant, S.; Merchant, U. An overview on pectins. Times Food Process. J. 2006, 23, 44–51. [Google Scholar]
- Da Silva, J.; Rao, M. 11 pectins: Structure, functionality, and uses. In Food Polysaccharides and Their Applications; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Begum, R.; Aziz, M.G.; Yusof, Y.A.; Saifullah, M.; Uddin, M.B. Evaluation of gelation properties of jackfruit (Artocarpus heterophyllus) waste pectin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100160. [Google Scholar] [CrossRef]
- Lootens, D.; Capel, F.; Durand, D.; Nicolai, T.; Boulenguer, P.; Langendorff, V. Influence of pH, Ca concentration, temperature and amidation on the gelation of low methoxyl pectin. Food Hydrocoll. 2003, 17, 237–244. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Coimbra, M.A.; Da Silva, J.L. Temperature dependence of the formation and melting of pectin–Ca2+ networks: A rheological study. Food Hydrocoll. 2003, 17, 801–807. [Google Scholar] [CrossRef]
- Gilsenan, P.; Richardson, R.; Morris, E. Thermally reversible acid-induced gelation of low-methoxy pectin. Carbohydr. Polym. 2000, 41, 339–349. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Mohite, P.B.; Adhav, S. A hydrogels: Methods of preparation and applications. Int. J. Adv. Pharm 2017, 6, 79–85. [Google Scholar]
- Mishra, R.; Majeed, A.; Banthia, A. Development and characterization of pectin/gelatin hydrogel membranes for wound dressing. Int. J. Plast. Technol. 2011, 15, 82–95. [Google Scholar] [CrossRef]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, D.; Alexander, A.; Ajazuddin; Badwaik, H.; Tripathy, M.; Tripathi, D.K. Biodegradable IPN hydrogel beads of pectin and grafted alginate for controlled delivery of diclofenac sodium. J. Mater. Sci. Mater. Med. 2013, 24, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Sutar, P.B.; Mishra, R.K.; Pal, K.; Banthia, A.K. Development of pH sensitive polyacrylamide grafted pectin hydrogel for controlled drug delivery system. J. Mater. Sci. Mater. Med. 2008, 19, 2247–2253. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Dai, S.; Wang, S.; Yan, H.; Xu, J.; Hu, H.; Ding, J.; Yuan, N. Stretchable and self-healable hydrogel-based capacitance pressure and strain sensor for electronic skin systems. Mater. Res. Express 2019, 6, 0850b0859. [Google Scholar] [CrossRef]
- Xu, L.; Cui, L.; Jia, M.; Li, Y.; Gao, J.; Jin, X. Self-assembly of flexible graphene hydrogel electrode based on crosslinked pectin-cations. Carbohydr. Polym. 2018, 195, 593–600. [Google Scholar] [CrossRef]
- Torpol, K.; Sriwattana, S.; Sangsuwan, J.; Wiriyacharee, P.; Prinyawiwatkul, W. Optimising chitosan–pectin hydrogel beads containing combined garlic and holy basil essential oils and their application as antimicrobial inhibitor. Int. J. Food Sci. Technol. 2019, 54, 2064–2074. [Google Scholar] [CrossRef]
- Song, K.; Hao, Y.; Liu, Y.; Cao, R.; Zhang, X.; He, S.; Wen, J.; Zheng, W.; Wang, L.; Zhang, Y. Preparation of pectin-chitosan hydrogels based on bioadhesive-design micelle to prompt bacterial infection wound healing. Carbohydr. Polym. 2023, 300, 120272. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Smirnov, V.; Khramova, D.; Paderin, N.; Chistiakova, E.; Ptashkin, D.; Vityazev, F. Effect of Hogweed Pectin on Rheological, Mechanical, and Sensory Properties of Apple Pectin Hydrogel. Gels 2023, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yi, J.; Ma, Y.; Bi, J. The role of amide groups in the mechanism of acid-induced pectin gelation: A potential pH-sensitive hydrogel based on hydrogen bond interactions. Food Hydrocoll. 2023, 141, 108741. [Google Scholar] [CrossRef]
- Elma, M.; Saraswati, N.K.D.A.; Simatupang, P.F.A.; Febriyanti, R.; Rahma, A.; Mustalifah, F.R. Hydrogel derived from water hyacinth and pectin from banana peel as a membrane layer. Mater. Today Proc. 2023, 87, 13–17. [Google Scholar] [CrossRef]
- Lee, Y.-e.; Kang, Y.-R.; Chang, Y.H. Effect of pectic oligosaccharide on probiotic survival and physicochemical properties of hydrogel beads for synbiotic encapsulation of Lactobacillus bulgaricus. Food Biosci. 2023, 51, 102260. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Y.; Lin, Y.; Shao, P. Facile fabrication of multifunctional citrus pectin aerogel fortified with cellulose nanofiber as controlled packaging of edible fungi. Food Chem. 2022, 374, 131763. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef]
- Méndez, D.A.; Schroeter, B.; Martínez-Abad, A.; Fabra, M.J.; Gurikov, P.; López-Rubio, A. Pectin-based aerogel particles for drug delivery: Effect of pectin composition on aerogel structure and release properties. Carbohydr. Polym. 2023, 306, 120604. [Google Scholar] [CrossRef]
- Horvat, G.; Žvab, K.; Knez, Ž.; Novak, Z. Hybrid Polylactic-Acid–Pectin Aerogels: Synthesis, Structural Properties, and Drug Release. Polymers 2023, 15, 407. [Google Scholar] [CrossRef]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Hu, X.-F.; Jia, Y.-J.; Pan, L.-H.; Zheng, Z.; Zhao, Y.-Y.; Mu, D.-D.; Zhong, X.-Y.; Jiang, S.-T. Camellia oil-based oleogels structuring with tea polyphenol-palmitate particles and citrus pectin by emulsion-templated method: Preparation, characterization and potential application. Food Hydrocoll. 2019, 95, 76–87. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Xue, C. Effect of interaction between ovotransferrin fibrils and pectin on properties of oleogel-based Pickering emulsions. Food Hydrocoll. 2023, 140, 108620. [Google Scholar] [CrossRef]
- Pan, L.H.; Wu, X.l.; Luo, S.Z.; He, H.Y.; Luo, J.P. Effects of tea polyphenol ester with different fatty acid chain length on camellia oil-based oleogels preparation and its effects on cookies properties. J. Food Sci. 2020, 85, 2461–2469. [Google Scholar] [CrossRef]
- Konovalova, M.V.; Kurek, D.V.; Litvinets, S.G.; Martinson, E.A.; Varlamov, V.P. Preparation and characterization of cryogels based on pectin and chitosan. Prog. Chem. Appl. Chitin Its Deriv. 2016, XXI, 114–121. [Google Scholar] [CrossRef]
- Ma, Y.; Bi, J.; Yi, J.; Feng, S.; Peng, J.; Zou, S.; Guo, S.; Wu, Z. Modulation of ice crystal formation behavior in pectin-sucrose hydrogel by freezing temperature: Effect on ice crystal morphology and drying properties. Dry. Technol. 2023, 41, 1771–1782. [Google Scholar] [CrossRef]
- Martinez, Y.N.; Piñuel, L.; Castro, G.R.; Breccia, J.D. Polyvinyl alcohol–pectin cryogel films for controlled release of enrofloxacin. Appl. Biochem. Biotechnol. 2012, 167, 1421–1429. [Google Scholar] [CrossRef]
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Muñoz, J. Studies on sorption, desorption, regeneration and reuse of sugar-beet pectin gels for heavy metal removal. J. Hazard. Mater. 2010, 178, 243–248. [Google Scholar] [CrossRef]
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Munoz, J. Optimization of the continuous biosorption of copper with sugar-beet pectin gels. J. Environ. Manag. 2009, 90, 1737–1743. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Formulation and characterization of hydrogel based on pectin and brea gum. Int. J. Biol. Macromol. 2019, 123, 784–791. [Google Scholar] [CrossRef]
- Kim, J.U.; Kim, B.; Shahbaz, H.M.; Lee, S.H.; Park, D.; Park, J. Encapsulation of probiotic Lactobacillus acidophilus by ionic gelation with electrostatic extrusion for enhancement of survival under simulated gastric conditions and during refrigerated storage. Int. J. Food Sci. Technol. 2017, 52, 519–530. [Google Scholar]
- de Moura, S.C.; Berling, C.L.; Germer, S.P.; Alvim, I.D.; Hubinger, M.D. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: Pigment stability during storage of microparticles. Food Chem. 2018, 241, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Dash, K.K.; Badwaik, L.S. Physicochemical and release behaviour of phytochemical compounds based on black jamun pulp extracts-filled alginate hydrogel beads through vibration dripping extrusion. Int. J. Biol. Macromol. 2022, 194, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.; Kamalapur, M.; Marapur, S.; Kadam, D. Ionotropic gelation and polyelectrolyte complexation: The novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A review. Dig. J. Nanomater. Biostructures 2010, 5, 241–248. [Google Scholar]
- Winkleman, A.; Bracher, P.J.; Gitlin, I.; Whitesides, G.M. Fabrication and manipulation of ionotropic hydrogels cross-linked by paramagnetic ions. Chem. Mater. 2007, 19, 1362–1368. [Google Scholar] [CrossRef]
- Mishra, R.K.; Datt, M.; Banthia, A.K. Synthesis and characterization of pectin/PVP hydrogel membranes for drug delivery system. Aaps Pharmscitech 2008, 9, 395–403. [Google Scholar] [CrossRef]
- Bušić, A.; Belščak-Cvitanović, A.; Cebin, A.V.; Karlović, S.; Kovač, V.; Špoljarić, I.; Mršić, G.; Komes, D. Structuring new alginate network aimed for delivery of dandelion (Taraxacum officinale L.) polyphenols using ionic gelation and new filler materials. Food Res. Int. 2018, 111, 244–255. [Google Scholar]
- Konovalova, M.V.; Markov, P.A.; Durnev, E.A.; Kurek, D.V.; Popov, S.V.; Varlamov, V.P. Preparation and biocompatibility evaluation of pectin and chitosan cryogels for biomedical application. J. Biomed. Mater. Res. Part A 2017, 105, 547–556. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar]
- Erkey, C.; Türk, M. Support materials. In Supercritical Fluid Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 8, pp. 19–30. [Google Scholar]
- Jiang, L.; Wen, Y.; Zhu, Z.; Liu, X.; Shao, W. A Double cross-linked strategy to construct graphene aerogels with highly efficient methylene blue adsorption performance. Chemosphere 2021, 265, 129169. [Google Scholar] [CrossRef]
- Erkey, C.; Turk, M. Synthesis of Nanostructured Materials in Near and/or Supercritical Fluids: Methods, Fundamentals and Modeling; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Czarnobaj, K. Preparation and characterization of silica xerogels as carriers for drugs. Drug Deliv. 2008, 15, 485–492. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B. Introduction to polymeric gels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–27. [Google Scholar]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo-and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-based systems for the delivery of bioactive compounds in foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2, 100058. [Google Scholar] [CrossRef]
- Farris, S.; Schaich, K.M.; Liu, L.; Piergiovanni, L.; Yam, K.L. Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: A review. Trends Food Sci. Technol. 2009, 20, 316–332. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C 2019, 102, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.; Schaich, K.M.; Liu, L.; Cooke, P.H.; Piergiovanni, L.; Yam, K.L. Gelatin–pectin composite films from polyion-complex hydrogels. Food Hydrocoll. 2011, 25, 61–70. [Google Scholar] [CrossRef]
- Miran, M.; Salami, M.; Emam-Djomeh, Z.; Moreno, F.J.; Montilla, A. Isolation and structural evaluation of pectin, pectin-based polymer blends, composites, IPNs and gels. Handb. Nat. Polym. 2023, 1, 369–398. [Google Scholar]
- Maciel, V.B.V.; Yoshida, C.M.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Xie, J.; Zheng, X.; Chen, W. Pectin-chitosan conjugated nanoliposome as a promising delivery system for neohesperidin: Characterization, release behavior, cellular uptake, and antioxidant property. Food Hydrocoll. 2019, 95, 432–444. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, S.; Shen, J.; Chen, Y.; Xiao, Y. A composite hydrogel based on pectin/cellulose via chemical cross-linking for hemorrhage. Front. Bioeng. Biotechnol. 2021, 8, 627351. [Google Scholar] [CrossRef]
- Taokaew, S. Recent Advances in Cellulose-Based Hydrogels Prepared by Ionic Liquid-Based Processes. Gels 2023, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Jia, X.; Zhang, Q.; Chen, H.; Zhu, Q.; Yin, L. Interpenetrating polymer network hydrogels of soy protein isolate and sugar beet pectin as a potential carrier for probiotics. Food Hydrocoll. 2021, 113, 106453. [Google Scholar] [CrossRef]
- Peng, H.; Chen, S.; Luo, M.; Ning, F.; Zhu, X.; Xiong, H. Preparation and self-assembly mechanism of bovine serum albumin–citrus peel pectin conjugated hydrogel: A potential delivery system for vitamin C. J. Agric. Food Chem. 2016, 64, 7377–7384. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, T.; Hu, Q.; Luo, Y. Low density lipoprotein/pectin complex nanogels as potential oral delivery vehicles for curcumin. Food Hydrocoll. 2016, 57, 20–29. [Google Scholar] [CrossRef]
- Jung, J.; Arnold, R.D.; Wicker, L. Pectin and charge modified pectin hydrogel beads as a colon-targeted drug delivery carrier. Colloids Surf. B Biointerfaces 2013, 104, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kaletunç, G. Dissolution kinetics of pH responsive alginate-pectin hydrogel particles. Food Res. Int. 2016, 88, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhou, L.; Lyu, F.; Liu, J.; Ding, Y. The complex of whey protein and pectin: Interactions, functional properties and applications in food colloidal systems–A review. Colloids Surf. B Biointerfaces 2022, 210, 112253. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Li, L.; Sun, L.; Jia, B.; Yang, H.; Zuo, F. Preparation of fat substitute based on the high-methoxyl pectin of citrus and application in moon-cake skin. Food Sci. Technol. 2021, 42, e92121. [Google Scholar] [CrossRef]
- Kavya, M.; Jacob, A.R.; Nisha, P. Pectin emulsions and emulgels: Bridging the correlation between rheology and microstructure. Food Hydrocoll. 2023, 143, 108868. [Google Scholar] [CrossRef]
- Lu, Y.; Rai, R.; Nitin, N. Image-based assessment and machine learning-enabled prediction of printability of polysaccharides-based food ink for 3D printing. Food Res. Int. 2023, 173, 113384. [Google Scholar] [CrossRef]
- Vancauwenberghe, V.; Katalagarianakis, L.; Wang, Z.; Meerts, M.; Hertog, M.; Verboven, P.; Moldenaers, P.; Hendrickx, M.E.; Lammertyn, J.; Nicolaï, B. Pectin based food-ink formulations for 3-D printing of customizable porous food simulants. Innov. Food Sci. Emerg. Technol. 2017, 42, 138–150. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Norcino, L.; Mendes, J.; Natarelli, C.; Manrich, A.; Oliveira, J.; Mattoso, L. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Otálora González, C.M.; De’Nobili, M.D.; Rojas, A.M.; Basanta, M.F.; Gerschenson, L.N. Development of functional pectin edible films with fillers obtained from red cabbage and beetroot. Int. J. Food Sci. Technol. 2021, 56, 3662–3669. [Google Scholar] [CrossRef]
- Dudnyk, I.; Janeček, E.-R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Muñoz-Labrador, A.; Moreno, R.; Villamiel, M.; Montilla, A. Preparation of citrus pectin gels by power ultrasound and its application as an edible coating in strawberries. J. Sci. Food Agric. 2018, 98, 4866–4875. [Google Scholar] [CrossRef]
- Pholsin, R.; Shiekh, K.A.; Jafari, S.; Kijpatanasilp, I.; Nan, T.N.; Suppavorasatit, I.; Assatarakul, K. Impact of pectin edible coating extracted from cocoa shell powder on postharvest quality attributes of tomato (Lycopersicon esculentum Mill.) fruit during storage. Food Control 2023, 155, 110023. [Google Scholar] [CrossRef]
- Tabatabaei Moradi, L.; Sharifan, A.; Larijani, K. The effect of multilayered chitosan–pectin–Mentha piperita and lemon essential oil on oxidation effects and quality of rainbow trout fillet (Oncorhynchus mykiss) during refrigeration at 4 ± 1 °C storage. Iran. J. Fish. Sci. 2020, 19, 2544–2559. [Google Scholar]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial, antioxidant, and sensorial impacts of oregano and rosemary essential oils over broccoli florets. J. Food Process. Preserv. 2019, 43, e13889. [Google Scholar]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; Moreira, M.d.R.; Roura, S.I.; Ayala-Zavala, J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Microbiol. 2014, 5, 699. [Google Scholar] [CrossRef] [PubMed]
- Nisar, T.; Yang, X.; Alim, A.; Iqbal, M.; Wang, Z.-C.; Guo, Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin based coatings enriched with clove essential oil during refrigeration. Int. J. Biol. Macromol. 2019, 124, 1156–1166. [Google Scholar] [CrossRef]
- Ranjitha, K.; Rao, D.S.; Shivashankara, K.; Oberoi, H.S.; Roy, T.K.; Bharathamma, H. Shelf-life extension and quality retention in fresh-cut carrots coated with pectin. Innov. Food Sci. Emerg. Technol. 2017, 42, 91–100. [Google Scholar] [CrossRef]




| Source | Pectin Yield (%) | Extraction Methods | References |
|---|---|---|---|
| Lime peel | 17.70–26.30 | EAE | [28] |
| Blood orange peel | 19.24 | MAE | [29] |
| Sour orange peel | 29.10 | MAE | [30] |
| Navel orange peel | 15.47–20.44 | CE, MAE, UHP | [31] |
| Apple pomace | 3.63–14.50 | SW, EAE | [32,33] |
| Citrus peel | 0.15–28.82 | CE, SW, UAE | [32,34,35,36] |
| Grapefruit peel | 23.50–27.34 | CE, UAE | [37] |
| Pineapple peel | 1.02–2.12 | CE, MAE | [38] |
| Apple peel | 3.60–6.40 | CE | [39] |
| Plantain peel | 6.20–13.40 | CE, EAE | [40] |
| Pumpkin peel | 8.08–10.03 | EAE | [41,42] |
| Mango peel | 1.55–21.82 | CE, UAE | [43,44] |
| Watermelon rind peel | 11.25–25.79 | CE, MAE | [45,46,47,48] |
| Dragon fruit peel | 7.50 | MAE | [49] |
| Cocoa husk | 8.00–11.31 | CE | [50,51] |
| Soy hull | 26.00–28.00 | CE | [52] |
| Potato pulp | 14.34 | CE | [53] |
| Banana peel | 15.89–24.08 | CE | [54] |
| Strawberry | 4.10–9.00 | CE, UAE, EAE | [55] |
| Redcurrent | 2.20–8.80 | CE, UAE, EAE | [55] |
| Blackberry | 4.30–9.10 | CE, UAE, EAE | [55] |
| Raspberry | 8.70–12.20 | CE, UAE, EAE | [55] |
| Types of Pectin Gel | Composite Material | Methods | Outcomes | Applications | References |
|---|---|---|---|---|---|
| Hydrogel | Pectin/chitosan/essential oils | Ionic gelation (Dripping method) | Good antimicrobial activity against six types of microorganism | - | [109] |
| Hydrogel | Pectin/chitosan | Ionic charge interaction | Good antibacterial and wound healing properties | Tissue regeneration | [110] |
| Hydrogel | LM apple pectin | - | Low toxicity, improved stability towards elastic and plastic deformation, ability to adhere to macrophages and the non-specific adsorption of blood plasma proteins | Scaffold for tissue engineering | [13] |
| Hydrogel | LM apple pectin/LM hogweed pectin | Ionotropic | Increased gel strength | - | [111] |
| Hydrogel | HM apple pectin/Glucono-δ-lactone | - | Great mechanical strength, stronger thermo-reversibility, and higher pH stability | [112] | |
| Hydrogel (membrane layer) | Banana peel pectin/Water hyacinth carboxymethyl cellulose | Casting | Increased hydrophobicity of hydrogel membrane | - | [113] |
| Hydrogel | LM pectin/Resistant starch/Lactobacillus bulgaricus | Filtration | High storage ability and protective effects on L. bulgaricus | Synbiotic encapsulation, protection, and delivery of probiotics | [114] |
| Aerogel | Citrus pectin/cellulose nanofiber | Freeze drying | Improved tensile and compressive properties | Edible fungus moisture-regulating packaging | [115] |
| Aerogel | LM pectin/alginate | Freeze drying | Strong antioxidant activity with good controlled released of proanthocyanidins | Matrix for the controlled release of proanthocyanidin compound | [116] |
| Aerogel | 1.Citrus pectin | Supercritical drying with CO2 | High specific surface and low bulk density | Matrix for the controlled release of vanillin compound | [117] |
| 2. Watermelon rind pectin | |||||
| Aerogel | Citrus pectin/PLA | Supercritical drying | Increased swelling and simulated body fluid (SBF) uptake | Active wound-healing materials | [118] |
| Aerogel | Pectin/TiO2 | Supercritical CO2 drying | Great mechanical, thermal, and antimicrobial properties | Temperature-sensitive food | [119] |
| Aerogel | Citrus pectin | Supercritical CO2 drying | Low density with high porosity and pore volume resulted in small pores size, mainly mesopores and small macropores | Matrix for the controlled release of theophylline compound | [120] |
| Oleogel | Citrus pectin/camellia oil/tea polyphenol-palmitate particles | Freeze drying | Improved oil binding capacity and gel strength | - | [121] |
| Oleogel | Citrus pectin/ ovotransferrin fibrils | Homogenizing | Better stability, smaller droplet size, more prominent gel-like structure, high viscosity, and superior texture properties | Matrix for the controlled release of curcumin compound | [122] |
| Oleogel | Citrus pectin/tea polyphenol ester | Freeze drying | Increased stability and viscoelasticity of emulsions, improved oil binding capacity and gel strength of the oleogels | Fat replacer in cookies product | [123] |
| Cryogel | 1. Apple pectin/chitosan | Cryotropic gelation (Freeze drying) | Possessed biocompatibility, biodegradability, and low toxicity | Potential medical purposes | [124] |
| 2. Heracleum pectin/chitosan | |||||
| Cryogel | LM pectin/sucrose | Freeze drying | Reduction of ice crystal in gel | - | [125] |
| Cryogel | Citrus pectin | Freeze drying | High loading efficiency of theophylline compound | Matrix for the controlled release of theophylline compound | [120] |
| Cryogel | LM, MM and HM pectin/polyvinyl alcohol | Film drying | Ability to keep the enrofloxacin antibiotic inside the matrix and control of the cargo amount in the gel | Can be used for different infectious pathologies and/or treatments | [126] |
| Xerogel | Citrus pectin | Oven drying | High density, low porosity, low pore volume and compact morphology | Matrix for the controlled release of theophylline compound | [120] |
| Xerogel | Sugar beet pectin | Air drying | Improved stability and reusability of the gels with good sorption capability of metal compounds | Heavy metal removal | [127] |
| Xerogel | Sugar beet pectin | Air drying | Good mechanical strength with high continuous biosorption and desorption of copper | Biosorbent for copper removal in a fixed-bed column | [128] |
| Xerogel | LM pectin/brea gum | Oven drying | Showed good compatibility between both polymers with high gel strength, while also able to respond to the changes in pH of the medium and modify dye release | Matrix for the controlled release of methylene blue dye | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. https://doi.org/10.3390/gels9090732
Said NS, Olawuyi IF, Lee WY. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels. 2023; 9(9):732. https://doi.org/10.3390/gels9090732
Chicago/Turabian StyleSaid, Nurul Saadah, Ibukunoluwa Fola Olawuyi, and Won Young Lee. 2023. "Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications" Gels 9, no. 9: 732. https://doi.org/10.3390/gels9090732






