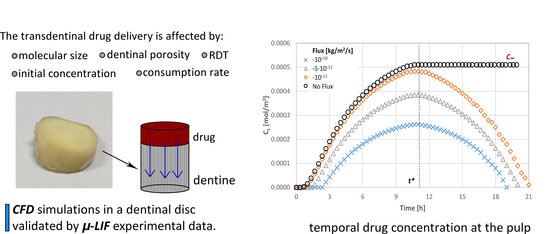
Computational Modelling for Efficient Transdentinal Drug Delivery
Abstract
:1. Introduction
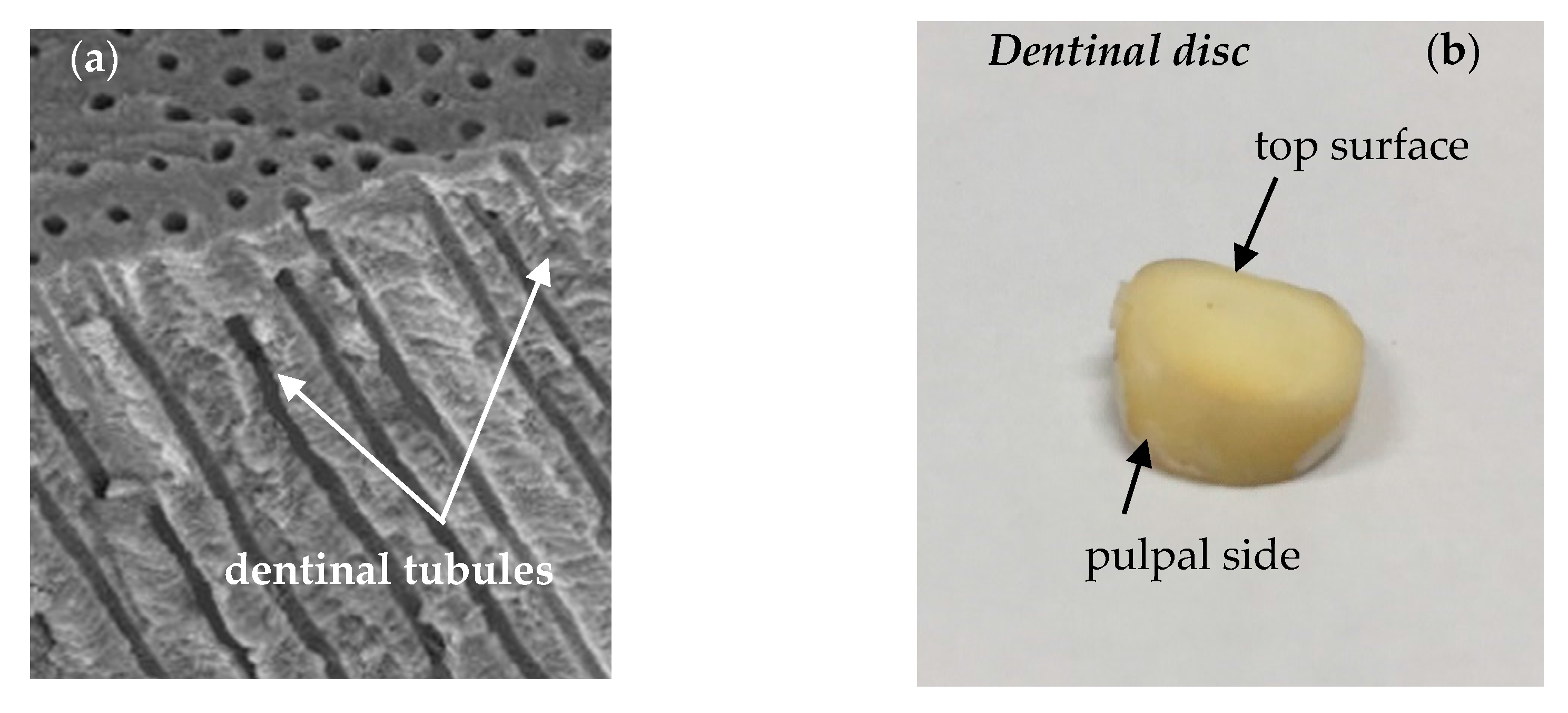
2. Transport of Therapeutic Compounds through the Dentinal Tissue
3. Materials and Methods
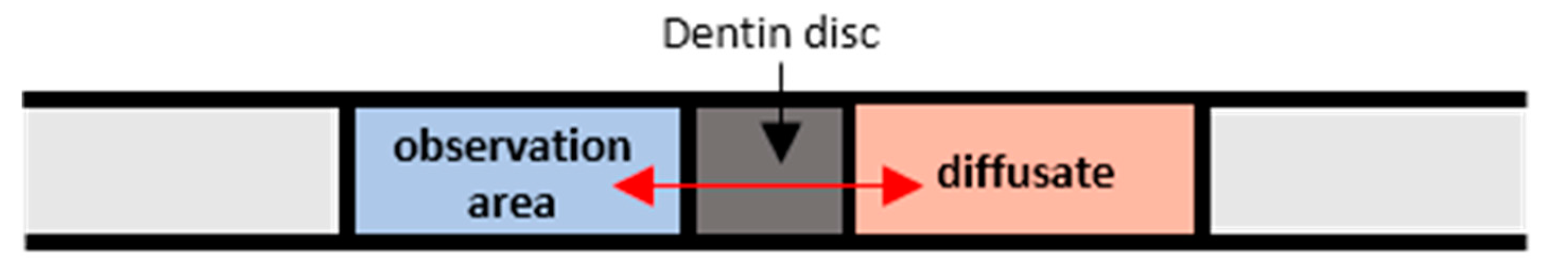
3.1. Code Validation
- Prior to the actual measurements, the system is calibrated using suitable solutions. In our case aqueous Rhodamine B solutions with known concentrations, namely, M = 0.05, 0.025 and 0.00 mg/L were employed, while, for each concentration Ci, an image C(x,y) is taken.
- To reduce noise, image masking of the acquired images is performed before defining an appropriate Region of Interest (ROI), at which the fluorescence intensity is measured.
- The relationship between the measured fluorescence intensity field I(x,t) and the concentration field C(x,y) is determined.
- A set of 20 images is acquired and the mean image is calculated.
- Each mean image is compared with the previously defined μ-LIF calibration curve.
3.2. Numerical Procedure
- the porosity of the tissue (ϕ),
- the thickness of the tissue (Remaining Dentinal Thickness, RDT),
- the initial concentration (M0) of the substances to be diffused,
- the molecular size of the substances to be diffused, i.e., their Diffusion Coefficient and
- the consumption rate (R) of the diffusate at the pulpal side
4. Results
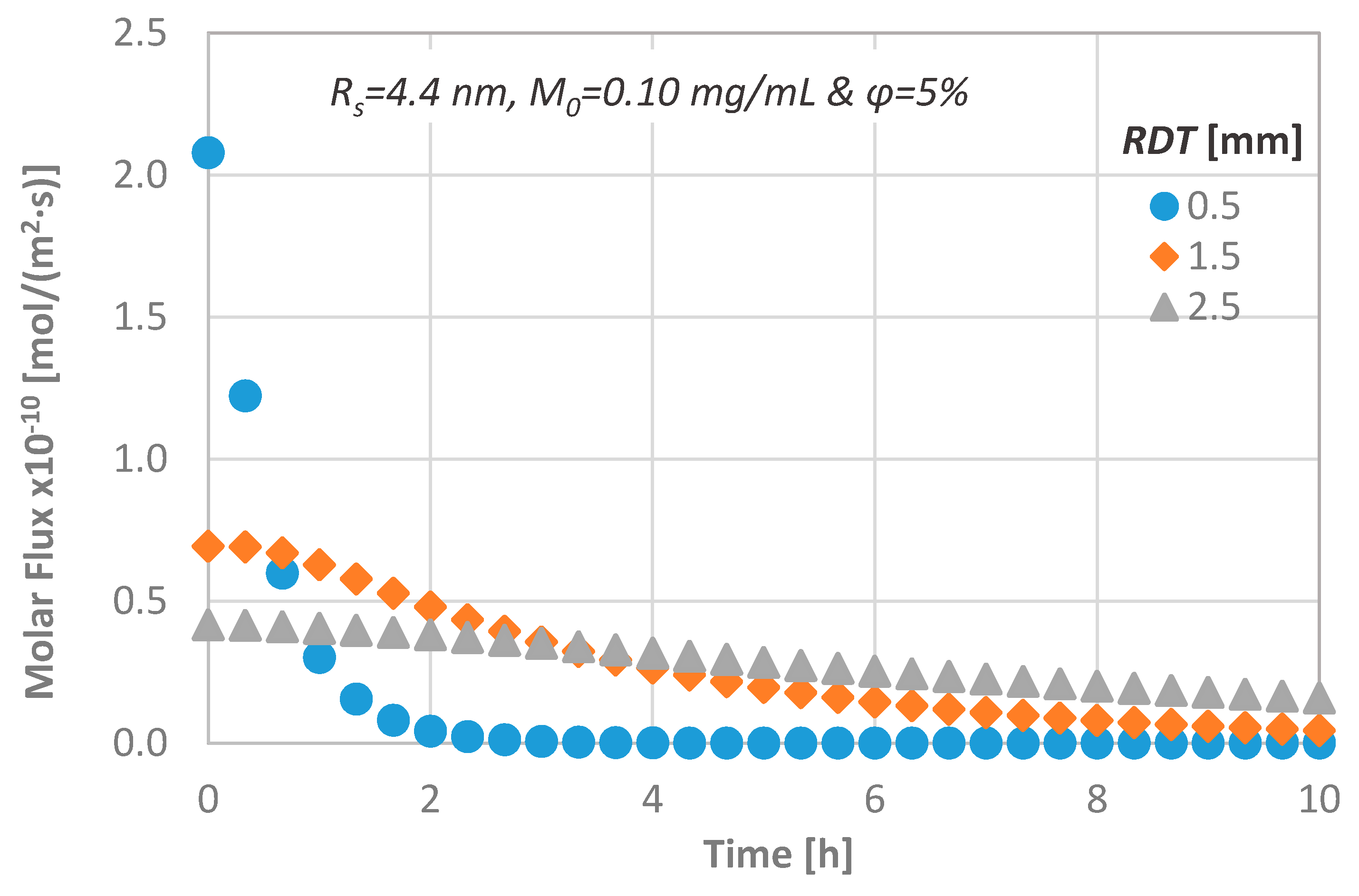
4.1. Effect of the Agent Consumption Rate at the Pulpal Side
4.2. Effect of Remaining Dentin Thickness (RDT)
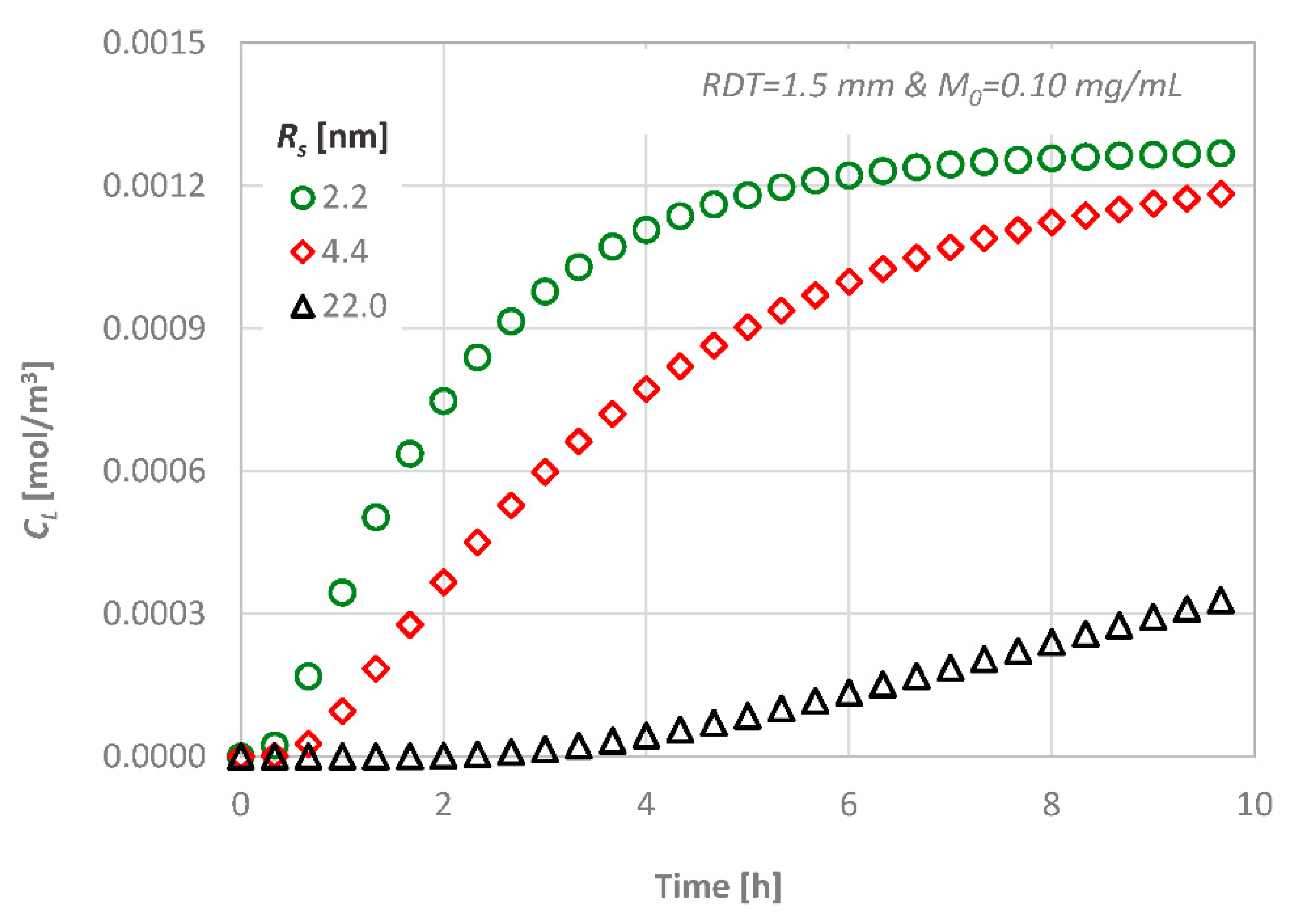
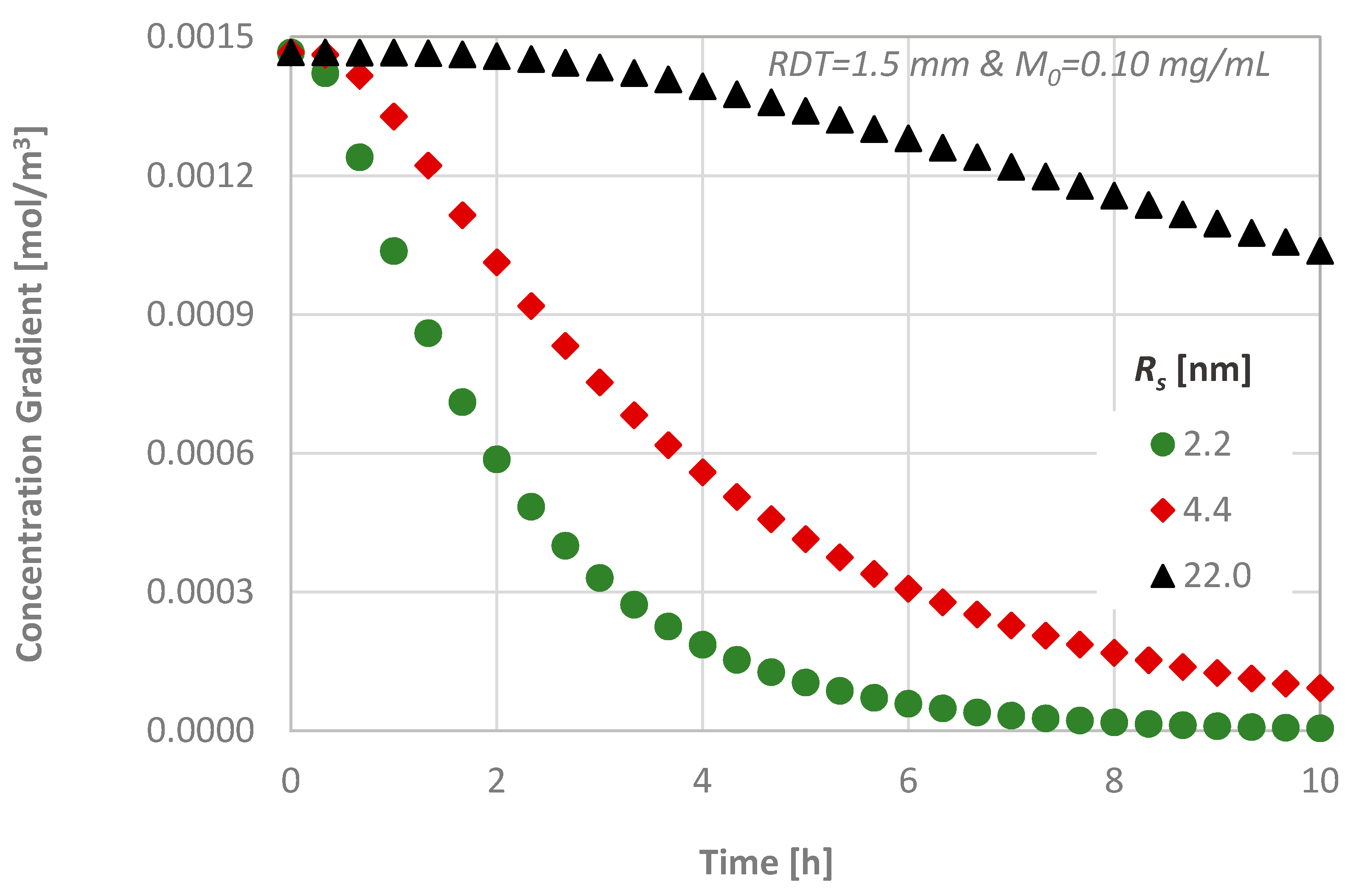
4.3. Effect of the Molecular Size
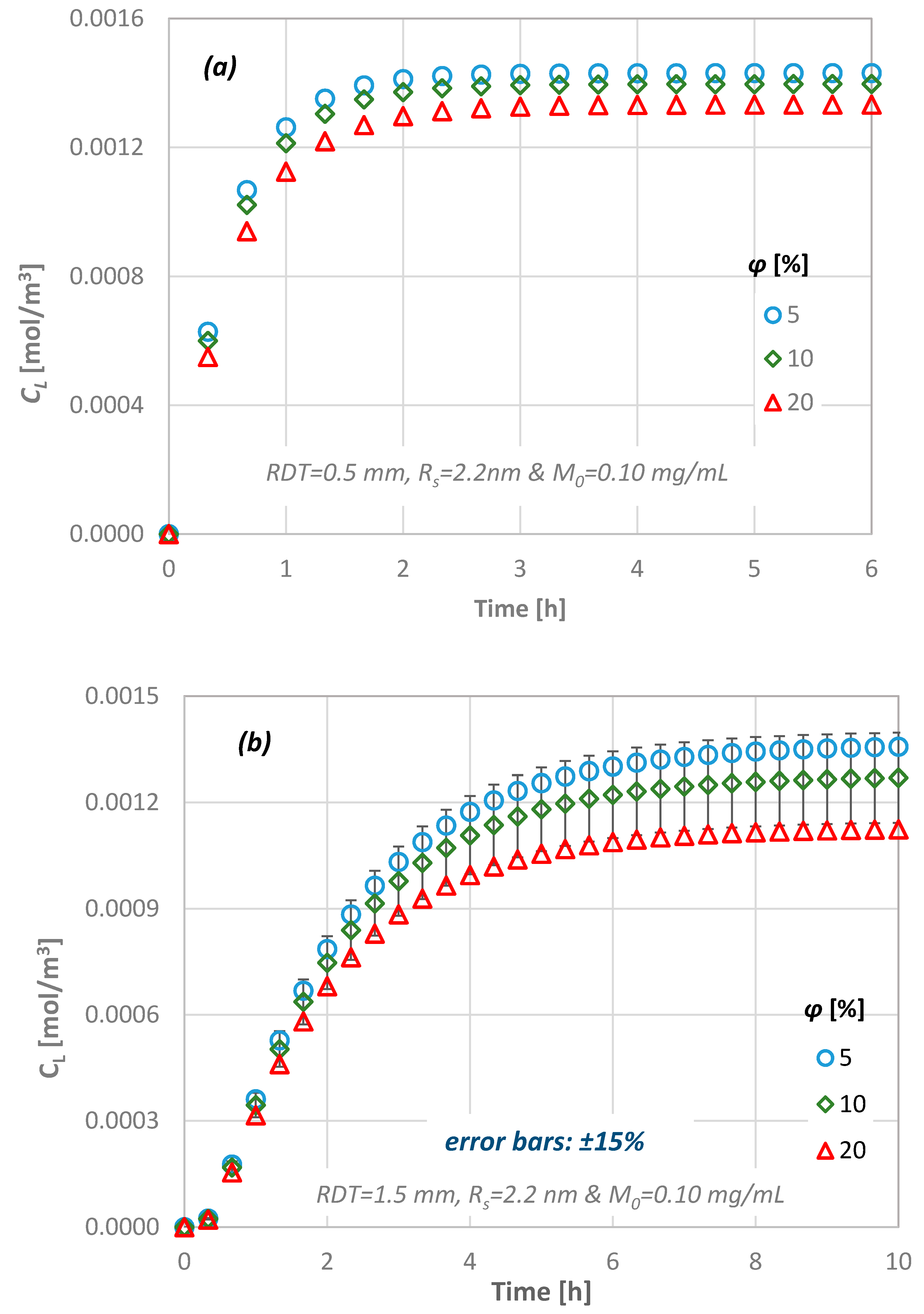
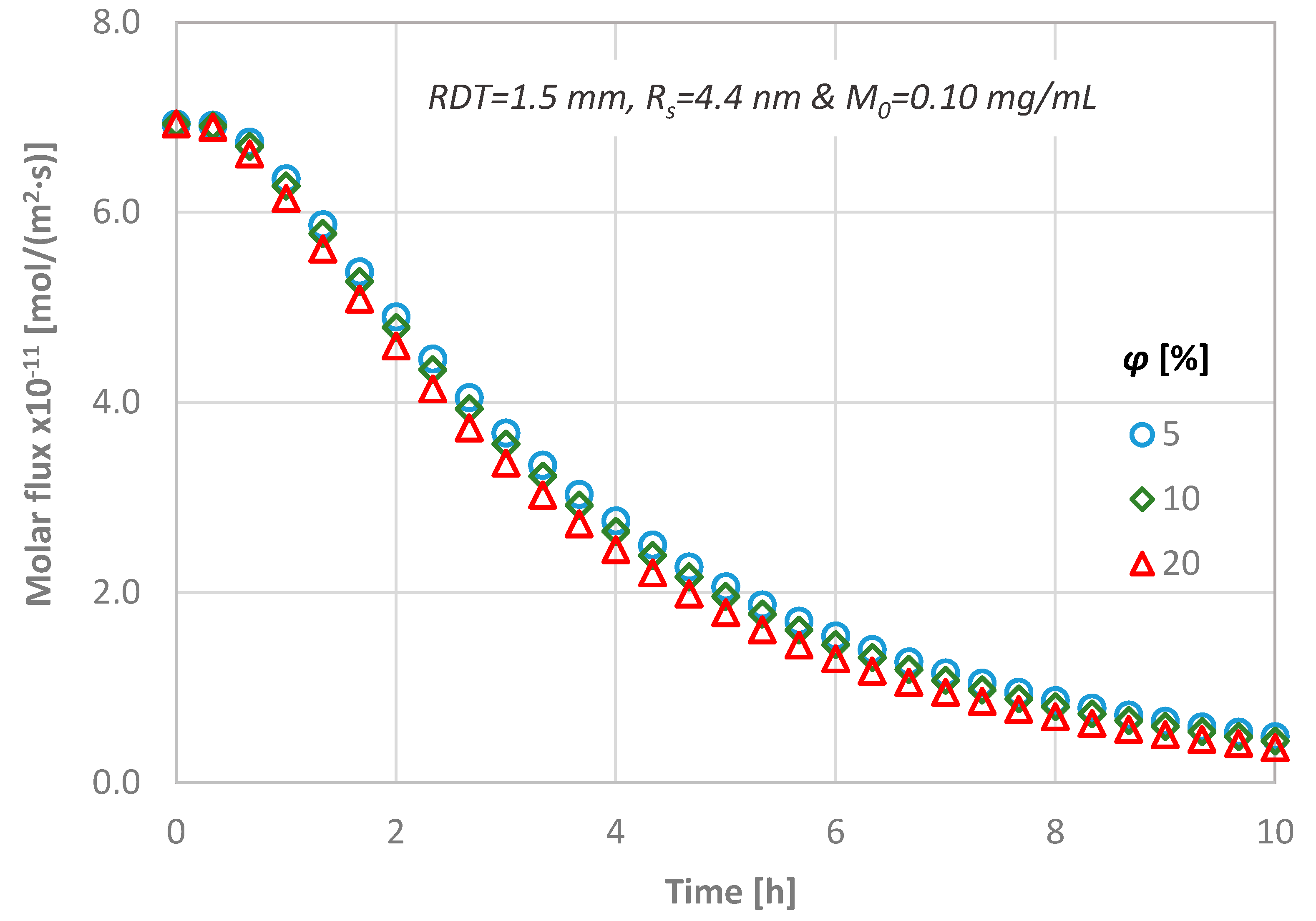
4.4. Effect of Dentine Porosity
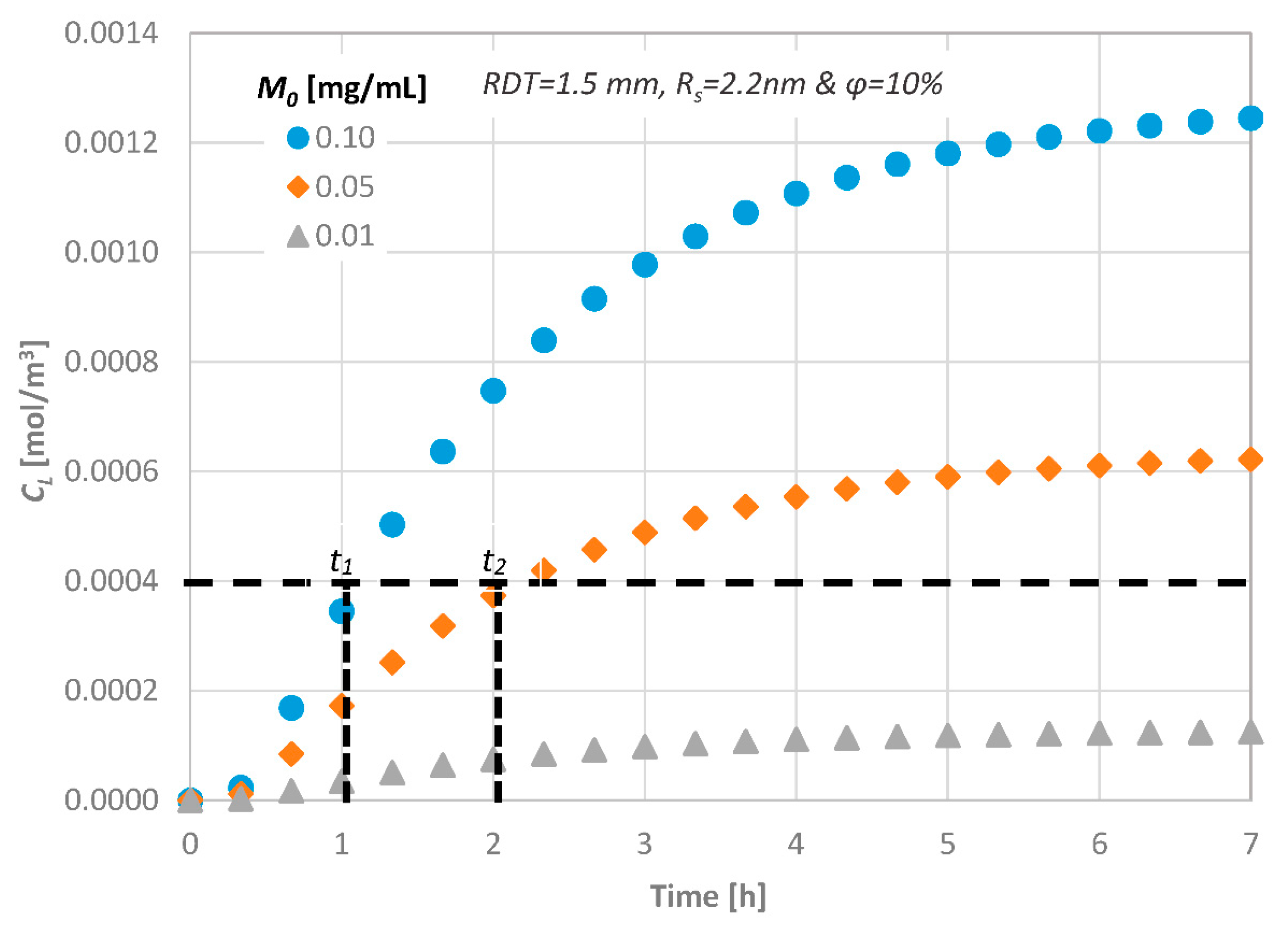
4.5. Effect of the Initial Diffusate Concentration
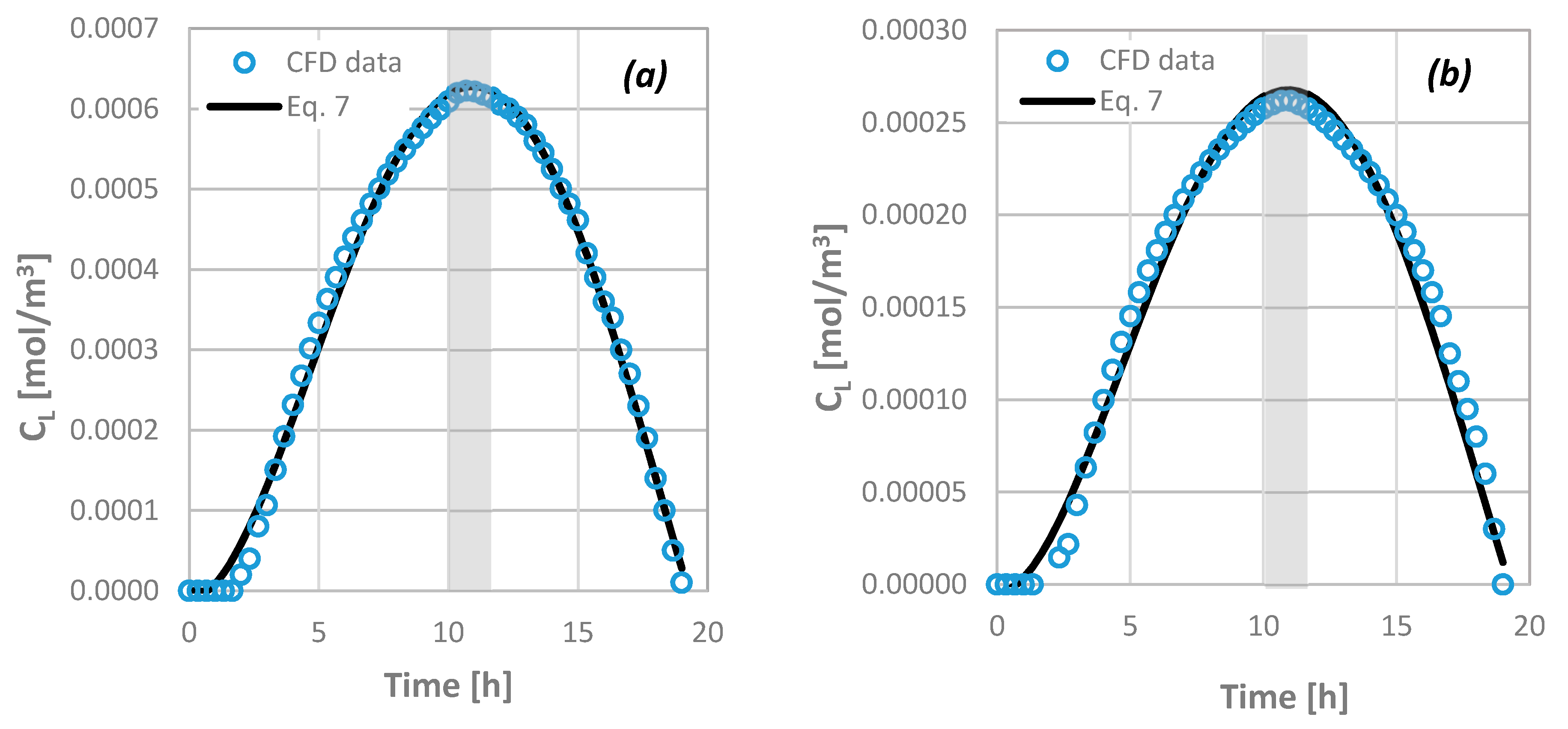
4.6. Prediction of the Drug Pulpal Concentration
5. Conclusions
- the transdentinal diffusion of drugs is mainly affected by the molecular size and the RDT, as it was expected,
- a porosity change of 5% to 20% results in less than ±15% CL difference,
- a variation of the agent consumption rate at the pulpal side between 0 and 10−10 kg/(m2·s), leads to a 100% CL decrease, while the consumption time is 18–25 h.
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| C | Molar concentration, mol/m3 |
| CL | Molar concentration at the bottom end, mol/m3 |
| Co | Initial molar concentration, mol/m3 |
| C∞ | Final molar concentration (for t = L2/D), mol/m3 |
| D | Coefficient of diffusion, m2/s |
| j | Concentration flux, mol/m2·s |
| L | Length, m |
| Μ0 | Mass concentration, g/m3 |
| Mw | Molecular weight, g/mol |
| R | Agent consumption rate, kg/(m2·s) |
| RDT | Remaining dentinal thickness, m |
| Rs | Stokes radius, m |
| r | Radius of conduit, m |
| T | Temperature, °C |
| t* | Dt/L2, dimensionless |
| t | Time, s |
| U | Velocity, m/s |
| x | Distance, m |
| μ | Viscosity, g/cm·s |
| ϕ | Porosity, % |
References
- De Peralta, T.L.; Nör, J.E. Regeneration of the living pulp. In The Dental Pulp: Biology, Pathology, and Regenerative Therapies; Springer: Berlin/Heidelberg, Germany, 2014; pp. 237–250. [Google Scholar]
- Tziafas, D.; Smith, A.J.; Lesot, H. Designing new treatment strategies in vital pulp therapy. J. Dent. 2000, 28, 77–92. [Google Scholar] [CrossRef]
- Rutherford, B.; Spangberg, L. Transdentinal stimulation of reparative dentine formation by osteogenic protein-1 in monkeys. Arch. Oral Biol. 1995, 40, 681–683. [Google Scholar] [CrossRef]
- Kalyva, M.; Papadimitriou, S.; Tziafas, D. Transdentinal stimulation of tertiary dentine formation and intratubular mineralization by growth factors. Int. Endod. J. 2010, 43, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Tobias, R.S.; Murray, P.E. Transdentinal stimulation of reactionary dentinogenesis in ferrets by dentine matrix components. J. Dent. 2001, 29, 341–346. [Google Scholar] [CrossRef]
- Pashley, D.H. Dentin Permeability: Theory and Practice; CRC Press Inc.: Boca Raton, FL, USA, 1990. [Google Scholar]
- Pashley, D.H. Dentin permeability and dentin sensitivity. Proc. Finn. Dent. Soc. 1992, 88 (Suppl. S1), 31–37. [Google Scholar] [PubMed]
- Murray, P.E.; Smith, A.J. Remaining dentine thickness and human pulp responses. Int. Endod. J. 2003, 36, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Tak, O.; Usumez, A. Diffusion of HEMA from resin cements through different dentin thicknesses in vitro. Am. J. Dent. 2015, 28, 285–291. [Google Scholar] [PubMed]
- Smith, A.J. Vitality of the dentin–pulp complex in health and disease: Growth factors as key mediators. J. Dent. Educ. 2003, 67, 678–689. [Google Scholar] [PubMed]
- Pashley, D.H.; Matthews, W.G. The effects of outward forced convective flow on inward diffusion in human dentine in vitro. Arch. Oral Biol. 1993, 38, 577–582. [Google Scholar] [CrossRef]
- Hanks, C.T.; Wataha, J.C. Permeability of biological and synthetic molecules through dentine. J. Oral Rehabil. 1994, 21, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Boutsioukis, C.; Lambrianidis, T.; Kastrinakis, E. Irrigant flow within a prepared root canal using various flow rates: A computational fluid dynamics study. Int. Endod. J. 2009, 42, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Boutsioukis, C.; Verhaagen, B.; Versluis, M.; Kastrinakis, E.; van der Sluis, L.W. Irrigant flow in the root canal: Experimental validation of an unsteady computational fluid dynamics model using high-speed imaging. Int. Endod. J. 2010, 43, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Haapasalo, M. Development and validation of a three-dimensional computational fluid dynamics model of root canal irrigation. J. Endod. 2009, 35, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Su, K.C.; Chuang, S.F. An investigation of dentinal fluid flow in dental pulp during food mastication: Simulation of fluid-structure interaction. Biomech. Model. Mechanobiol. 2014, 13, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Passos, A.D.; Tziafas, D.; Mouza, A.A.; Paras, S.V. Study of the transdentinal diffusion of bioactive molecules. Med. Eng. Phys. 2016, 38, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Ingle, J.I.; Bakland, L.K.; Baumgartner, J.C. Ingle’s Endodontics 6; BC Decker: Hamilton, ON, Canada, 2008. [Google Scholar]
- Garberoglio, R.; Brannstrom, M. Scanning electron microscopic investigation of human dentinal tubules. Arch. Oral Biol. 1976, 21, 355–362. [Google Scholar] [CrossRef]
- Andrew, D.; Matthews, B. Displacement of the contents of dentinal tubules and sensory transduction in intradental nerves of the cat. J. Physiol. 2000, 529 Pt 3, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Fearnhead, R.W. Histological evidence for the innervation of human dentine. J. Anat. 1957, 91, 267–277. [Google Scholar] [PubMed]
- Hildebrand, C.; Fried, K. Teeth and tooth nerves. Prog. Neurobiol. 1995, 45, 165–222. [Google Scholar] [CrossRef]
- Gendron, P.O.; Avaltroni, F.; Wilkinson, K.J. Diffusion coefficients of several rhodamine derivatives as determined by pulsed field gradient-nuclear magnetic resonance and fluorescence correlation spectroscopy. J. Fluoresc. 2008, 18, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Bindhu, C.V.; Harilal, S.S. Effect of the excitation source on the quantum-yield measurements of rhodamine B laser dye studied using thermal-lens technique. Anal. Sci. 2001, 17, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, J.; Adrian, R.J. Whole field measurement of temperature in water using two-color laser induced fluorescence. Exp. Fluids 1999, 26, 7–15. [Google Scholar] [CrossRef]
- Murphy, D.B. Fundamentals of Light Microscopy and Electronic Imaging; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Mortensen, N.A.; Okkels, F.; Bruus, H. Reexamination of Hagen-Poiseuille flow: Shape dependence of the hydraulic resistance in microchannels. Phys. Rev. E 2005, 71, 057301. [Google Scholar] [CrossRef] [PubMed]
- Paterson, M.S. The equivalent channel model for permeability and resistivity in fluid-saturated rock—A re-appraisal. Mech. Mater. 1983, 2, 345–352. [Google Scholar] [CrossRef]
- Panda, M.N.; Lake, L.W. Estimation of single-phase permeability from parameters of particle-size distribution. AAPG Bull. Am. Assoc. Pet. Geol. 1994, 78, 1028–1039. [Google Scholar]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Vedantam, S. Hydromechanics in dentine: Role of dentinal tubules and hydrostatic pressure on mechanical stress-strain distribution. Dent. Mater. 2007, 23, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Stogiannis, I.A.; Mouza, A.A.; Paras, S.V. Study of a micro-structured PHE for the thermal management of a fuel cell. Appl. Therm. Eng. 2013, 59, 717–724. [Google Scholar] [CrossRef]
- Outhwaite, W.C.; Livingston, M.J.; Pashley, D.H. Effects of changes in surface area, thickness, temperature and post-extraction time on human dentine permeability. Arch. Oral Biol. 1976, 21, 599–603. [Google Scholar] [CrossRef]





| Parameter | Lower Bound | Upper Bound |
|---|---|---|
| Porosity (ϕ), % | 5 | 20 |
| Remaining Dentinal Thickness (RDT), mm | 0.5 | 2.5 |
| Initial Concentration (M0), mg/mL | 0.01 | 0.10 |
| Molecular Size (Rs), nm | 2.2 | 22.0 |
| Consumption Rate (R), kg/(m2·s) | 0 | 10−10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passos, A.D.; Tziafas, D.; Mouza, A.A.; Paras, S.V. Computational Modelling for Efficient Transdentinal Drug Delivery. Fluids 2018, 3, 4. https://doi.org/10.3390/fluids3010004
Passos AD, Tziafas D, Mouza AA, Paras SV. Computational Modelling for Efficient Transdentinal Drug Delivery. Fluids. 2018; 3(1):4. https://doi.org/10.3390/fluids3010004
Chicago/Turabian StylePassos, Agathoklis D., Dimitris Tziafas, Aikaterini A. Mouza, and Spiros V. Paras. 2018. "Computational Modelling for Efficient Transdentinal Drug Delivery" Fluids 3, no. 1: 4. https://doi.org/10.3390/fluids3010004
APA StylePassos, A. D., Tziafas, D., Mouza, A. A., & Paras, S. V. (2018). Computational Modelling for Efficient Transdentinal Drug Delivery. Fluids, 3(1), 4. https://doi.org/10.3390/fluids3010004