Fluid Flow and Mass Transport in Brain Tissue
Abstract
:1. Introduction
2. Background
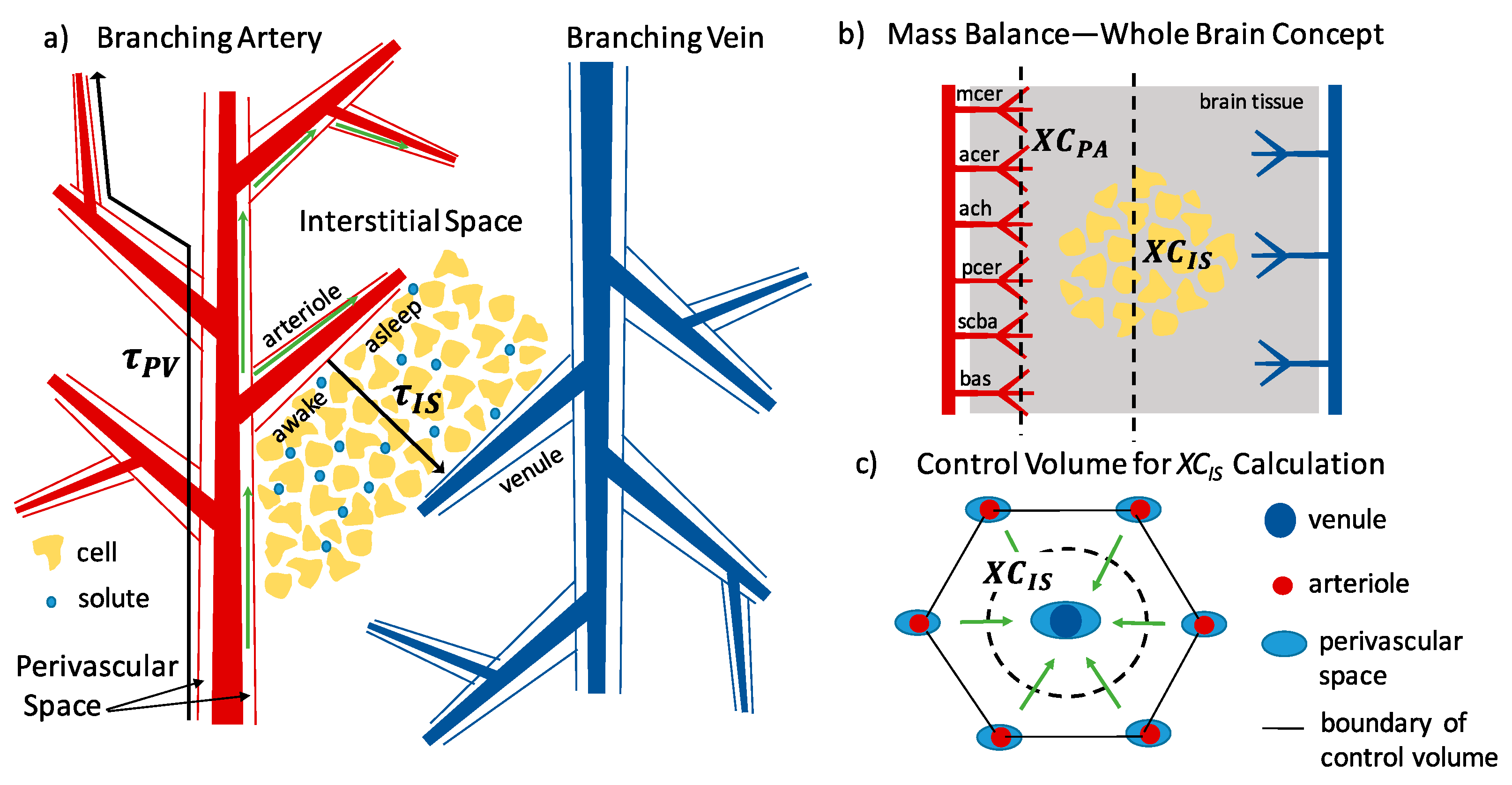
2.1. Relevant Physiology
2.2. Relevant Transport
2.2.1. Diffusion and Advection
- = relevant length scale,
- = velocity, and
- = diffusivity.
2.2.2. Osmotics
2.2.3. Transport Equations for Interstitial Space (Porous Media)
- = concentration in the ISF,
- = free diffusivity,
- = tortuosity,
- = apparent diffusivity,
- = source term,
- = void volume = VECS/Vtotal,
- = cellular uptake and adsorption,
- = superficial velocity,
- = hydraulic conductivity, and
- = pressure.
2.2.4. Equations of Flow for Perivascular Space
- intrinsic velocity;
- effective viscosity;
- density;
- pressure;
- void volume;
- gravitational acceleration, and
- hydraulic conductivity
2.2.5. Transport Parameters
3. Evolution of the Field
3.1. Key Experimental Work
3.2. Glymphatic Hyothesis
- CSF from the subarachnoid space moves along periarterial spaces into the brain (in the same direction as blood flow, termed antegrade) by advective transport;
- From the periarterial space the fluid moves into the brain interstitium, facilitated by AQP4 channels on astrocytic endfeet comprising the perivascular wall;
- The fluid flows across the interstitium, dissolving or entraining waste molecules, and
- Carries them to the perivenous space, where they are transported out of the brain via primary perivenous drainage pathways.
3.3. Transport in the Whole Brain: Dynamic Contrast-Enhanced (DCE) MRI
3.4. Efflux Routes and Meningeal Lymphatic Vessels
3.4.1. Efflux Routes
3.4.2. Meningeal Lymph Vessels
3.5. Sleep Enhances Glymphatic Function
3.6. Unanswered Questions
3.6.1. Perivascular Flow: Influx
- slow neural waves that occur during non-rapid eye movement (NREM) sleep;
- followed by a decrease in cerebral blood flow;
- followed by an increase in CSF production.
3.6.2. Interstitial Flow
3.6.3. Transport between Perivascular Space (PVS) and Interstitium
3.6.4. Perivascular Flow: Efflux
- Perivenous efflux to the CSF, but subsequent exit through a proximal route (glymphatic hypothesis); or
- Efflux via an intramural periarterial route that passes through the subarachnoid space, but remains physically separated from the CSF, and ultimately connects to the cervical lymph.
3.7. Glymphatic Debate Is Focussing
3.8. Early Human Results
4. Discussion
4.1. Transport Time-Scale Analysis
4.2. Mass/Volumetric Flow Balance
- Flow rates for transport in tissues are most frequently reported as either velocity or volumetric flow rate per gram of tissue, known as rate of perfusion, and
- Fluids in the brain are incompressible and at nearly constant temperature, making mass and volume equivalent.
4.3. Discussion Summary
5. Conclusions and Areas of Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lundgaard, I.; Li, B.; Xie, L.; Kang, H.; Sanggaard, S.; Haswell, J.D.R.; Sun, W.; Goldman, S.; Blekot, S.; Nielsen, M.; et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 2015, 6, 6807. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.P.; Spacek, J.; Bartol, T.M.; Bajaj, C.L.; Harris, K.M.; Sejnowski, T.J. Extracellular sheets and tunnels modulate glutamate diffusion in hippocampal neuropil. J. Comp. Neurol. 2013, 521, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, D.M.; Min, M.Y.; Asztely, F.; Rusakov, D.A. Extracellular glutamate diffusion determines the occupancy of glutamate receptors at CA1 synapses in the hippocampus. Philos. Tran. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 395–402. [Google Scholar] [CrossRef]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 379–394. [Google Scholar] [CrossRef]
- Benveniste, H.; Heerdt, P.M.; Fontes, M.; Rothman, D.L.; Volkow, N.D. Glymphatic System Function in Relation to Anesthesia and Sleep States. Anesth. Analg. 2019, 128, 747–758. [Google Scholar] [CrossRef]
- Maneshi, M.M.; Maki, B.; Gnanasambandam, R.; Belin, S.; Popescu, G.K.; Sachs, F.; Hua, S.Z. Mechanical stress activates NMDA receptors in the absence of agonists. Sci. Rep. 2017, 7, 39610. [Google Scholar] [CrossRef]
- Wolak, D.J.; Thorne, R.G. Diffusion of Macromolecules in the Brain: Implications for Drug Delivery. Mol. Pharm. 2013, 10, 1492–1504. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.J.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of Glymphatic Pathway Function Promotes Tau Pathology after Traumatic Brain Injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Piantino, J.; Lim, M.M.; Newgard, C.D.; Iliff, J. Linking Traumatic Brain Injury, Sleep Disruption and Post-Traumatic Headache: A Potential Role for Glymphatic Pathway Dysfunction. Curr. Pain Headache Rep. 2019, 23, 62. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Labbadia, J.; Morimoto, R.I. The Biology of Proteostasis in Aging and Disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Elimination of substances from the brain parenchyma: Efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 2018, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Goulay, R.; Flament, J.; Gauberti, M.; Naveau, M.; Pasquet, N.; Gakuba, C.; Emery, E.; Hantraye, P.; Vivien, D.; Aron-Badin, R.; et al. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke 2017, 48, 2301–2305. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Ding, G.L.; Davoodi-Bojd, E.; Li, Q.J.; Li, L.; Sadry, N.; Nedergaard, M.; Chopp, M.; Zhang, Z.G. Impairment of the glymphatic system after diabetes. J. Cereb. Blood Flow Metab. 2017, 37, 1326–1337. [Google Scholar] [CrossRef]
- Xia, M.S.; Yang, L.; Sun, G.F.; Qi, S.; Li, B.M. Mechanism of depression as a risk factor in the development of Alzheimer's disease: The function of AQP4 and the glymphatic system. Psychopharmacology 2017, 234, 365–379. [Google Scholar] [CrossRef]
- Fournier, A.P.; Gauberti, M.; Quenault, A.; Vivien, D.; Macrez, R.; Docagne, F. Reduced spinal cord parenchymal cerebrospinal fluid circulation in experimental autoimmune encephalomyelitis. J. Cereb. Blood Flow Metab. 2019, 39, 1258–1265. [Google Scholar] [CrossRef]
- Lundgaard, I.; Wang, W.; Eberhardt, A.; Vinitsky, H.S.; Reeves, B.C.; Peng, S.S.; Lou, N.H.; Hussain, R.; Nedergaard, M. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci. Rep. 2018, 8, 2246. [Google Scholar] [CrossRef]
- Schain, A.J.; Melo-Carrillo, A.; Strassman, A.M.; Burstein, R. Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J. Neurosci. 2017, 37, 2904–2915. [Google Scholar] [CrossRef]
- Chary, S.R.; Jain, R.K. Direct Measurement of Interstitial Convection and Diffusion of Albumin in Normal and Neoplastic Tissues by Fluorescence Photobleaching. Proc. Natl. Acad. Sci. USA 1989, 86, 5385–5389. [Google Scholar] [CrossRef]
- Swartz, M.A.; Fleury, M.E. Interstitial flow and its effects in soft tissues. Annu. Rev. Biomed. Eng. 2007, 9, 229–256. [Google Scholar] [CrossRef] [PubMed]
- Cserr, H.F.; Cooper, D.N.; Suri, P.K.; Patlak, C.S. Efflux of radiolabeled polyethylene glycols and albumin from rat-brain. Am. J. Physiol. 1981, 240, F319–F328. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Woollam, D.H.M.; Millen, J.W. Perivascular spaces of the mammalian central nervous system. Biol. Rev. 1954, 29, 251–283. [Google Scholar] [CrossRef]
- Pizzo, M.E.; Wolak, D.J.; Kumar, N.N.; Brunette, E.; Brunnquell, C.L.; Hannocks, M.-J.; Abbott, N.J.; Meyerand, M.E.; Sorokin, L.; Stanimirovic, D.B.; et al. Intrathecal antibody distribution in the rat brain: Surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol. Lond. 2018, 596, 445–475. [Google Scholar] [CrossRef]
- Hannocks, M.J.; Pizzo, M.E.; Huppert, J.; Deshpande, T.; Abbott, N.J.; Thorne, R.G.; Sorokin, L. Molecular characterization of perivascular drainage pathways in the murine brain. J. Cereb. Blood Flow Metab. 2018, 38, 669–686. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Raper, D.; Louveau, A.; Kipnis, J. How Do Meningeal Lymhatic Vessels Drain the CNS? Trends Neurosci. 2016, 39, 581–586. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a 'glymphatic' system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef]
- Sykova, E.; Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef]
- Bloomfield, I.G.; Johnston, I.H.; Bilston, L.E. Effects of proteins, blood cells and glucose on the viscosity of cerebrospinal fluid. Pediatr. Neurosurg. 1998, 28, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Greiner, S.T.; Gashev, A.A.; Cote, G.L.; Moore, J.E.; Zawieja, D.C. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 2006, 13, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Khaled, A.R.A.; Vafai, K. The role of porous media in modeling flow and heat transfer in biological tissues. Int. J. Heat Mass Transf. 2003, 46, 4989–5003. [Google Scholar] [CrossRef]
- Brinkman, H.C. A calculation of the viscous force exerted by a flowing fluid on a dense swarm of particles. Appl. Sci. Res. Sect. A Mech. Heat Chem. Eng. Math. Methods 1947, 1, 27–34. [Google Scholar] [CrossRef]
- Nicholson, C.; Hrabetova, S. Brain Extracellular Space: The Final Frontier of Neuroscience. Biophys. J. 2017, 113, 2133–2142. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O'Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, C. Diffusion and related transport mechanisms in brain tissue. Rep. Prog. Phys. 2001, 64, 815–884. [Google Scholar] [CrossRef]
- Mestre, H.; Tithof, J.; Du, T.; Song, W.; Peng, W.G.; Sweeney, A.M.; Olyeda, G.; Thomas, J.H.; Nedergaard, M.; Kelley, D.H. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 2018, 9, 4878. [Google Scholar] [CrossRef]
- Bedussi, B.; Almasian, M.; de Vos, J.; VanBavel, E.; Bakker, E. Paravascular spaces at the brain surface: Low resistance pathways for cerebrospinal fluid flow. J. Cereb. Blood Flow Metab. 2018, 38, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Asgari, M.; de Zelicourt, D.; Kurtcuoglu, V. Glymphatic solute transport does not require bulk flow. Sci. Rep. 2016, 6, 38635. [Google Scholar] [CrossRef] [Green Version]
- Basser, P.J. Interstitial pressure, volume, and flow during infusion into brain-tissue. Microvasc. Res. 1992, 44, 143–165. [Google Scholar] [CrossRef]
- Smith, J.H.; Humphrey, J.A.C. Interstitial transport and transvascular fluid exchange during infusion into brain and tumor tissue. Microvasc. Res. 2007, 73, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.-J.; Smith, A.J.; Verkman, A.S. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J. Gen. Physiol. 2016, 148, 489–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holter, K.E.; Kehlet, B.; Devor, A.; Sejnowski, T.J.; Dale, A.M.; Omholt, S.W.; Ottersen, O.P.; Nagelhus, E.A.; Mardal, K.A.; Pettersen, K.H. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc. Natl. Acad. Sci. USA 2017, 114, 9894–9899. [Google Scholar] [CrossRef] [Green Version]
- Ray, L.; Iliff, J.J.; Heys, J.J. Analysis of convective and diffusive transport in the brain interstitium. Fluids Barriers CNS 2019, 16, 6. [Google Scholar] [CrossRef] [Green Version]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The Perivascular Astroglial Sheath Provides a Complete Covering of the Brain Microvessels: An Electron Microscopic 3D Reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef]
- Korogod, N.; Petersen, C.C.H.; Knott, G.W. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. Elife 2015, 4, e05793. [Google Scholar] [CrossRef]
- Cserr, H.F.; Ostrach, L.H. Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp. Neurol. 1974, 45, 50–60. [Google Scholar] [CrossRef]
- Rennels, M.L.; Gregory, T.F.; Blaumanis, O.R.; Fujimoto, K.; Grady, P.A. Evidence for a paravascular fluid circulation in the mammalian central nervous-system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985, 326, 47–63. [Google Scholar] [CrossRef]
- Wisniewski, H.M.; Wegiel, J. Beta-Amyloid Formation by Myocytes of Leptomeningeal Vessels. Acta Neuropathol. 1994, 87, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Massey, A.; Newman, T.A.; Hutchings, M.; Kuo, Y.M.; Roher, A.E. Cerebral amyloid angiopathy: Amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am. J. Pathol. 1998, 153, 725–733. [Google Scholar] [CrossRef]
- Preston, S.D.; Steart, P.V.; Wilkinson, A.; Nicoll, J.A.R.; Weller, R.O. Capillary and arterial cerebral amyloid angiopathy in Alzheimer's disease: Defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol. Appl. Neurobiol. 2003, 29, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.R.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Schley, D.; Carare-Nnadi, R.; Please, C.P.; Perry, V.H.; Weller, R.O. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 2006, 238, 962–974. [Google Scholar] [CrossRef]
- Weller, R.O.; Djuanda, E.; Yow, H.Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef]
- Sharp, M.K.; Diem, A.K.; Weller, R.O.; Carare, R.O. Peristalsis with Oscillating Flow Resistance: A Mechanism for Periarterial Clearance of Amyloid Beta from the Brain. Ann. Biomed. Eng. 2016, 44, 1553–1565. [Google Scholar] [CrossRef]
- Coloma, M.; Schaffer, J.D.; Carare, R.O.; Chiarot, P.R.; Huang, P. Pulsations with reflected boundary waves: A hydrodynamic reverse transport mechanism for perivascular drainage in the brain. J. Math. Biol. 2016, 73, 469–490. [Google Scholar] [CrossRef]
- Faghih, M.M.; Sharp, M.K. Is bulk flow plausible in perivascular, paravascular and paravenous channels? Fluids Barriers CNS 2018, 15, 17. [Google Scholar] [CrossRef]
- Nielsen, S.; Nagelhus, E.A.; AmiryMoghaddam, M.; Bourque, C.; Agre, P.; Ottersen, O.P. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997, 17, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Yao, X.M.; Dix, J.A.; Jin, B.J.; Verkman, A.S. Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 2017, 6, e27679. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Ringstad, G.; Vatnehol, S.A.S.; Eide, P.K. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017, 140, 2691–2705. [Google Scholar] [CrossRef] [Green Version]
- Bedussi, B.; van der Wel, N.N.; de Vos, J.; van Veen, H.; Siebes, M.; VanBavel, E.; Bakker, E. Paravascular channels, cisterns, and the subarachnoid space in the rat brain: A single compartment with preferential pathways. J. Cereb. Blood Flow Metab. 2017, 37, 1374–1385. [Google Scholar] [CrossRef] [Green Version]
- Trumbore, C.N. Shear-Induced Amyloid Formation in the Brain: III. The Roles of Shear Energy and Seeding in a Proposed Shear Model. J. Alzheimers Dis. 2018, 65, 47–70. [Google Scholar] [CrossRef] [Green Version]
- Albargothy, N.J.; Johnston, D.A.; MacGregor-Sharp, M.; Weller, R.O.; Verma, A.; Hawkes, C.A.; Carare, R.O. Convective influx/glymphatic system: Tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018, 136, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Arbel-Ornath, M.; Hudry, E.; Eikermann-Haerter, K.; Hou, S.; Gregory, J.L.; Zhao, L.Z.; Betensky, R.A.; Frosch, M.P.; Greenberg, S.M.; Bacskai, B.J. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer's disease mouse models. Acta Neuropathol. 2013, 126, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, M.W.B.; Cserr, H.F.; Westrop, R.J. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol. 1981, 240, F329–F336. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gakuba, C.; Gaberel, T.; Goursaud, S.; Bourges, J.; Di Palma, C.; Quenault, A.; de Lizarrondo, S.M.; Vivien, D.; Gauberti, M. General Anesthesia Inhibits the Activity of the “Glymphatic System”. Theranostics 2018, 8, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Dobson, H.; Sharp, M.M.; Cumpsty, R.; Criswell, T.P.; Wellman, T.; Finucane, C.; Sullivan, J.M.; Weller, R.O.; Verma, A.; Carare, R.O. The perivascular pathways for influx of cerebrospinal fluid are most efficient in the midbrain. Clin. Sci. 2017, 131, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Stemklein, J.M.; Waldman, L.; Zhou, X.; Filippi, C.G. Measuring Glymphatic Flow in Man Using Quantitative Contrast-Enhanced MRI. Am. J. Neuroradiol. 2019, 40, 648–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokri-Kojori, E.; Wang, G.J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. Beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef] [Green Version]
- de Leon, M.J.; Li, Y.; Okamura, N.; Tsui, W.H.; Saint-Louis, L.A.; Glodzik, L.; Osorio, R.S.; Fortea, J.; Butler, T.; Pirraglia, E.; et al. Cerebrospinal Fluid Clearance in Alzheimer Disease Measured with Dynamic PET. J. Nucl. Med. 2017, 58, 1471–1476. [Google Scholar] [CrossRef] [Green Version]
- Plog, B.A.; Mestre, H.; Olveda, G.E.; Sweeney, A.M.; Kenney, H.M.; Cove, A.; Dholakia, K.Y.; Tithof, J.; Nevins, T.D.; Lundgaard, I.; et al. Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, M.E.; Thorne, R.G. The Extracellular and Perivascular Spaces of the Brain. In Brain Edema: From Molecular Mechanisms to Clinical Practice; Badaut, J., Plesnila, N., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 105–127. [Google Scholar]
- Louveau, A.; Plog, B.A.; Antila, S.; Alitalo, K.; Nedergaard, M.; Kipnis, J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Investig. 2017, 127, 3210–3219. [Google Scholar] [CrossRef] [Green Version]
- Koh, L.; Zakharov, A.; Johnston, M. Integration of the subarachnoid space and lymphatics: Is it time to embrace a new concept of cerebrospinal fluid absorption? Fluids Barriers CNS 2005, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Kida, S.; Pantazis, A.; Weller, R.O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993, 19, 480–488. [Google Scholar] [CrossRef]
- Kaminski, M.; Bechmann, I.; Pohland, M.; Kiwit, J.; Nitsch, R.; Glumm, J. Migration of monocytes after intracerebral injection at entorhinal cortex lesion site. J. Leukoc. Biol. 2012, 92, 31–39. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Hawkes, C.A.; Jayakody, N.; Johnston, D.A.; Bechmann, I.; Carare, R.O. Failure of Perivascular Drainage of beta-amyloid in Cerebral Amyloid Angiopathy. Brain Pathol. 2014, 24, 396–403. [Google Scholar] [CrossRef]
- Zhang, E.T.; Inman, C.B.E.; Weller, R.O. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J. Anat. 1990, 170, 111–123. [Google Scholar]
- Cserr, H.F.; Harlingberg, C.J.; Knopf, P.M. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992, 2, 269–276. [Google Scholar] [CrossRef]
- Cserr, H.F.; Knopf, P.M. Cervical lymphatics, the blood-brain-barrier and the immunoreactivity of the brain: A new view. Immunol. Today 1992, 13, 507–512. [Google Scholar] [CrossRef]
- Antila, S.; Karaman, S.; Nurmi, H.; Airavaara, M.; Voutilainen, M.H.; Mathivet, T.; Chilov, D.; Li, Z.L.; Koppinen, T.; Park, J.H.; et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017, 214, 3645–3667. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Brain Drain (vol 314, pg 44, 2016). Sci. Am. 2016, 315, 6. [Google Scholar]
- Vorisek, I.; Sykova, E. Measuring diffusion parameters in the brain: Comparing the real-time iontophoretic method and diffusion-weighted magnetic resonance. Acta Physiol. 2009, 195, 101–110. [Google Scholar] [CrossRef]
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.A.; Rosen, B.R.; Polimeni, J.R.; Lewis, L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631. [Google Scholar] [CrossRef]
- Sweetman, B.; Xenos, M.; Zitella, L.; Linninger, A.A. Three-dimensional computational prediction of cerebrospinal fluid flow in the human brain. Comput. Biol. Med. 2011, 41, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral Arterial Pulsation Drives Paravascular CSF-Interstitial Fluid Exchange in the Murine Brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.H.; Jaffrin, M.Y.; Weinberg, S.L. Peristaltic pumping with long wavelengths at low reynolds number. J. Fluid Mech. 1969, 37, 799–825. [Google Scholar] [CrossRef]
- Bilston, L.E.; Fletcher, D.F.; Brodbelt, A.R.; Stoodley, M.A. Arterial Pulsation-driven Cerecrospinal Fluid Flow in the Perivascular Space: A Computational Model. Comput. Methods Biomech. Biomed. Eng. 2003, 6, 235–241. [Google Scholar] [CrossRef]
- Mitchell, G.F.; van Buchem, M.A.; Sigurdsson, S.; Gotal, J.D.; Jonsdottir, M.K.; Kjartansson, O.; Garcia, M.; Aspelund, T.; Harris, T.B.; Gudnason, V.; et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility-Reykjavik Study. Brain 2011, 134, 3398–3407. [Google Scholar] [CrossRef]
- Croci, M.; Vinje, V.; Rognes, M.E. Uncertainty quantification of parenchymal tracer distribution using random diffusion and convective velocity fields. Fluids Barriers CNS 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Mestre, H.; Kostrikov, S.; Mehta, R.I.; Nedergaard, M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. 2017, 131, 2257–2274. [Google Scholar] [CrossRef] [Green Version]
- Thorne, R.G.; Nicholson, C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl. Acad. Sci. USA 2006, 103, 5567–5572. [Google Scholar] [CrossRef] [Green Version]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of Paravascular Clearance Pathways in the Aging Brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Cserr, H.F.; Depasquale, M.; Nicholson, C.; Patlak, C.S.; Pettigrew, K.D.; Rice, M.E. Extracellular volume decreases while cell volume is maintained by ion uptake in rat brain during acute hypernatremia. J. Physiol. Lond. 1991, 442, 277–295. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Hrabetova, S.; Nicholson, C.; Manley, G.T. Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J. Neurosci. 2008, 28, 5460–5464. [Google Scholar] [CrossRef] [Green Version]
- Binder, D.K.; Papadopoulos, M.C.; Haggie, P.M.; Verkman, A.S. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J. Neurosci. 2004, 24, 8049–8056. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Manley, G.T.; Krishna, S.; Verkman, A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004, 18, 1291–1293. [Google Scholar] [CrossRef]
- Asgari, M.; de Zelicourt, D.; Kurtcuoglu, V. How astrocyte networks may contribute to cerebral metabolite clearance. Sci. Rep. 2015, 5, 15024. [Google Scholar] [CrossRef] [Green Version]
- Ringstad, G.; Valnes, L.M.; Dale, A.M.; Pripp, A.H.; Vatnehol, S.A.S.; Emblem, K.E.; Mardal, K.A.; Eide, P.K. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Absinta, M.; Ha, S.K.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 2017, 6, e29738. [Google Scholar] [CrossRef]
- Mazel, T.; Simonova, Z.; Sykova, E. Diffusion heterogeneity and anisotropy in rat hippocampus. Neuroreport 1998, 9, 1299–1304. [Google Scholar] [CrossRef]
- Ooms, S.; Overeem, S.; Besse, K.; Rikkert, M.O.; Verbeek, M.; Claassen, J. Effect of 1 Night of Total Sleep Deprivation on Cerebrospinal Fluid beta-Amyloid 42 in Healthy Middle-Aged Men A Randomized Clinical Trial. Jama Neurol. 2014, 71, 971–977. [Google Scholar] [CrossRef]
- Ju, Y.E.; Ooms, S.J.; Sutphen, C.; Macauley, S.L.; Zangrilli, M.A.; Jerome, G.; Fagan, A.M.; Mignot, E.; Zempel, J.M.; Claassen, J.; et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain 2017, 140, 2104–2111. [Google Scholar] [CrossRef]
- Nakamura, K.; Brown, R.A.; Narayanan, S.; Collins, D.L.; Arnold, D.L.; Alzheimer’s Dis, N. Diurnal fluctuations in brain volume: Statistical analyses of MRI from large populations. Neuroimage 2015, 118, 126–132. [Google Scholar] [CrossRef]
- Bejan, A. Shape and Structure, from Engineering to Nature; Cambridge University Press: New York, NY, USA, 2000. [Google Scholar]
- Levenspiel, O.; (Oregon State University, Corvallis, OR, USA). Personal communication, 1979.
- Cieslicki, K. Experimental and Numerical Modeling of flow in the Human Cerebral Arteries. J. Med. Informat. Technol. 2004, 7, 17–26. [Google Scholar]
- Kamath, S. Observations on the length and diameter of vessels forming the circle of Willis. J. Anat. 1981, 133, 419–423. [Google Scholar]
- Groothuis, D.R.; Vavra, M.W.; Schlageter, K.E.; Kang, E.W.Y.; Itskovich, A.C.; Hertzler, S.; Allen, C.V.; Lipton, H.L. Efflux of drugs and solutes from brain: The interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J. Cereb. Blood Flow Metab. 2007, 27, 43–56. [Google Scholar] [CrossRef]
- Xiong, B.; Li, A.; Lou, Y.; Chen, S.; Long, B.; Peng, J.; Yang, Z.; Xu, T.; Yang, X.; Li, X.; et al. Precise Cerebral Vascular Atlas in Stereotaxic Coordinates of Whole Mouse Brain. Front. Neuroanat. 2017, 11, 128. [Google Scholar] [CrossRef] [Green Version]





| Acronym | Description | Acronym | Description |
|---|---|---|---|
| ADCw | apparent diffusion coefficient of water, as measured by MRI | iNHP | idiopathic normal-pressure hydrocephalus |
| AQP | aquaporin, protein channel for water transport | IOI | integrated optical imaging |
| AQP4 | aquaporin-4 | ISF | interstitial fluid |
| BBB | blood–brain barrier | KO | knock-out, genetically altered animal model |
| CSF | cerebrospinal fluid | NREM | non-rapid eye movement sleep |
| DCE MRI | dynamic contrast-enhanced MRI | Pe | Peclet number, advective rate divided by diffusive rate |
| DTI MRI | diffusion tensor imaging MRI | PET | positron emission tomography |
| ECM | extracellular matrix | PVS | perivascular space |
| ECoG | electrocorticography | PVW | perivascular wall |
| ECS | extracellular space | Re | Reynolds number, inertial forces divided by viscous forces |
| EM | electron micrograph | RTI | real-time iontophoresis |
| EMG | electromyography | SAS | sub-arachnoid space |
| GAG | glycosaminoglycans | TBI | traumatic brain injury |
| ICP | intracranial pressure | WT | wild-type, unaltered animal model |
| Parameter | Symbol | Description | Values, References |
|---|---|---|---|
| Void Volume | Percentage of tissue volume that is extracellular | 20% (anesthetized) [36] 14% (awake) [37] | |
| Tortuosity | λ | Degree to which molecular transport is slowed by the porous medium | 1.6 (small molecules) [36] 1.5–2.5 (large molecules) [38] |
| Perivascular Velocity | Average fluid velocity in the perivascular space | 17–19 μm s−1 (experiment) [39,40] ≈0 μm s−1 (computation) [41] | |
| Hydraulic Conductivity (Interstitial) | Ease with which a fluid can move through a porous media. Equation (2). | 2 × 10−6–2 × 10−8 cm2 mmHg−1 s−1 [42,43,44,45] | |
| Perivascular Wall Permeability | Quality of a material that allows liquids or gases to pass through it. | 0.6% [46,47] [46,48] 3 × 10−5 − 5 × 10−3 cm s−1 for Dex 3 kDa |
| State | Void Volume | Hydraulic Conductivity (cm2 mmHg−1 s−1) | Pavg (mmHg) | |
|---|---|---|---|---|
| Asleep | 0.23 [37] | 2 × 10−6 [43,44] | 0.5 | 15 [46] |
| Awake | 0.14 [37] | 2 × 10−7 | 0.5 | <1 [46] |
| Bilston et al. [95] (Computational Model) | Asgari et al. [41] (Computational Model) | Mestre et al. [39] (Experimental Observations) | |
|---|---|---|---|
| Model Dimensions | 2-D | 3-D axisymmetric | - |
| Theoretical Equation | Navier–Stokes | Navier–Stokes with porous media term | - |
| Solution Method | Finite-volume, moving mesh (CFX4) | Finite-volume (OpenFOAM) | Particle tracking of fluorescent microspheres |
| Boundary Conditions | Inlet pressure set | Zero net flow | Pressure not measured |
| Perivascular Geometry | Shape = Annular PV Width = 25 μm 100 μm | Shape = Annular PV Width = 10 μm 23 μm | Shape = Annular, with an elliptical outer surface with a high eccentricity PV Width = 40 μm 40 μm |
| Arterial Pulse Wave | Amplitude = 10% of arterial radius; Speed = 5 m/s; Wavelength = 100 μm; Shape = sinusoidal | Amplitude = 4% of arterial radius; Frequency = 10/s; Speed = 1 m/s; Wavelength = 0.1 m; Shape = sinusoidal | Amplitude = 2% of arterial radius; Frequency = 5/s; Shape = fast increase during systole and slow decrease during diastole (not sinusoidal) |
| Results | - | - | - |
| Flow Rate and Velocity | Q = 1.35 mm3 s−1 1.4 × 105 μm s−1 | Q = 3.7 × 10−10 mm3 s−1 = negligible | Q = 0.005 mm3 s−1 = 18.7 1 μm s−1 |
| Conclusions | Arterial pulsations are able to drive perivascular flow (for a much shorter than observed wavelength). | Arterial pulsation unlikely to drive perivascular flow; Dispersion may accelerate transport beyond diffusion only. | Measured periarterial flow was pulsatile, correlated with the cardiac cycle, parabolic (laminar), and net antegrade (in the direction of blood flow). |
| Jin et al. [44] | Holter et al. [45] | Ray et al. [46] | |
|---|---|---|---|
| Model Dimensions | 2-D | 3-D | 3-D |
| Theoretical Equation | Navier–Stokes in reconstructed ECS | Navier–Stokes in reconstructed ECS | Darcy’s Law, combined with Mass Transfer in porous media |
| Solution Method | Finite-element (COMSOL) | Finite-element (FEniCS) | Finite-element (FEniCS) |
| Boundary Conditions | Pressure Gradient = 1 mmHg mm−1 | Pressure Difference = 0–8 mmHg | Pressure Difference = 0–10 mmHg |
| Interstitial Geometry | For flow: EM of Kinney et al. [2] adjusted to increase void volume to around 20% For mass: vascular separation = 280 μm (center-to-center) = 30 μm | For flow: EM of Kinney et al. [2] adjusted to increase void volume to around 20% For mass: vascular separation = 285 μm (center-to-center) 30 μm 40 μm 24 nm | For flow and mass: vascular separation = 255–305 μm (center-to-center) 30 μm 30 μm 24 nm 18–23% (depending on experimental data) = 2 × 10−6 − 2 × 10−8 cm2 mmHg−1 s−1 |
| Validation | Comparison to experimental values of , which were found to be significantly higher. Mass transport results compatible with Xie et al. [37] tracer studies. | Mass transport results compatible with Iliff et al. [71] tracer studies and Thorne et al. [99] IOI experiments. | Quantitative comparison to published experimental RTI curve replicates [37,100,101]. |
| Results | - | - | - |
| Hydraulic Conductivity | = 2 × 10−8 cm2 mmHg−1 s−1 | = 1.2 × 10−6 cm2 mmHg−1 s−1 | - |
| Interstitial Velocity | 0.1 μm min−1 | - | 10 μm min−1 |
| Conclusions | Interstitial transport is dominated by diffusion. | Interstitial transport observations adequately explained by diffusion. | RTI experimental data range is consistent with simulations for velocities of order 10 μm min−1. |
| Hydraulic Conductivity (cm2 mmHg−1 s−1) | For Pavg = 0.2 mmHg | For Pavg = 0.8 mmHg | For Pavg = 2.4 mmHg | For Pavg = 8 mmHg |
|---|---|---|---|---|
| 2 × 10−6 [43,44] 1 | 5 [44], 5 | 22 [44], 25 | 65 [44], 75 | 220 [44], 250 |
| 2 × 10−7 [42] | 0.5 | 2.5 | 7.5 | 25 |
| 2 × 10−8 [45] | 0.1 [45], 0.05 | 0.25 | 0.75 | 2.5 |
| Description | Periarterial Advection | Interstitial Advection | Interstitial Diffusion |
|---|---|---|---|
| Length (mm) | 9.5 1 | 0.2 | 0.2 |
| Velocity (μm s−1) | 18 [39,40] | 0.2 [46] | |
| Apparent Diffusivity (cm2 s−1) | 10−8–10−5 | ||
| Characteristic time (τ) (min) | 9 | 19 | 1–10 small molecules 10–1000 large molecules |
| Description | Periarterial | Interstitial—Anesthetized/Asleep | Interstitial—Awake |
|---|---|---|---|
| Total Cross-Sectional Area (mm2) | 0.2 | 200–500 | 200–500 |
| Velocity (um/sec) | 18 [39,40] | 0.2 [46] | 0.01 [46] |
| Total Volumetric Flow Rate (μL min−1) | 0.2 | 2–6 | 0.2–0.4 |
| Calculated Perfusion 1 Rate (μL g−1 min−1) | 0.5 | 5–15 | 0.4–1 |
| Literature Perfusion Rate1 (μL g−1 min−1) | 0.3–5.5 [37] 0.2–1.2 [116] | 2.8–5.5 [37] 0.8–1.2 [116] | 0.3–1.5 [37] 0.2–0.5 [116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, L.A.; Heys, J.J. Fluid Flow and Mass Transport in Brain Tissue. Fluids 2019, 4, 196. https://doi.org/10.3390/fluids4040196
Ray LA, Heys JJ. Fluid Flow and Mass Transport in Brain Tissue. Fluids. 2019; 4(4):196. https://doi.org/10.3390/fluids4040196
Chicago/Turabian StyleRay, Lori A., and Jeffrey J. Heys. 2019. "Fluid Flow and Mass Transport in Brain Tissue" Fluids 4, no. 4: 196. https://doi.org/10.3390/fluids4040196





