Crosstalk between Non-Coding RNAs and Wnt/β-Catenin Signaling in Head and Neck Cancer: Identification of Novel Biomarkers and Therapeutic Agents
Abstract
:1. Introduction
2. Wnt Signaling and Cancer
3. Wnt Signaling in HNC
4. Crosstalk between ncRNAs and WBC Signaling in HNSCC
4.1. Interplay between ncRNAs and the WBC Pathway in Modulating Cell Proliferation and Survival
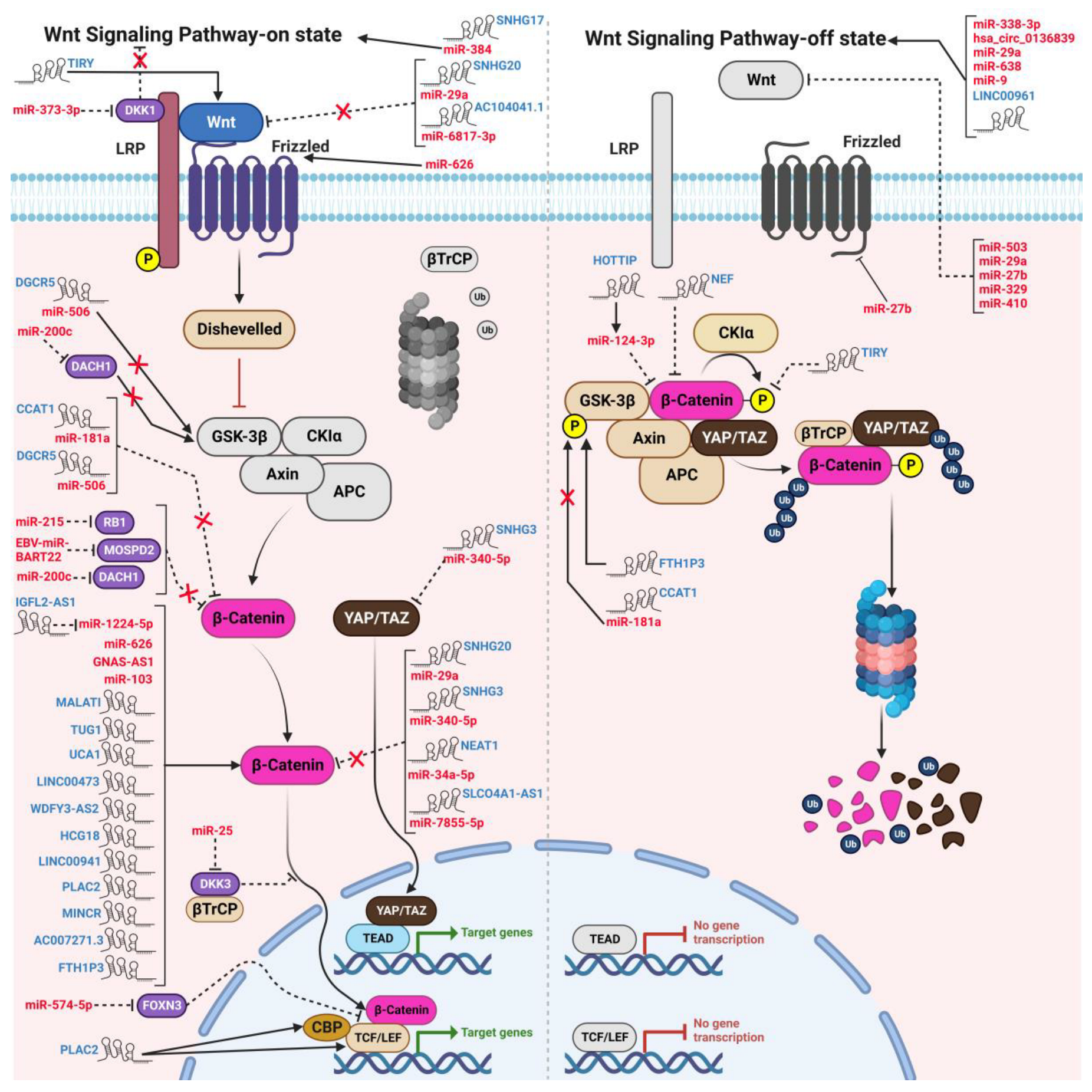

| ncRNA | Type of Study | Cell Line/Cancer Model | Target | Mechanism/Mode of Action | Reference |
|---|---|---|---|---|---|
| Nasopharyngeal Cancer | |||||
| EBV-miR-BART22 b | In vitro | CNE1, CNE2, SUNE1 (Overexpression) | MOSPD2 | ↑Cell migration, invasion, N-cadherin, vimentin, Snail, β-catenin, EMT ↓E-cadherin, MOSPD2 | [105] |
| EBV-miR-BART22 b | In vitro | C666-1 (siRNA-mediated knockdown) | - | ↑E-cadherin, MOSPD2 ↓Cell invasion, migration, N-cadherin, vimentin, Snail, β-catenin, EMT | [105] |
| EBV-miR-BART22 b | In vivo | Hepatic metastasis BALB/c nude mice model (Overexpression) | - | ↑Cell motility, tumor invasiveness | [105] |
| miR-25 b | In vitro | HONE-1 (miRNA inhibitor) | DKK3 | ↑Apoptosis, DKK3 ↓Colony formation | [74] |
| miR-25 b | In vitro | HONE-1 (Overexpression) | - | ↑TCF4, c-Myc, Cyclin D1 | [74] |
| miR-215 b | In vitro | C666-1 (Overexpression) | RB1 | ↑Cell proliferation, migration, EMT, N-cadherin, vimentin, p-β-catenin ↓RB1, E-cadherin | [75] |
| miR-215 b | In vitro | C666-1 (miRNA inhibitor) | RB1 | ↑RB1, E-cadherin ↓Cell proliferation, migration, p-β-catenin, N-cadherin, vimentin | [75] |
| miR-103 b | In vitro | CNE1, SUNE1 (Overexpression) | TIMP3 | ↑β-catenin, CyclinD1, invasion, migration, proliferation ↓TIMP3 | [106] |
| GNAS-AS1 a | In vitro | SUNE1 (siRNA-mediated knockdown) | β-catenin | ↓Cell proliferation, c-Myc, Cyclin D, MMP-2, β-catenin, invasion, migration | [107] |
| miR-574-5p b | In vitro | C666-1 (Overexpression) | FOXN3 | ↑Cell viability, β-catenin, TCF4, invasion, metastasis ↓ FOXN3 | [108] |
| HCG18 a | In vitro | SUNE1, CNE2 (siRNA-mediated knockdown) | miR-140 | ↑miR-140, apoptosis, Caspase-3 and 9 ↓Cell growth, migration, invasion, Cyclin D1, β-catenin, c-Myc, Hedgehog signaling | [80] |
| miR-140 b | In vitro | SUNE1, CNE2 (Overexpression) | HCG18 | ↓HCG18, Cyclin D1 | [80] |
| miR-200c b | In vitro | CNE2, SUNE1 (miR-200c-inhibitor) | DACH1 | ↑DACH1 ↓Cell proliferation, colony number, migration, β-catenin, c-Myc, GSK3β, Cyclin D1 | [83] |
| NEAT1 a | In vitro | CNE1, CNE2, SUNE1, SUNE2, 5-8F | miR-34a-5p | ↓miR-34a-5p | [109] |
| NEAT1 a | In vitro | 5-8F (siRNA-mediated knockdown) | miR-34a-5p | ↑ miR-34a-5p, E-cadherin ↓β-catenin, Cyclin D1, and c-Myc, N-cadherin, vimentin, cell proliferation, invasion, migration, EMT | [109] |
| NEAT1 a | In vivo | SCID mouse xenografts (5-8F (shRNA mediated knockdown) xenografts) | miR-34a-5p | ↑ miR-34a-5p, E-cadherin ↓Tumor growth, β-catenin, Cyclin D1, c-Myc, N-cadherin, vimentin | [109] |
| Laryngeal Cancer | |||||
| SLCO4A1-AS1 a | In vitro | SNU46, TU177 (shRNA-mediated knockdown) | miR-7855-5p | ↑miR-7855-5p ↓Cell proliferation, colony formation, β-catenin, Cyclin D1, c-Myc | [70] |
| SNHG3 a | In vitro | TU177, AMC-HN-8 (shRNA-mediated knockdown) | - | ↑Apoptosis, miR-340-5p, E-cadherin ↓Cell viability, glycolysis, YAP1, β-catenin, c-Myc, Bcl-2 | [85] |
| SNHG3 a | In vivo | BALB/c nude mice xenograft (shRNA-mediated knockdown) | - | ↑miR-340-5p ↓Tumor volume, weight, YAP1 | [85] |
| UCA1 a | In vitro | AMC-HN-8 (Overexpression) | - | ↑Cell proliferation, invasion, migration, β-catenin ↓ p-GSK3β | [76] |
| UCA1 a | In vitro | AMC-HN-8 (siRNA-mediated knockdown) | - | ↓Cell proliferation, invasion, migration | [76] |
| DGCR5 a | In vitro | Hep2R | miR-506 | ↑DGCR5, ↓miR-506, CSC-like phenotype | [87] |
| DGCR5 a | In vitro | Hep2R (siRNA-mediated knockdown) | miR-506 | ↑GSK3β, ↓Sox2, Oct4, Nanog, spheroid formation, β-catenin, Cyclin D1 | [87] |
| DGCR5 a | In vitro | Hep2R (siRNA-mediated knockdown and Radiation) | - | ↓Radioresistance | [87] |
| miR-506 b | In vitro | Hep2R (Overexpression) | - | ↓Sox2, Oct4, Nanog, β-catenin, Cyclin D1 | [87] |
| miR-506 b | In vitro | Hep2R (Overexpression and Radiation) | - | ↓Radioresistance | [87] |
| LINC00473 a | In vitro | SCC25, CAL27 (shRNA-mediated knockdown) | - | ↑Apoptosis, Bax, ↓Cell viability, colony number, Bcl-2, β-catenin, c-Myc | [65] |
| LINC00473 a | In vitro | SCC9 (Overexpression) | - | ↑ Cell viability, colony number, Bcl-2 ↓Bax, Apoptosis | [65] |
| LINC00473 a | In vitro | SCC25, CAL27 (shRNA-mediated knockdown and radiation) | - | ↑Apoptosis, Bax, ↓Cell viability, colony number, Bcl-2, β-catenin, c-Myc | [65] |
| Oral Cancer | |||||
| WDFY3-AS2 a | In vitro | CAL27, SCC9 (siRNA-mediated knockdown) | - | ↑E-cadherin ↓Cell proliferation, invasion, migration, vimentin, β-catenin, Myc, Slug | [72] |
| IGFL2-AS1 a | In vitro | CAL-27, SCC-15, SCC-9, SCC-4 (shRNA-mediated knockdown) | miR-1224-5p | ↑E-cadherin ↓Cell proliferation, invasion, migration, EMT, nuclear β-catenin, c-Myc, Cyclin D1, MMP-7 | [71] |
| HCG18 a | In vitro | HN30, SCC-4 (Overexpression) | - | ↑Cell proliferation, migration, invasion, Cyclin D1 | [62] |
| HCG18 a | In vitro | HN30, SCC-4 (siRNA-mediated knockdown) | - | ↓Cell invasion, migration, AXIN2, c-Myc, survivin, Cyclin D1, β-catenin | [62] |
| HCG18 a | In vivo | Nude mice xenograft (Overexpression) | - | ↑Tumor weight, volume | [62] |
| SNHG17 a | In vitro | YD-38, SCC-9 (siRNA-mediated knockdown) | miR-384 | ↑Apoptosis ↓Cell proliferation, viability, CTNNB1, ELF1, Wnt/β-catenin signaling | [78] |
| miR-626 b | In vitro | Ca9-22, HSC2 (miRNA inhibitor) | - | ↑RASSF4, E-cadherin ↓vimentin, N-cadherin, invasion, migration, FZD1, β-catenin | [110] |
| miR-626 b | In vitro | Ca9-22, HSC2 (Overexpression) | RASSF4 | ↑Invasion, migration, N-cadherin, β-catenin, FZD1 ↓E-cadherin | [110] |
| IGF2BP2-AS1 a | In vitro | CAL27, SCC-9 (knockdown) | - | ↑G1 phase arrest, apoptosis, Bax ↓Cell proliferation, colony formation, β-catenin, Cyclin D1, Bcl-2, MMP-2 | [111] |
| LINC00941 a | In vitro | HSC-3, OSC-19 (dCas9 tagged with KRAB-MeCP2) | - | ↓Cell proliferation, colony formation, cell number, CAPRIN2, β-catenin, p-LRP6, MYC, CCND1, SOX9 | [69] |
| LINC00941 a | In vivo | Nude mice (HSC-3 xenograft dCas9 tagged with KRAB-MeCP2) | - | ↓ Tumor formation, tumor weight | [69] |
| SNHG20 a | In vitro | SCC-9 (siRNA-mediated knockdown) | miR-29a | ↑Apoptosis, miR-29a ↓Cell viability, invasion, migration, Wnt-3a, β-catenin | [82] |
| miR-29a b | In vitro | SCC-9 (Overexpression) | - | ↓Cell viability, invasion, migration, Wnt-3a, β-catenin | [82] |
| miR-29a b | In vitro | SCC-9 (miRNA inhibitor) | - | ↑SNHG20 | [82] |
| TIRY a | In vitro | Oral CAFs (Overexpression) | - | ↑Snail, Zeb1, α-SMA, β-catenin ↓miR-14 | [77] |
| TIRY a | In vitro | Tca8113 (CAF-conditioned media) (Overexpression) | - | ↑Invasion, metastasis, Snail, Wnt-3a ↓Phosphorylation of β-catenin | [77] |
| TIRY a | In vitro | Tca8113 (CAF-conditioned media) (siRNA-mediated knockdown) | - | ↑miR-14 | [77] |
| miR-14 a | In vitro | Tca8113 (CAF-conditioned media) (Overexpression) | - | ↓Invasion, metastasis | [77] |
| HOTTIP a | In vitro | SCC25, UM1 (siRNA-mediated knockdown) | miR-124-3p | ↑miR-124-3p, E-cadherin ↓Cell growth, invasion, migration, β-catenin, c-Myc | [84] |
| HOTTIP a | In vivo | Nude mice (sh-HOTTIP OTSCC xenografts) | - | ↑miR-124-3p, E-cadherin ↓Tumor weight, tumor volume, β-catenin, c-Myc, HMGA2 | [84] |
| AC104041.1 a | In vitro | SCC4 (shRNA-mediated knockdown) | miR-6817-3p | ↓Cell viability, migration, Wnt-2b, β-catenin, c-Myc, vimentin | [79] |
| AC104041.1 a | In vitro | CAL27 (Overexpression) | miR-6817-3p | ↑Cell viability, migration, Wnt-2b | [79] |
| AC104041.1 a | In vivo | BALB/c nude mice (SCC4 xenografts) (shRNA-mediated knockdown) | - | ↓Tumor volume | [79] |
| AC104041.1 a | In vivo | BALB/c nude mice (CAL27 xenografts) (Overexpression) | - | ↑Tumor volume | [79] |
| CCAT1 a | In vitro | KB, Cal-27 (shRNA-mediated knockdown) | miR-181a | ↑Apoptosis, Bax, miR-181a, Caspase-3 and -9 ↓Cell proliferation, colony formation, Bcl-2, Cyclin D1, CDK4, invasion, migration, p-GSK3β, β-catenin and c-Myc | [81] |
| CCAT1 a | In vivo | BALB/c mice with Cal-27 xenograft (shRNA-mediated knockdown) | - | ↓Tumor size, weight, p-GSK-3β, β-catenin, c-Myc, Cyclin D1, Ki-67 | [81] |
| PLAC2 a | In vitro | SCC-9 (Overexpression) | - | ↑Cell proliferation, Ki-67, invasion, migration, β-catenin, TCF-4, MMP-7 and -9, Cyclin D1 | [63] |
| PLAC2 a | In vitro | CAL-27 (siRNA-mediated knockdown) | - | ↓Cell proliferation, Ki-67, Migration, Invasion, β-catenin, TCF-4, MMP-7 and -9, Cyclin D1 | [63] |
| PLAC2 a | In vivo | BALB/c nude mice (SCC-9 xenograft) (Overexpression) | - | ↑Tumor volume, metastasis, PLAC2, CBP, β-catenin | [63] |
| MINCR a | In vitro | SCC-25, TSCCA (shRNA-mediated knockdown) | - | ↑Apoptosis, G0/G1 cell cycle arrest, Cleaved caspase-3 and -9, E-cadherin ↓Cell proliferation, migration, invasion, N-cadherin, β-catenin, c-Myc, Cyclin D1 | [67] |
| AC007271.3 a | In vitro | SCC-9, SCC-15 (siRNA-mediated knockdown) | - | ↑Apoptosis ↓Cell proliferation, cell growth, Colony formation, invasion, migration, β-catenin, c-Myc, Cyclin D1, Bcl-2 | [68] |
| AC007271.3 a | In vitro | SCC-9, SCC-15 (Overexpression) | - | ↑β-catenin, c-Myc, Cyclin D1, Bcl-2 | [68] |
| AC007271.3 a | In vivo | SCC-9 nude mice xenograft (Overexpression) | - | ↑Keratinization, abnormal nuclear division, Ki-67, CD44, β-catenin, c-Myc, Cyclin D1, Bcl-2 | [68] |
| FTH1P3 a | In vitro | SCC-4, SCC-25 (siRNA-mediated knockdown) | - | ↓Cell viability, invasion, β-catenin, p-AKT, p-GSK3β | [112] |
| miR-373-p b | In vitro | SCC-9, UM1 (Overexpression) | DKK1 | ↑N-cadherin, vimentin, Cell invasion, viability, β-catenin ↓E-cadherin, CK18, DKK1 | [113] |
| miR-373-3p b | In vitro | SCC-9, UM1 (miRNA inhibitor) | - | ↓N-cadherin, vimentin, invasion, cell viability, β-catenin | [113] |
| miR-218 b | In vitro | UM1cis, Cal-27cis (anti-miR) | PPP2R5A | ↑Cisplatin sensitivity, apoptosis, PPP2R5A ↓Cell viability, MRP1, ABCG2, p-gp, TopoIIβ, EZH2 | [114] |
| miR-218 b | In vitro | UM1cis (Overexpression) | PPP2R5A | ↑ β-catenin, GSK3β, MRP1, ABCG2, p-gp, TopoIIβ, EZH2, Cell viability, cell growth ↓PPP2R5A | [114] |
| MALAT1 a | In vitro | TSCC (shRNA-mediated knockdown) | - | ↑E-cadherin, Bax, Apoptosis ↓Cell growth, invasion, migration, vimentin, β-catenin | [73] |
| MALAT1 a | In vitro | TSCC (Overexpression) | - | ↑Cell growth, invasion, migration, vimentin, β-catenin ↓E-cadherin, Bax, apoptosis | [73] |
| TUG1 a | In vitro | Tca8113, TSCCA (siRNA-mediated knockdown) | - | ↑Apoptosis, Caspase-3 activity, Cleaved caspase-3 and -9, Bax ↓Cell proliferation, growth, colony formation, invasion, Bcl-2, β-catenin, c-Myc, Cyclin D1 | [66] |
| ncRNA | Type of Study | Cell Line/Cancer Model | Target | Mechanism/Mode of Action | Reference |
|---|---|---|---|---|---|
| Hypopharyngeal Cancer | |||||
| miR-503 b | In vitro | FaDu (Overexpression) | - | ↓Cell invasion, WNT-3A, BCL11B, and CCND2, MMP-3, -7, and -9, FGF7, CTGF | [60] |
| miR-338-3p b | In vitro | FaDu (Overexpression) | ADAM17 | ↓Cell proliferation, ADAM17, cell migration, invasion, cyclin D1, MMP-2, nuclear β-catenin, p-pRb, Wnt/β-catenin | [92] |
| miR-338-3p b | In vitro | FaDu (Inhibitor) | - | ↑β-catenin, cyclin D1, p-pRb, MMP-2, sox-2, Nanog | [92] |
| Laryngeal Cancer | |||||
| miR-384 b | In vitro | TU212, TU686 | WISP1 | ↑Cell apoptosis, DNA fragmentation, Caspase-3 ↓Cell proliferation, WISP-1 | [91] |
| miR-384 b | In vitro | TU212, TU686 (Inhibitor) | - | ↓Caspase-3, DNA fragmentation | [91] |
| NEF a | In vitro | UM-SCC-17A (Overexpression) | - | ↑Cell apoptosis ↓Cell proliferation, β-catenin | [93] |
| Nasopharyngeal Cancer | |||||
| hsa_circ_0136839 c | In vitro | CNE2 (Overexpression) | - | ↓Cell proliferation, invasion, migration colony formation, G0/G1 cell cycle arrest, β-catenin | [56] |
| hsa_circ_0136839 c | In vitro | C666-1 (siRNA-mediated knockdown) | - | ↑Cell proliferation, invasion, migration colony formation, β-catenin, c-Jun, LEF1, CD44, cyclin D1 | [56] |
| Oral Cancer | |||||
| miR-503 b | In vitro | SAS, OECM1 | - | ↓Cell invasion, WNT-3A, BCL11B, CCND2, MMP-3, 7, and 9, FGF7, CTGF | [60] |
| miR-638 b | In vitro | SCC-9 (Overexpression) | PLD1 | ↓Cell proliferation, invasion, migration, PLD1, β-catenin, c-Myc, Cyclin D1 | [115] |
| miR-638 b | In vitro | SCC-9 (Inhibitor) | PLD1 | ↑PLD1, β-catenin, c-Myc, Cyclin D1 | [115] |
| LINC00961 a | In vitro | SCC-1 (Overexpression) | - | ↑E-cadherin ↓Cell proliferation, invasion, migration, vimentin, N-cadherin, Snail, β-catenin | [90] |
| LINC00961 a | In vitro | SCC-1 (shRNA-mediated knockdown) | - | ↑Cell proliferation, Wnt/β-catenin signaling | [90] |
| miR-27b b | In vitro | Tca8113, SCC-4 (Overexpression) | FZD7 | ↓Cell proliferation, FZD7, Wnt, Cyclin D1, c-Myc | [88] |
| miR-9 b | In vitro | Tca8113, SCC-9 (Overexpression) | CXCR4 | ↑Cell apoptosis, G1/S cell cycle arrest ↓Cell proliferation, colony formation, cell invasion, CXCR4, β-catenin, Bcl-2, c-Myc | [86] |
| miR-9 b | In vitro | Nude mice xenograft (Overexpression) | CXCR4 | ↓Tumor growth, CXCR4, Ki-67 | [86] |
| miR-329b/miR-410 b | In vitro | OEC-M1, SCC-15 (Overexpression) | Wnt-7b | ↓Wnt-7b, TCF/LEF1transcriptional activity, cell proliferation, invasion, colony formation, β-catenin, p-GSK3β, c-Myc, Cyclin D1 | [89] |
| miR-329b/miR-410 b | In vitro | OC-3, SCC-4 (miR329-inhibitor/ miR410-inhibitor) | Wnt-7b | ↑Wnt-7b, TCF/LEF1transcriptional activity, β-catenin, c-Myc, Cyclin D1 | [89] |
| miR-329b/miR-410 b | In vivo | OEC-M1 xenograft (overexpression of miR329/miR410) | Wnt-7b | ↓Tumor weight, volume, Wnt-7b, β-catenin | [89] |
4.2. Interplay between ncRNAs and the WBC Pathway in the Modulating EMT, Invasion, and Migration
4.3. Interplay between ncRNAs and the WBC Pathway in Modulating Chemoresistance and Radioresistance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | Alpha smooth muscle actin |
| β-TrCP | Beta-transducin repeat-containing protein |
| ABC | ATP-binding cassette |
| ADAM17 | ADAM metallopeptidase domain 17 |
| AKT | Protein kinase B |
| APC | Adenomatous polyposis coli |
| Bax | Bcl-2-associated X protein |
| BCL11B | B-cell lymphoma/leukemia 11B |
| Bcl-2 | B-cell leukemia/lymphoma 2 protein |
| CD44 | Cluster of differentiation 44 |
| CILA1 | Chemotherapy-induced long non-coding RNA 1 |
| CK1 | Casein kinase 1 |
| c-Myc | Cellular myelocytomatosis oncogene |
| CTGF | Connective tissue growth factor |
| CXCR4 | CXC chemokine receptor 4 |
| DACH1 | Dachshund family transcription factor 1 |
| DGCR5 | DiGeorge syndrome critical region gene 5 |
| DKK1 | Dickkopf-1 |
| DKK3 | Dickkopf Wnt signaling pathway inhibitor 3 |
| ELF1 | E74 Like ETS Transcription Factor 1 |
| EZH2 | Enhancer of zeste homolog 2 |
| FGF7 | Fibroblast growth factor 7 |
| FZD7 | Frizzled 7 |
| GSK3β | Glycogen synthase kinase-3 beta |
| HCG18 | HLA complex group 18 |
| HMGA2 | High mobility group A2 |
| HNC | Head and neck cancer |
| HNSCC | Head and neck squamous cell carcinoma |
| HPV | Human papilloma virus |
| LC | Laryngeal cancer |
| LEF1 | Lymphoid enhancer binding factor 1 |
| LRP | Lipoprotein receptor-related protein |
| LNEC | Laryngeal neuroendocrine carcinoma |
| LNM | Lymph node metastasis |
| MALAT1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 |
| MMP | Matrix metalloproteinase |
| MOSPD2 | Motile sperm domain containing 2 |
| MRP1 | Multidrug resistance-associated protein 1 |
| ncRNA | Non-coding RNA |
| NUAK1 | NUAK family SNF1-like kinase 1 |
| p-gp | P-glycoprotein |
| PLAC2 | Placenta-specific protein 2 |
| PLD1 | Phospholipase D1 |
| PPP2R5A | Protein phosphatase 2 regulatory subunit B’alpha |
| RASSF4 | Ras association domain family member 4 |
| SETD7 | SET domain-containing 7 histone lysine methyl transferase |
| SLCO4A1 | AS1-solute carrier organic anion transporter family member 4A1 antisense RNA 1 |
| snoRNA | Small nucleolar RNA |
| SNHG1 | Small nucleolar RNA host gene 17 |
| SNHG3 | Small nucleolar RNA host gene 3 |
| TCF/LEF | T-cell factor/lymphoid enhancer factor 1 |
| TCF4 | Transcription factor 4 |
| TIMP-3 | Tissue inhibitor of metalloproteinases-3 |
| TopoII | topoisomerase IIβ |
| TSCC | Tongue squamous cell carcinoma |
| TUG1 | Taurine upregulated gene 1 |
| UCA1 | Urothelial Carcinoma Associated 1 |
| WBC | Wnt/β-catenin |
| WIF1 | Wnt inhibitory factor-1 |
| WISP1 | Wnt-induced secreted protein-1 |
| YAP/TAZ | Yes-associated protein 1/transcriptional coactivator with PDZ-binding motif |
References
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Roy, N.K.; Bordoloi, D.; Padmavathi, G.; Banik, K.; Khwairakpam, A.D.; Kunnumakkara, A.B.; Sukumar, P. Orai-1 and Orai-2 regulate oral cancer cell migration and colonisation by suppressing Akt/mTOR/NF-κB signalling. Life Sci. 2020, 261, 118372. [Google Scholar] [CrossRef] [PubMed]
- Monisha, J.; Roy, N.K.; Padmavathi, G.; Banik, K.; Bordoloi, D.; Khwairakpam, A.D.; Arfuso, F.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A. NGAL is downregulated in oral squamous cell carcinoma and leads to increased survival, proliferation, migration and chemoresistance. Cancers 2018, 10, 228. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Haddad, R.I.; Shin, D.M. Recent advances in head and neck cancer. N. Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case–control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- Liu, Y.; Tergaonkar, V.; Krishna, S.; Androphy, E.J. Human papillomavirus type 16 E6-enhanced susceptibility of L929 cells to tumor necrosis factor α correlates with increased accumulation of reactive oxygen species. J. Biol. Chem. 1999, 274, 24819–24827. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N. Engl. J. Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Girisa, S.; Kumar, A.; Rana, V.; Parama, D.; Daimary, U.D.; Warnakulasuriya, S.; Kumar, A.P.; Kunnumakkara, A.B. From simple mouth cavities to complex oral mucosal disorders—Curcuminoids as a promising therapeutic approach. ACS Pharmacol. Transl. Sci. 2021, 4, 647–665. [Google Scholar] [CrossRef]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P. Targeting AKT/mTOR in oral cancer: Mechanisms and advances in clinical trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef]
- Monisha, J.; Padmavathi, G.; Roy, N.K.; Deka, A.; Bordoloi, D.; Anip, A.; B Kunnumakkara, A. NF-κB blockers gifted by mother nature: Prospectives in cancer cell chemosensitization. Curr. Pharm. Des. 2016, 22, 4173–4200. [Google Scholar] [CrossRef] [PubMed]
- Kishor Roy, N.; Bordoloi, D.; Monisha, J.; Padmavathi, G.; Kotoky, J.; Golla, R.; B Kunnumakkara, A. Specific targeting of Akt kinase isoforms: Taking the precise path for prevention and treatment of cancer. Curr. Drug Targets 2017, 18, 421–435. [Google Scholar] [CrossRef]
- Khattar, E.; Maung, K.Z.Y.; Chew, C.L.; Ghosh, A.; Mok, M.M.H.; Lee, P.; Zhang, J.; Chor, W.H.J.; Cildir, G.; Wang, C.Q. Rap1 regulates hematopoietic stem cell survival and affects oncogenesis and response to chemotherapy. Nat. Commun. 2019, 10, 5349. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Wong, E.; Kua, N.; Ling Teo, H.; Tergaonkar, V.; Lane, D. Hexamethylene bisacetamide (HMBA) simultaneously targets akt and mapk pathway and represses NF-κB activity: Implications for cancer therapy. Cell Cycle 2008, 7, 3759–3767. [Google Scholar] [CrossRef]
- Dey, A.; Wong, E.; Bist, P.; Tergaonkar, V.; Lane, D. Nutlin-3 inhibits the NFκB pathway in a p53 dependent manner: Implications in lung cancer therapy. Cell Cycle 2007, 6, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.B.; Li, Y.; Tergaonkar, V. Current insights to regulation and role of telomerase in human diseases. Antioxidants 2017, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Deldar Abad Paskeh, M.; Mirzaei, S.; Ashrafizadeh, M.; Zarrabi, A.; Sethi, G. Wnt/β-Catenin signaling as a driver of hepatocellular carcinoma progression: An emphasis on molecular pathways. J. Hepatocell. Carcinoma 2021, 8, 1415–1444. [Google Scholar] [CrossRef]
- Hiremath, I.S.; Goel, A.; Warrier, S.; Kumar, A.P.; Sethi, G.; Garg, M. The multidimensional role of the Wnt/β-catenin signaling pathway in human malignancies. J. Cell. Physiol. 2022, 237, 199–238. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef]
- Chen, D.; Xie, R.; Shu, B.; Landay, A.L.; Wei, C.; Reiser, J.; Spagnoli, A.; Torquati, A.; Forsyth, C.B.; Keshavarzian, A.; et al. Wnt signaling in bone, kidney, intestine, and adipose tissue and interorgan interaction in aging. Ann. N. Y. Acad. Sci. 2019, 1442, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Perugorria, M.J.; Olaizola, P.; Labiano, I.; Esparza-Baquer, A.; Marzioni, M.; Marin, J.J.G.; Bujanda, L.; Banales, J.M. Wnt-beta-catenin signalling in liver development, health and disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Skronska-Wasek, W.; Mutze, K.; Baarsma, H.A.; Bracke, K.R.; Alsafadi, H.N.; Lehmann, M.; Costa, R.; Stornaiuolo, M.; Novellino, E.; Brusselle, G.G.; et al. Reduced Frizzled Receptor 4 Expression Prevents WNT/beta-Catenin-driven Alveolar Lung Repair in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 172–185. [Google Scholar] [CrossRef]
- Salyakina, D.; Tsinoremas, N.F. Non-coding RNAs profiling in head and neck cancers. NPJ Genomic Med. 2016, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.; Barker, D.; Birney, E.; Cameron, G.; Chen, Y.; Clark, L.; Cox, T.; Cuff, J.; Curwen, V.; Down, T. The Ensembl genome database project. Nucleic Acids Res. 2002, 30, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Mostafavi, E.; Aref, A.R.; Sethi, G.; Wang, L.; Tergaonkar, V. Non-coding RNA-based regulation of inflammation. Semin. Immunol. 2022, 59, 101606. [Google Scholar] [CrossRef]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef]
- Ricciardiello, F.; Falco, M.; Tortoriello, G.; Riccardi, F.; Pellini, R.; Iorio, B.; Russo, G.; Longo, G.; Coppola, C.; Takeuchi, T.; et al. Poorly Differentiated Neuroendocrine Larynx Carcinoma: Clinical Features and miRNAs Signature-A New Goal for Early Diagnosis and Therapy? J. Clin. Med. 2021, 10, 2019. [Google Scholar] [CrossRef]
- Kawasaki, H.; Takeuchi, T.; Ricciardiello, F.; Lombardi, A.; Biganzoli, E.; Fornili, M.; De Bortoli, D.; Mesolella, M.; Cossu, A.M.; Scrima, M.; et al. Definition of miRNA Signatures of Nodal Metastasis in LCa: miR-449a Targets Notch Genes and Suppresses Cell Migration and Invasion. Mol. Ther.—Nucleic Acids 2020, 20, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Wingless a new mutant in Drosophila melanogaster. Drosoph. Inf. Serv. 1973, 50, 134. [Google Scholar]
- Nusse, R.; Varmus, H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982, 31, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, K.W.; Nilbert, M.C.; Su, L.K.; Vogelstein, B.; Bryan, T.M.; Levy, D.B.; Smith, K.J.; Preisinger, A.C.; Hedge, P.; McKechnie, D.; et al. Identification of FAP locus genes from chromosome 5q21. Science 1991, 253, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zheng, S.; Jin, S.H.; Zhang, S.Z. Somatic mutations of APC gene in carcinomas from hereditary non-polyposis colorectal cancer patients. World J. Gastroenterol. 2004, 10, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.P.; Pandith, A.A.; Hussain, M.U.; Yousuf, A.; Khan, M.S.; Wani, K.A.; Mudassar, S. Novelty of Axin 2 and lack of Axin 1 gene mutation in colorectal cancer: A study in Kashmiri population. Mol. Cell. Biochem. 2011, 355, 149–155. [Google Scholar] [CrossRef]
- Guezguez, B.; Almakadi, M.; Benoit, Y.D.; Shapovalova, Z.; Rahmig, S.; Fiebig-Comyn, A.; Casado, F.L.; Tanasijevic, B.; Bresolin, S.; Masetti, R.; et al. GSK3 Deficiencies in Hematopoietic Stem Cells Initiate Pre-neoplastic State that Is Predictive of Clinical Outcomes of Human Acute Leukemia. Cancer Cell 2016, 29, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Voeller, H.J.; Truica, C.I.; Gelmann, E.P. β-Catenin mutations in human prostate cancer. Cancer Res. 1998, 58, 2520–2523. [Google Scholar]
- Zhong, Z.; Virshup, D.M. Wnt Signaling and Drug Resistance in Cancer. Mol. Pharmacol. 2020, 97, 72–89. [Google Scholar] [CrossRef]
- Galluzzi, L.; Spranger, S.; Fuchs, E.; Lopez-Soto, A. WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol. 2019, 29, 44–65. [Google Scholar] [CrossRef]
- Nsengimana, J.; Laye, J.; Filia, A.; O’Shea, S.; Muralidhar, S.; Pozniak, J.; Droop, A.; Chan, M.; Walker, C.; Parkinson, L.; et al. beta-Catenin-mediated immune evasion pathway frequently operates in primary cutaneous melanomas. J. Clin. Investig. 2018, 128, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Akasu, M.; Shimada, S.; Kabashima, A.; Akiyama, Y.; Shimokawa, M.; Akahoshi, K.; Kudo, A.; Yamaoka, S.; Tanabe, M.; Tanaka, S. Intrinsic activation of beta-catenin signaling by CRISPR/Cas9-mediated exon skipping contributes to immune evasion in hepatocellular carcinoma. Sci. Rep. 2021, 11, 16732. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Morris, L.G.; Lee, W.; Chan, T.A. Unraveling the molecular genetics of head and neck cancer through genome-wide approaches. Genes Dis. 2014, 1, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zeng, Q.; Yu, G.; Li, S.; Wang, C.Y. Wnt/beta-catenin signaling inhibits death receptor-mediated apoptosis and promotes invasive growth of HNSCC. Cell. Signal. 2006, 18, 679–687. [Google Scholar] [CrossRef]
- Iwai, S.; Yonekawa, A.; Harada, C.; Hamada, M.; Katagiri, W.; Nakazawa, M.; Yura, Y. Involvement of the Wnt-beta-catenin pathway in invasion and migration of oral squamous carcinoma cells. Int. J. Oncol. 2010, 37, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.A.; Tola, R.; Rashid, K.; Ali, A.H.; Dowlah, T.; Malik, U.A.; Zia, S.; Saleem, M.; Anjali, F.; Irfan, M. Clinicopathological Parameters Predicting Nodal Metastasis in Head and Neck Squamous Cell Carcinoma. Cureus 2023, 15, e40744. [Google Scholar] [CrossRef]
- Moon, J.H.; Lee, S.H.; Lim, Y.C. Wnt/beta-catenin/Slug pathway contributes to tumor invasion and lymph node metastasis in head and neck squamous cell carcinoma. Clin. Exp. Metastasis 2021, 38, 163–174. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Seyhan, A.A. Circulating microRNAs as Potential Biomarkers in Pancreatic Cancer-Advances and Challenges. Int. J. Mol. Sci. 2023, 24, 13340. [Google Scholar] [CrossRef]
- Thakur, K.K.; Kumar, A.; Banik, K.; Verma, E.; Khatoon, E.; Harsha, C.; Sethi, G.; Gupta, S.C.; Kunnumakkara, A.B. Long noncoding RNAs in triple-negative breast cancer: A new frontier in the regulation of tumorigenesis. J. Cell. Physiol. 2021, 236, 7938–7965. [Google Scholar] [CrossRef] [PubMed]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef]
- Huang, J.; Cai, Y.; Guo, L.; Huang, W.; Yan, J.; Lai, J.; Wang, Y.; Jiang, D.; Peng, L. hsa_circ_0136839 regulates the malignant phenotypes of nasopharyngeal carcinoma via the Wnt/beta-catenin signaling pathway. Pathol. Res. Pract. 2023, 245, 154433. [Google Scholar] [CrossRef] [PubMed]
- Pisignano, G.; Michael, D.C.; Visal, T.H.; Pirlog, R.; Ladomery, M.; Calin, G.A. Going circular: History, present, and future of circRNAs in cancer. Oncogene 2023, 42, 2783–2800. [Google Scholar] [CrossRef]
- Obayashi, M.; Yoshida, M.; Tsunematsu, T.; Ogawa, I.; Sasahira, T.; Kuniyasu, H.; Imoto, I.; Abiko, Y.; Xu, D.; Fukunaga, S.; et al. microRNA-203 suppresses invasion and epithelial-mesenchymal transition induction via targeting NUAK1 in head and neck cancer. Oncotarget 2016, 7, 8223–8239. [Google Scholar] [CrossRef] [PubMed]
- Rishabh, K.; Khadilkar, S.; Kumar, A.; Kalra, I.; Kumar, A.P.; Kunnumakkara, A.B. MicroRNAs as modulators of oral tumorigenesis—A focused review. Int. J. Mol. Sci. 2021, 22, 2561. [Google Scholar] [CrossRef]
- Tang, S.J.; Fan, K.H.; You, G.R.; Huang, S.F.; Kang, C.J.; Huang, Y.F.; Huang, Y.C.; Chang, J.T.; Cheng, A.J. Tumor Suppressor miRNA-503 Inhibits Cell Invasion in Head and Neck Cancer through the Wnt Signaling Pathway via the WNT3A/MMP Molecular Axis. Int. J. Mol. Sci. 2022, 23, 15900. [Google Scholar] [CrossRef]
- Yan, H.; Bu, P. Non-coding RNA in cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar] [CrossRef]
- Mao, B.; Wang, F.; Zhang, J.; Li, Q.; Ying, K. Long non-coding RNA human leucocyte antigen complex group-18 HCG18 (HCG18) promoted cell proliferation and migration in head and neck squamous cell carcinoma through cyclin D1-WNT pathway. Bioengineered 2022, 13, 9425–9434. [Google Scholar] [CrossRef]
- Chen, F.; Qi, S.; Zhang, X.; Wu, J.; Yang, X.; Wang, R. lncRNA PLAC2 activated by H3K27 acetylation promotes cell proliferation and invasion via the activation of Wnt/beta-catenin pathway in oral squamous cell carcinoma. Int. J. Oncol. 2019, 54, 1183–1194. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Lu, Y.G.; Zheng, D.L. Roles of the Wnt Signaling Pathway in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2020, 7, 590912. [Google Scholar] [CrossRef]
- Han, P.B.; Ji, X.J.; Zhang, M.; Gao, L.Y. Upregulation of lncRNA LINC00473 promotes radioresistance of HNSCC cells through activating Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7305–7313. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, S.; Wang, P.; Yang, C.; Shang, C.; Yang, J.; Wang, J. LncRNA, TUG1 regulates the oral squamous cell carcinoma progression possibly via interacting with Wnt/beta-catenin signaling. Gene 2017, 608, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Jin, L.; Yang, X.; Zhang, F. LncRNA MINCR activates Wnt/beta-catenin signals to promote cell proliferation and migration in oral squamous cell carcinoma. Pathol. Res. Pract. 2019, 215, 924–930. [Google Scholar] [CrossRef]
- Shao, T.R.; Zheng, Z.N.; Chen, Y.C.; Wu, Q.Q.; Huang, G.Z.; Li, F.; Zeng, W.S.; Lv, X.Z. LncRNA AC007271.3 promotes cell proliferation, invasion, migration and inhibits cell apoptosis of OSCC via the Wnt/beta-catenin signaling pathway. Life Sci. 2019, 239, 117087. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Wu, S.; Zou, C.; Wei, H. LINC00941 promotes oral squamous cell carcinoma progression via activating CAPRIN2 and canonical WNT/beta-catenin signaling pathway. J. Cell. Mol. Med. 2020, 24, 10512–10524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Mao, D.; He, Z.; Li, W.; Zhang, X.; Li, L. SLCO4A1-AS1 regulates laryngeal squamous cell carcinoma cell phenotypes via the Wnt pathway. Oral Dis. 2023, 29, 390–401. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, S.; Tan, L.; Li, H.; Liu, J.; Zhang, S. IGFL2-AS1 facilitates tongue squamous cell carcinoma progression via Wnt/beta-catenin signaling pathway. Oral Dis. 2023, 29, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ding, J.M.; Zheng, X.Z.; Chen, J.G. Immunity-related long noncoding RNA WDFY3-AS2 inhibited cell proliferation and metastasis through Wnt/beta-catenin signaling in oral squamous cell carcinoma. Arch. Oral Biol. 2023, 147, 105625. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liang, L.; Ouyang, K.; Li, Z.; Yi, X. MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/beta-catenin signaling pathway. J. Oral Pathol. Med. 2017, 46, 98–105. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yuan, K.; Chen, W. Effect of miR-25 on Proliferation of Nasopharyngeal Carcinoma Cells through Wnt/beta-Catenin Signaling Pathway. BioMed Res. Int. 2021, 2021, 9957161. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Li, X. MicroRNA-215 promoted the progression of nasopharyngeal carcinoma through targeting RB1 and activating Wnt/beta-catenin pathway. J. Balk. Union Oncol. 2020, 25, 1579–1586. [Google Scholar]
- Sun, S.; Gong, C.; Yuan, K. LncRNA UCA1 promotes cell proliferation, invasion and migration of laryngeal squamous cell carcinoma cells by activating Wnt/beta-catenin signaling pathway. Exp. Ther. Med. 2019, 17, 1182–1189. [Google Scholar] [CrossRef]
- Jin, N.; Jin, N.; Bu, W.; Li, X.; Liu, L.; Wang, Z.; Tong, J.; Li, D. Long non-coding RNA TIRY promotes tumor metastasis by enhancing epithelial-to-mesenchymal transition in oral cancer. Exp. Biol. Med. 2020, 245, 585–596. [Google Scholar] [CrossRef]
- Qiao, C.; Qiao, T.; Yang, S.; Liu, L.; Zheng, M. SNHG17/miR-384/ELF1 axis promotes cell growth by transcriptional regulation of CTNNB1 to activate Wnt/beta-catenin pathway in oral squamous cell carcinoma. Cancer Gene Ther. 2022, 29, 122–132. [Google Scholar] [CrossRef]
- Li, M.; Ding, X.; Zhang, Y.; Li, X.; Zhou, H.; Yang, L.; Li, Y.; Yang, P.; Zhang, X.; Hu, J.; et al. Antisense oligonucleotides targeting lncRNA AC104041.1 induces antitumor activity through Wnt2B/beta-catenin pathway in head and neck squamous cell carcinomas. Cell Death Dis. 2020, 11, 672. [Google Scholar] [CrossRef]
- Li, L.; Ma, T.T.; Ma, Y.H.; Jiang, Y.F. LncRNA HCG18 contributes to nasopharyngeal carcinoma development by modulating miR-140/CCND1 and Hedgehog signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10387–10399. [Google Scholar] [CrossRef]
- Li, G.H.; Ma, Z.H.; Wang, X. Long non-coding RNA CCAT1 is a prognostic biomarker for the progression of oral squamous cell carcinoma via miR-181a-mediated Wnt/beta-catenin signaling pathway. Cell Cycle 2019, 18, 2902–2913. [Google Scholar] [CrossRef]
- Chen, Z.F.; Wang, Y.; Sun, L.L.; Ding, S.Y.; Jinag, H. LncRNA SNHG20 enhances the progression of oral squamous cell carcinoma by regulating the miR-29a/DIXDC1/Wnt regulatory axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5436–5445. [Google Scholar] [CrossRef]
- Cao, W.; Sun, J. MicroRNA-200c promotes tumor cell proliferation and migration by directly targeting dachshund family transcription factor 1 by the Wnt/beta-catenin signaling pathway in nasopharyngeal carcinoma. Anticancer Drugs 2019, 30, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Tang, Y.; Tang, J.; Liu, Z.; Wang, X. Downregulation of lncRNA HOTTIP Suppresses the Proliferation, Migration, and Invasion of Oral Tongue Squamous Cell Carcinoma by Regulation of HMGA2-Mediated Wnt/beta-Catenin Pathway. Cancer Biother. Radiopharm. 2020, 35, 720–730. [Google Scholar] [CrossRef]
- Kang, R.; Yao, D.F.; Xu, G.Z.; Zhou, Y.H. The knockdown of SNHG3 inhibits the progression of laryngeal squamous cell carcinoma by miR-340-5p/YAP1 axis and Wnt/beta-catenin pathway. Neoplasma 2020, 67, 1094–1105. [Google Scholar] [CrossRef]
- Yu, T.; Liu, K.; Wu, Y.; Fan, J.; Chen, J.; Li, C.; Yang, Q.; Wang, Z. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/beta-catenin signaling pathway. Oncogene 2014, 33, 5017–5027. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Shan, G. DGCR5 promotes cancer stem cell-like properties of radioresistant laryngeal carcinoma cells by sponging miR-506 via Wnt pathway. J. Cell. Physiol. 2019, 234, 18423–18431. [Google Scholar] [CrossRef]
- Liu, B.; Chen, W.; Cao, G.; Dong, Z.; Xu, J.; Luo, T.; Zhang, S. MicroRNA-27b inhibits cell proliferation in oral squamous cell carcinoma by targeting FZD7 and Wnt signaling pathway. Arch. Oral Biol. 2017, 83, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Shiah, S.G.; Hsiao, J.R.; Chang, W.M.; Chen, Y.W.; Jin, Y.T.; Wong, T.Y.; Huang, J.S.; Tsai, S.T.; Hsu, Y.M.; Chou, S.T.; et al. Downregulated miR329 and miR410 promote the proliferation and invasion of oral squamous cell carcinoma by targeting Wnt-7b. Cancer Res. 2014, 74, 7560–7572. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, L.; Hu, Y. Long noncoding RNA LINC00961 inhibited cell proliferation and invasion through regulating the Wnt/beta-catenin signaling pathway in tongue squamous cell carcinoma. J. Cell. Biochem. 2019, 120, 12429–12435. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Cao, H. MicroRNA-384 regulates cell proliferation and apoptosis through directly targeting WISP1 in laryngeal cancer. J. Cell. Biochem. 2019, 120, 3018–3026. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, X.; Liang, Z.; Xu, Z.; Li, Y.; Pan, Y. MiR-338-3p inhibits cell migration and invasion in human hypopharyngeal cancer via downregulation of ADAM17. Anticancer Drugs 2020, 31, 925–931. [Google Scholar] [CrossRef]
- Cui, X.; Fang, N.; Cui, Y.; Xiao, D.; Wang, X. Long non-coding RNA NEF inhibits proliferation and promotes apoptosis of laryngeal squamous cell carcinoma cells by inhibiting Wnt/beta-catenin signaling. Oncol. Lett. 2019, 17, 4928–4934. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the therapeutic potential of apigenin against cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, M.; Banik, K.; Parama, D.; Sasikumar, P.; Harsha, C.; Joseph, A.G.; Sherin, D.R.; Thanathu, M.K.; Kunnumakkara, A.B.; Vasu, R.K. Exploring the cytotoxic effects of the extracts and bioactive triterpenoids from dillenia indica against oral squamous cell carcinoma: A scientific interpretation and validation of indigenous knowledge. ACS Pharmacol. Transl. Sci. 2021, 4, 834–847. [Google Scholar] [CrossRef]
- Babu, B.; Jayram, H.; Nair, M.; Ajaikumar, K.; Padikkala, J. Free radical scavenging, antitumor and anticarcinogenic activity of gossypin. J. Exp. Clin. Cancer Res. 2003, 22, 581–589. [Google Scholar] [PubMed]
- Banik, K.; Khatoon, E.; Harsha, C.; Rana, V.; Parama, D.; Thakur, K.K.; Bishayee, A.; Kunnumakkara, A.B. Wogonin and its analogs for the prevention and treatment of cancer: A systematic review. Phytother. Res. 2022, 36, 1854–1883. [Google Scholar] [CrossRef]
- Bordoloi, D.; Kunnumakkara, A.B. The potential of curcumin: A multitargeting agent in cancer cell chemosensitization. In Role of Nutraceuticals in Cancer Chemosensitization; Elsevier: Amsterdam, The Netherlands, 2018; pp. 31–60. [Google Scholar]
- Devi Khwairakpam, A.; Monisha, J.; Roy, N.K.; Bordoloi, D.; Padmavathi, G.; Banik, K.; Khatoon, E.; Kunnumakkara, A.B. Vietnamese coriander inhibits cell proliferation, survival and migration via suppression of Akt/mTOR pathway in oral squamous cell carcinoma. J. Basic Clin. Physiol. Pharmacol. 2019, 31, 20190162. [Google Scholar] [CrossRef]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015, 22, 1163–1171. [Google Scholar] [CrossRef]
- Sajeev, A.; Hegde, M.; Daimary, U.D.; Kumar, A.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Modulation of diverse oncogenic signaling pathways by oroxylin A: An important strategy for both cancer prevention and treatment. Phytomedicine 2022, 105, 154369. [Google Scholar] [CrossRef]
- Shabnam, B.; Padmavathi, G.; Banik, K.; Girisa, S.; Monisha, J.; Sethi, G.; Fan, L.; Wang, L.; Mao, X.; Kunnumakkara, A.B. Sorcin a potential molecular target for cancer therapy. Transl. Oncol. 2018, 11, 1379–1389. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Zhu, L.; Zhang, C.; Zhou, J. Curcumin inhibits oral squamous cell carcinoma SCC-9 cells proliferation by regulating miR-9 expression. Biochem. Biophys. Res. Commun. 2014, 454, 576–580. [Google Scholar] [CrossRef]
- Wang, T.T.; Chen, Z.Z.; Xie, P.; Zhang, W.J.; Du, M.Y.; Liu, Y.T.; Zhu, H.Y.; Guo, Y.S. Isoliquiritigenin suppresses the proliferation and induced apoptosis via miR-32/LATS2/Wnt in nasopharyngeal carcinoma. Eur. J. Pharmacol. 2019, 856, 172352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Z.; Deng, J.; Xu, K.; Che, D.; Lin, J.; Jiang, P.; Gu, X.; Xu, B. Epstein-Barr virus-encoded microRNA BART22 serves as novel biomarkers and drives malignant transformation of nasopharyngeal carcinoma. Cell Death Dis. 2022, 13, 664. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Wang, Y. MicroRNA-103 promotes nasopharyngeal carcinoma through targeting TIMP-3 and the Wnt/beta-catenin pathway. Laryngoscope 2020, 130, E75–E82. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Xu, H.; Wang, C.H.; Xie, H. Long non-coding RNA GNAS-AS1 promotes cell migration and invasion via regulating Wnt/beta-catenin pathway in nasopharyngeal carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, M.; Wan, Y.; Lei, L.; Ruan, H. miR-574-5p Targets FOXN3 to Regulate the Invasion of Nasopharyngeal Carcinoma Cells via Wnt/beta-Catenin Pathway. Technol. Cancer Res. Treat. 2020, 19, 1533033820971659. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, M.; Li, X.; Cui, F. The Long Noncoding RNA NEAT1 Targets miR-34a-5p and Drives Nasopharyngeal Carcinoma Progression via Wnt/beta-Catenin Signaling. Yonsei Med. J. 2019, 60, 336–345. [Google Scholar] [CrossRef]
- Cui, S.H.; Hu, X.D.; Yan, Y. Wnt/beta-catenin signaling pathway participates in the effect of miR-626 on oral squamous cell carcinoma by targeting RASSF4. J. Oral Pathol. Med. 2021, 50, 1005–1017. [Google Scholar] [CrossRef]
- Tong, S.; Wang, X.; Guo, X.; Lu, Z. Knockdown of lncRNA IGF2BP2-AS1 inhibits proliferation and migration of oral squamous cell carcinoma cells via the Wnt/beta-catenin pathway. J. Oral Pathol. Med. 2022, 51, 272–280. [Google Scholar] [CrossRef]
- Liu, M.; Gao, X.; Liu, C.L. Increased expression of lncRNA FTH1P3 promotes oral squamous cell carcinoma cells migration and invasion by enhancing PI3K/Akt/GSK3b/ Wnt/beta-catenin signaling. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8306–8314. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Zhang, H.; Wang, C.; Liang, J.; Chen, G.; Li, W.; Tang, H.; Hou, J. miR-373-3p Targets DKK1 to Promote EMT-Induced Metastasis via the Wnt/beta-Catenin Pathway in Tongue Squamous Cell Carcinoma. BioMed Res. Int. 2017, 2017, 6010926. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Hu, F.; Hu, J.; Wang, C.; Hou, J.; Yu, Z.; Wang, T.T.; Liu, X.; Huang, H. MicroRNA-218 promotes cisplatin resistance in oral cancer via the PPP2R5A/Wnt signaling pathway. Oncol. Rep. 2017, 38, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Tang, H.Y.; Du, Y.; Tian, T.; Xiong, S.J. MiR-638 suppresses the progression of oral squamous cell carcinoma through wnt/beta-catenin pathway by targeting phospholipase D1. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3278–3285. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Cano, A.; Perez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Takkunen, M.; Grenman, R.; Hukkanen, M.; Korhonen, M.; Garcia de Herreros, A.; Virtanen, I. Snail-dependent and -independent epithelial-mesenchymal transition in oral squamous carcinoma cells. J. Histochem. Cytochem. 2006, 54, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Hinerman, R.W.; Amdur, R.J.; Malyapa, R.S.; Lansford, C.D.; Werning, J.W.; Villaret, D.B. Postoperative radiotherapy for squamous cell carcinoma of the head and neck. Clin. Med. Res. 2006, 4, 200–208. [Google Scholar] [CrossRef]
- Anderson, G.; Ebadi, M.; Vo, K.; Novak, J.; Govindarajan, A.; Amini, A. An updated review on head and neck cancer treatment with radiation therapy. Cancers 2021, 13, 4912. [Google Scholar] [CrossRef]
- Morton, R.; Rugman, F.; Dorman, E.; Stoney, P.; Wilson, J.; McCormick, M.; Veevers, A.; Stell, P. Cisplatinum and bleomycin for advanced or recurrent squamous cell carcinoma of the head and neck: A randomised factorial phase III controlled trial. Cancer Chemother. Pharmacol. 1985, 15, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Li, Y.; Murphy, B.A.; Langer, C.J.; Forastiere, A.A. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J. Clin. Oncol. 2004, 22, 262–268. [Google Scholar] [CrossRef]
- Guan, G.F.; Zhang, D.J.; Zheng, Y.; Wen, L.J.; Yu, D.J.; Lu, Y.Q.; Zhao, Y. Abnormal Wnt signaling and overexpression of ABCG2 contributes to drug efflux properties of side population cells in nasopharyngeal carcinoma. Mol. Med. Rep. 2015, 12, 4352–4357. [Google Scholar] [CrossRef]
- Wickstrom, M.; Dyberg, C.; Milosevic, J.; Einvik, C.; Calero, R.; Sveinbjornsson, B.; Sanden, E.; Darabi, A.; Siesjo, P.; Kool, M.; et al. Wnt/beta-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat. Commun. 2015, 6, 8904. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sun, L.; Xie, S.; Zhang, S.; Fan, S.; Li, Q.; Chen, W.; Pan, G.; Wang, W.; Weng, B.; et al. Chemotherapy-Induced Long Non-coding RNA 1 Promotes Metastasis and Chemo-Resistance of TSCC via the Wnt/beta-Catenin Signaling Pathway. Mol. Ther. 2018, 26, 1494–1508. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajeev, A.; BharathwajChetty, B.; Vishwa, R.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. Crosstalk between Non-Coding RNAs and Wnt/β-Catenin Signaling in Head and Neck Cancer: Identification of Novel Biomarkers and Therapeutic Agents. Non-Coding RNA 2023, 9, 63. https://doi.org/10.3390/ncrna9050063
Sajeev A, BharathwajChetty B, Vishwa R, Alqahtani MS, Abbas M, Sethi G, Kunnumakkara AB. Crosstalk between Non-Coding RNAs and Wnt/β-Catenin Signaling in Head and Neck Cancer: Identification of Novel Biomarkers and Therapeutic Agents. Non-Coding RNA. 2023; 9(5):63. https://doi.org/10.3390/ncrna9050063
Chicago/Turabian StyleSajeev, Anjana, Bandari BharathwajChetty, Ravichandran Vishwa, Mohammed S. Alqahtani, Mohamed Abbas, Gautam Sethi, and Ajaikumar B. Kunnumakkara. 2023. "Crosstalk between Non-Coding RNAs and Wnt/β-Catenin Signaling in Head and Neck Cancer: Identification of Novel Biomarkers and Therapeutic Agents" Non-Coding RNA 9, no. 5: 63. https://doi.org/10.3390/ncrna9050063





