Novel Morphology for NiWMo Carbides Obtained by Mechanical Alloying and Quenching
Abstract
:1. Introduction
2. Materials and Methods
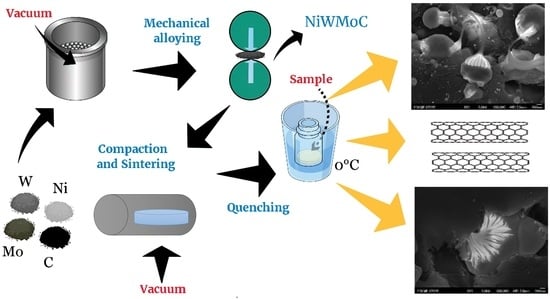
2.1. Synthesis
2.2. Characterization
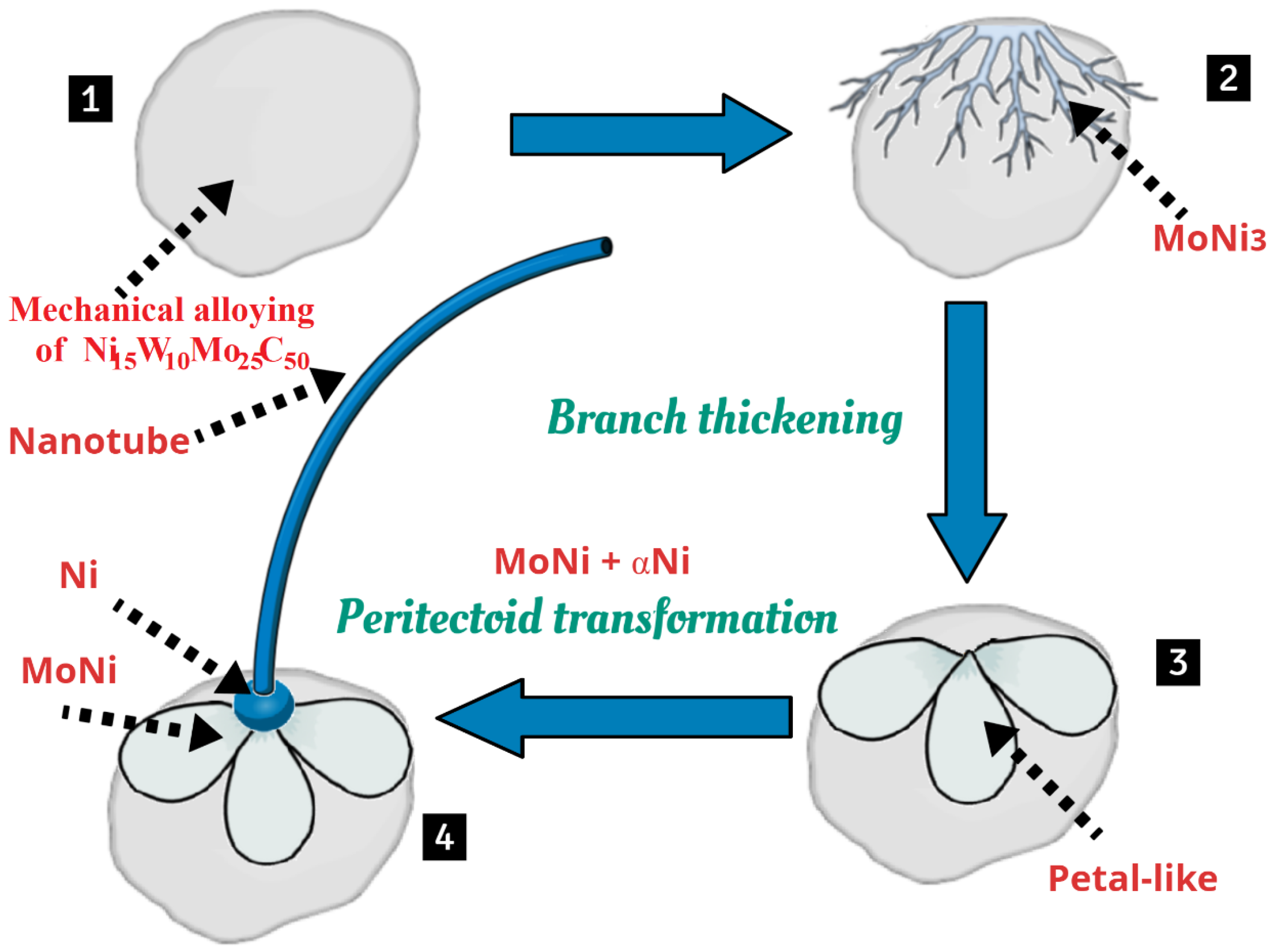
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostorz, G. Phase Transformations in Materials; Wiley-VCH: Weinheim, Germany, 2001; Chapter 6; ISBN 3527302565. [Google Scholar]
- Vvedensky, D.D. Transformations of Materials; IOP Publishing: Bristol, UK, 2019; ISBN 1643276212. [Google Scholar]
- Jurgen Buschow, K.H.; Cahn, R.W. Encyclopedia of Materials: Science and Technology; Elsevier Publishing: Amsterdam, The Netherlands, 2001; ISBN 978-0-08-043152-9. [Google Scholar]
- Reedijk, J.; Poeppelmeier, K. Comprehensive Inorganic Chemistry II, 2nd ed.; Elsevier Publishing: Amsterdam, The Netherlands, 2013; ISBN 978-0-08-096529-1. [Google Scholar]
- Rudraraju, S.; Van der Ven, A.; Garikipati, K.T. Mechanochemical spinodal decomposition: A phenomenological theory of phase transformations in multi-component crystalline solids. NPJ Comput. Mater. 2016, 2, 16012. [Google Scholar] [CrossRef]
- Sun, L.; Vasina, R.N.; Islamov, A.K.; Bobrikova, I.A.; Sumnikova, S.V.; Balagurov, A.M.; Guo, W.; Cheng, W.C.; Golovin, I.S. Spinodal decomposition in ternary Mn-Cu-Cr alloy and its influence on martensitic transition temperatures. J. Alloys Comp. 2021, 884, 161082. [Google Scholar] [CrossRef]
- Zhou, X.; Kamachali, R.D.; Boyce, B.L.; Clark, B.G.; Raabe, D.; Thompson, G.B. Spinodal Decomposition in Nanocrystalline Alloys. Acta Mater. 2021, 221, 117054. [Google Scholar] [CrossRef]
- Chen, W.; Yu, G.; Li, K.; Wang, Y.; Zhang, J.; Sun, J. Plastic instability in Ti–6Cr–5Mo–5V–4Al metastable β-Ti alloy containing the β-spinodal decomposition structures. Mater. Sci. Eng. A 2021, 811, 141052. [Google Scholar] [CrossRef]
- Velázquez, D.; Chaparro, M.A.E.; Böhnel, H.N.; Romero, R.; Lanzini, F. Spinodal decomposition, chemical and magnetic ordering in Cu–Al–Mn shape memory alloys. Mater. Chem. Phys. 2020, 246, 122793. [Google Scholar] [CrossRef]
- Schultz, A.H.; Stubican, V.S. Modulated structures in the system TiO2-SnO2. Philos. Mag. 1968, 18, 155. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwanga, J.; Sun, Y. Transition metal carbide-based materials: Synthesis and applications in electrochemical energy storage. J. Mater. Chem. 2016, 4, 10379–10393. [Google Scholar] [CrossRef]
- Shahzad, F.; Iqbal, A.; Kim, H.; Koo, C.M. 2D Transition Metal Carbides (MXenes): Applications as an Electrically Conducting Material. Adv. Mater. 2020, 32, 2002159. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y. 2D Early Transition Metal Carbides (MXenes) for Catalysis. Nano micro-Small. Adv. Mater. 2019, 15, 1804736. [Google Scholar]
- Vasilevich, A.V.; Baklanova, O.N.; Lavrenov, A.V. Molybdenum Carbides: Synthesis and Application in Catalysis. Solid Fuel Chem. 2020, 54, 354–361. [Google Scholar] [CrossRef]
- Plata, F.J.C.; Olvera, J.N.R.; Febles, V.G.; Barriga, L.G.D. Thermodynamic analysis and microstructural evolution of the W-Mo-Ni-C system produced by mechanical alloying. Phys. B Phys. Cond. Matt. 2022, 644, 414126. [Google Scholar] [CrossRef]
- Pierson, H.O. Properties, Characteristics, Processing and Applications (Materials Science and Process Technology; William Andrew Publishing: Norwich, NY, USA, 1996; ISBN 978-0815513926. [Google Scholar]
- Patel, P.; Kim, I.S.; Kumta, P.N. Nanocomposites of silicon/titanium carbide synthesized using high-energy mechanical milling for use as anodes in lithium-ion batteries. Mater. Sci. Eng. B 2005, 116, 347–352. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Progress in Materials Science. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Díaz-Barriga Arceo, L.; Rendón-Vázquez, L.; Orozco, E.; Garibay-Febles, V.; Palacios Gonzalez, E.; Leyte Guerrero, F. Nanotubes Obtained from Mono and Bimetallic Carbides Produced by Mechanical Alloying. Mater. Sci. Forum. 2007, 539–543, 2731–2736. [Google Scholar] [CrossRef]
- Padmanaban, D.B.; Mohan, L.; Giri, P.; Bera, P.; An an, C.; Barshilia, H.C. Effect of Molybdenum Content on Mechanical and Tribological Properties of Diamond-Like Carbon Coatings over Titanium β-21S Alloy. J. Carbon. Res. 2021, 7, 1. [Google Scholar] [CrossRef]
- Czaplicka, N.; Rogala, A.; Wysocka, I. Metal (Mo, W, Ti) Carbide Catalysts: Synthesis and Application as Alternative Catalysts for Dry Reforming of Hydrocarbons—A Review. Int. J. Mol. Sci. 2021, 22, 12337. [Google Scholar] [CrossRef]
- Bao, X.; Wang, T.; Yang, Y. Recent progress in bimetallic carbide-based electrocatalysts for water splitting. Mater. Chem. Front. 2024. [Google Scholar] [CrossRef]
- Balbino, N.A.N.; Correa, E.O.; Huanca, D.R.; Flávio Amaury de Freitas Matos and Livio de Carvalho Valeriano. Comparative Study of Corrosion Behaviors of WC-NiMo and WC-Co Cemented Carbides. Materials 2023, 16, 4480. [Google Scholar] [CrossRef]
- JOlvera, e.N.R.; Gutiérrez, J.; Serrano, J.A.R.; Ovando, M.; Febles, V.G.; Arceo, L.D.B. Use of unsupported, mechanically alloyed NiWMoC nanocatalyst to reduce the viscosity of aquathermolysis reaction of heavy oil. Catal. Commun. 2014, 43, 131–135. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Prentice Hall: Englewood Cliffs, NJ, USA, 2001; ISBN 0201610914. [Google Scholar]
- Martinez Ruiz, M.; Rivera Olvera, J.N.; Morales Davila, R.; González Reyes, L.; Garibay Febles, V.; Garcia Martinez, J.; Diaz Barriga Arceo, L.G. Synthesis and Characterization of Mechanically Alloyed, Nanostructured Cubic MoW Carbide. Appl. Sci. 2020, 10, 9114. [Google Scholar] [CrossRef]
- Olvera, J.N.R.; Paredes, G.G.; Serrano, A.R.; López, E.R.; Franco, E.M.; Meza, P.T.; Arceo, L.D.B. Synthesis and characterization of a MoWC-WC-NiC nanocomposite via mechanical alloying and sintering. Powder Technol. 2015, 271, 292–300. [Google Scholar] [CrossRef]
- Akbarpour, M.R. Effects of mechanical milling time on densification, microstructural characteristics and hardness of Cu–SiC nanocomposites prepared by conventional sintering process. Mater. Chem. Phys. 2021, 261, 124205. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformations in Metals and Alloys; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group LLC: New York, NY, USA, 2009; Chapter 5; pp. 302–309. ISBN 9781420062106. [Google Scholar]
- Carter, C.B. Solid-State Phase Transformations and Reactions. In Ceramic Materials; Springer: New York, NY, USA, 2007; ISBN 978-0-387-46271-4_25. [Google Scholar]
- Illescas, C.R.E. Analysis of Phase Separation in Aged Alloys by the Phase Field Method. Master’s Thesis, National Polytechnic Institute of Mexico, Mexico City, Mexico, 2018; p. 112, Figure C. Available online: https://tesis.ipn.mx/handle/123456789/25979 (accessed on 3 January 2024).
- C-Ni Phase Diagram. Available online: https://www.crct.polymtl.ca/fact/phase_diagram.php?file=C-Ni.jpg&dir=SGTE2014 (accessed on 3 January 2024).
- Corletto, A.; Shapter, J.G. Nanoscale Patterning of Carbon Nanotubes: Techniques, Applications, and Future. Adv. Sci. 2021, 8, 2001778. [Google Scholar] [CrossRef] [PubMed]
- Egerton, R.F. Physical Principles of Electron Microscopy: An Introduction to TEM, SEM, and AEM; Springer Publishing: Berlin/Heidelberg, Germany, 2016; ISBN 978-3319398761. [Google Scholar]
- Zhang, L.; Peng, Y.; Zhang, L.; Lei, X.; Yao, W.; Wang, N. Temperature and initial composition dependence of pattern formation and dynamic behavior in phase separation under deep-quenched conditions. RSC Adv. 2019, 9, 10670–10678. [Google Scholar] [CrossRef]
- Mo-Ni Phase Diagram. Available online: http://www.factsage.cn/fact/phase_diagram.php?file=Mo-Ni.jpg&dir=SGTE (accessed on 3 January 2024).
- Mitcheson, P.D.; Miao, P.; Stark, B.H.; Yeatman, E.M.; Holmes, A.S.; Green, T.C. MEMS electrostatic micropower generator for low frequency operation Green. Sens. Actuators A 2004, 115, 523–529. [Google Scholar] [CrossRef]

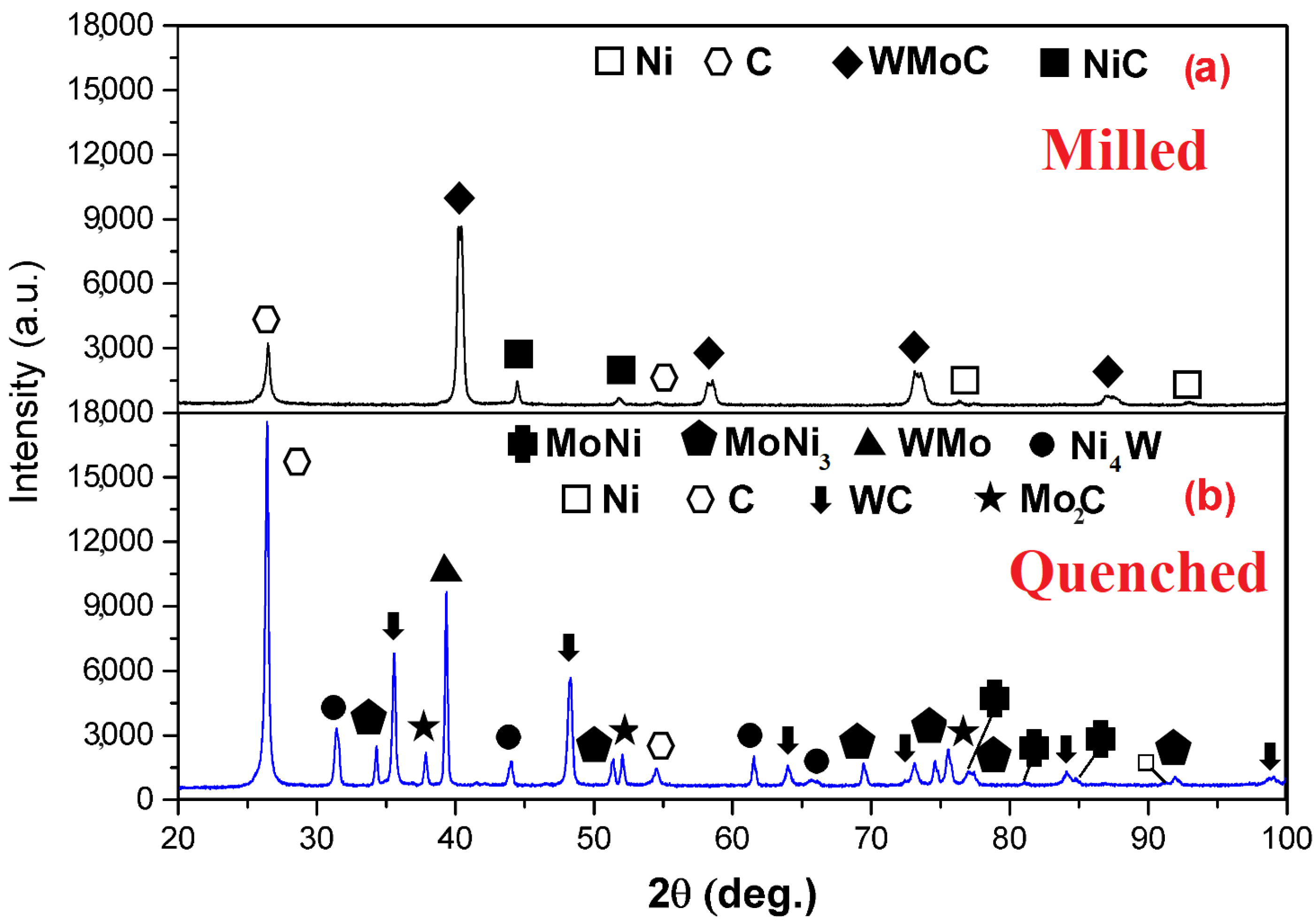

| Phases (M—Milled and Q—Quenched) | Crystalline Structure | Lattice Parameter (nm) | Phase Percentage % | ICDD Card * |
|---|---|---|---|---|
| Cubic | a = 0.3148 | 70.28 | Ref. [26] | |
| Hexagonal | a = 0.2464, c = 0.6736 | 20.26 | 120212 | |
| Cubic | a = 0.3523 | 8.96 | 040850 | |
| Cubic | a = 0.3539 | 0.50 | 140020 | |
| Hexagonal | a = 0.2900, c = 0.2831 | 23.44 | 654539 | |
| - | - | 16.04 | Unknown | |
| Orthorhombic | a = 0.5064, b = 0.4224, c = 0.4448 | 12.44 | 652587 | |
| Orthorhombic | a = 0.9128, b = 0.9134, c = 0.8835 | 1.16 | 481745 | |
| Cubic | a = 0.3523 | 0.32 | 040850 | |
| Hexagonal | a = 0.3012, c = 0.4735 | 8.00 | 350787 | |
| Tetragonal | a = 0.5730, c = 0.3553 | 7.46 | 652673 | |
| Hexagonal | a = 0.2464, c = 0.6736 | 31.14 | 120212 |
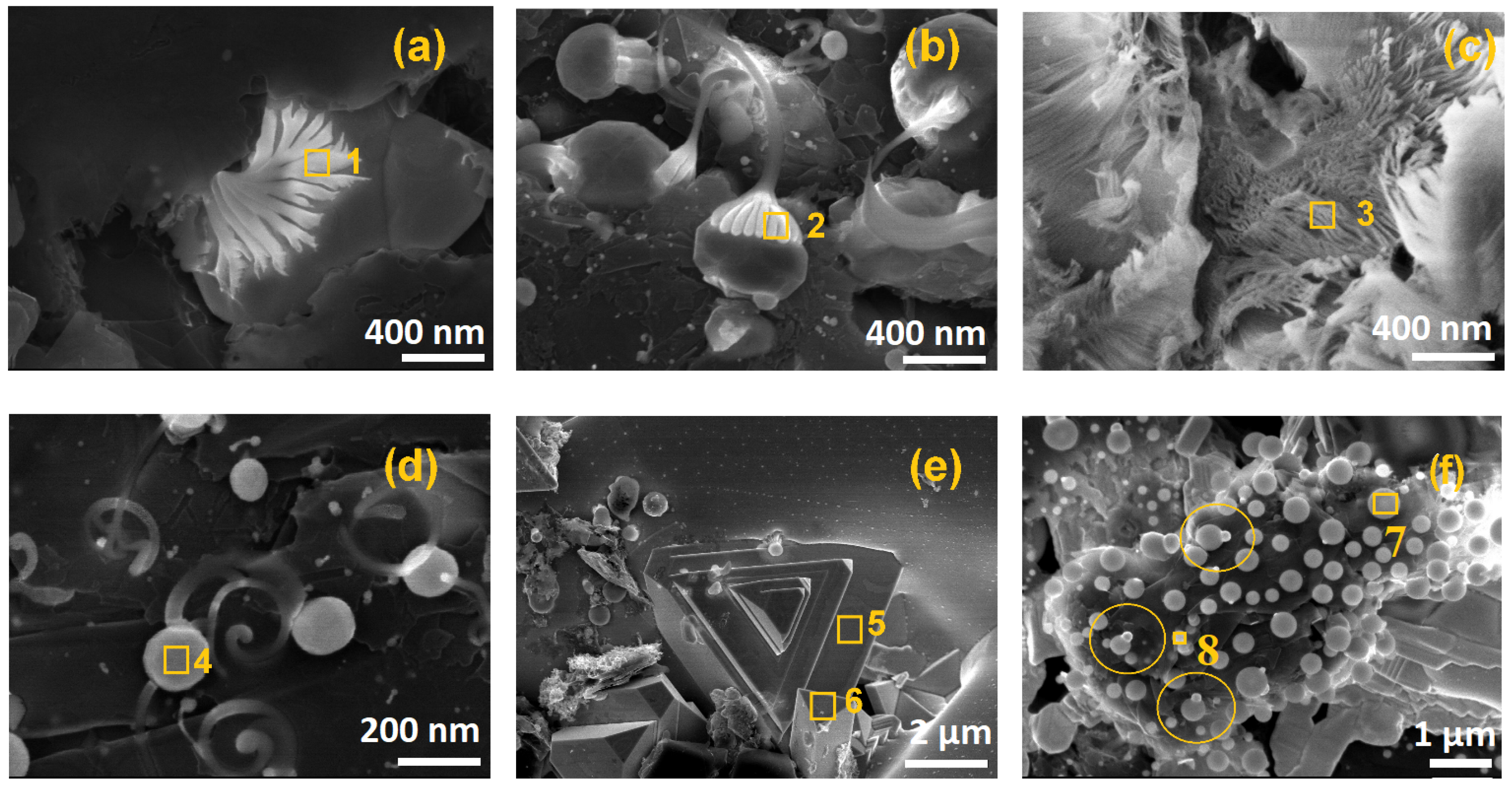
| SEM–EDS * (Figure 4) | C (at.%) | Mo (at.%) | Ni( at.%) | W (at.%) | O (at.%) | Fe (at.%) |
|---|---|---|---|---|---|---|
| Spot 1 | 2.67 ± 0.04 | 22.98 ± 0.41 | 73.34 ± 0.84 | 0.98 ± 0.09 | 0.23 ± 0.01 | - |
| Spot 2 | 12.18 ± 0.13 | 66.48± 0.54 | 15.99± 1.02 | 5.22 ± 0.01 | 0.13 ± 0.04 | - |
| Spot 3 | 97.42 ± 0.75 | 0.95 ± 0.01 | 0.32 ± 0.02 | 0.44 ± 0.01 | 0.87 ± 0.03 | - |
| Spot 4 | 1.26 ± 0.03 | 65.78 ± 1.64 | 14.05 ± 0.67 | 18.91 ± 0.86 | - | - |
| Spot 5 | 93.80 ± 2.01 | - | 0.99 ± 0.01 | 4.35 ± 0.22 | 0.35 ± 0.02 | 0.51 ± 0.04 |
| Spot 6 | 78.15 ± 1.78 | 4.10 ± 0.52 | - | 17.58 ± 0.75 | 0.17 ± 0.02 | - |
| Spot 7 | 72.56 ± 1.57 | 0.18 ± 0.03 | - | 27.26 ± 0.75 | - | - |
| Spot 8 | 1.04 ± 0.01 | 34.3 ± 0.43 | 64.52 ± 0.74 | - | - | 0.14 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera Olvera, J.N.; Hernández Maya, L.; Diaz Barriga Arceo, L.G. Novel Morphology for NiWMo Carbides Obtained by Mechanical Alloying and Quenching. C 2024, 10, 11. https://doi.org/10.3390/c10010011
Rivera Olvera JN, Hernández Maya L, Diaz Barriga Arceo LG. Novel Morphology for NiWMo Carbides Obtained by Mechanical Alloying and Quenching. C. 2024; 10(1):11. https://doi.org/10.3390/c10010011
Chicago/Turabian StyleRivera Olvera, Jesús Noé, Luis Hernández Maya, and Lucia Graciela Diaz Barriga Arceo. 2024. "Novel Morphology for NiWMo Carbides Obtained by Mechanical Alloying and Quenching" C 10, no. 1: 11. https://doi.org/10.3390/c10010011
APA StyleRivera Olvera, J. N., Hernández Maya, L., & Diaz Barriga Arceo, L. G. (2024). Novel Morphology for NiWMo Carbides Obtained by Mechanical Alloying and Quenching. C, 10(1), 11. https://doi.org/10.3390/c10010011