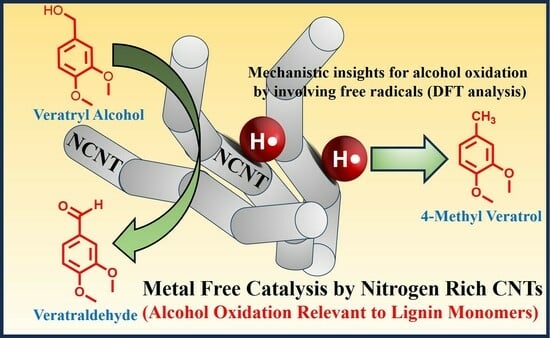
Metal-Free Catalytic Conversion of Veratryl and Benzyl Alcohols through Nitrogen-Enriched Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Catalyst
2.3. Catalytic Oxidation Test
2.4. Characterization of the Catalysts
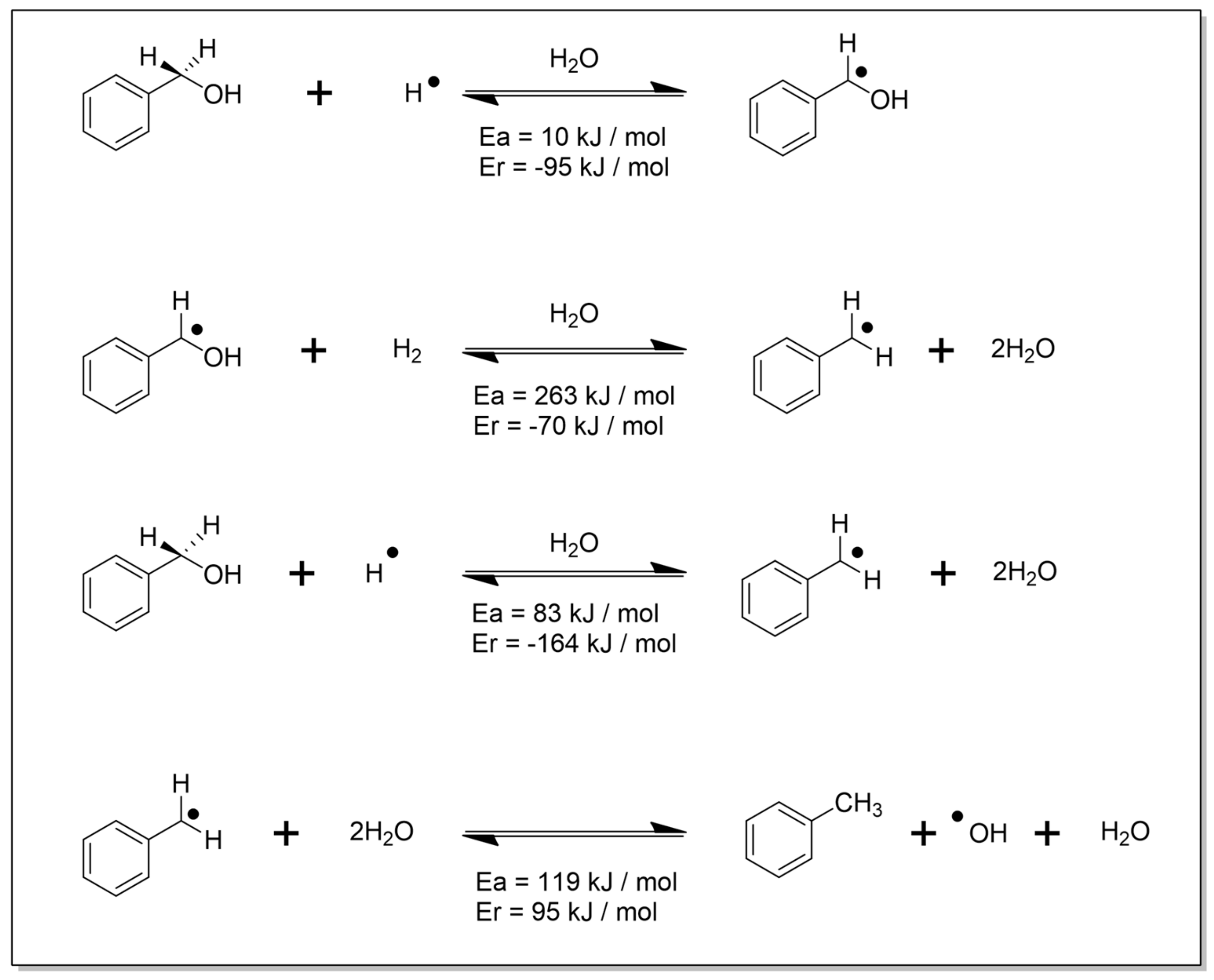
2.5. Quantum Chemical Calculations
3. Results
4. Discussion
5. Future Perspective
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Getahun, T.; Verma, M.; Villa, A.; Gupta, N. Carbon Based Catalysts for the Hydrodeoxygenation of Lignin and Related Molecules: A Powerful Tool for the Generation of Non-Petroleum Chemical Products Including Hydrocarbons. Renew. Sustain. Energy Rev. 2020, 133, 110280. [Google Scholar] [CrossRef]
- Klein, I.; Saha, B.; Abu-Omar, M.M. Lignin Depolymerization over Ni/C Catalyst in Methanol, a Continuation: Effect of Substrate and Catalyst Loading. Catal. Sci. Technol. 2015, 5, 3242–3245. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin Depolymerization (LDP) in Alcohol over Nickel-Based Catalysts via a Fragmentation-Hydrogenolysis Process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Zakzeski, J.; Jongerius, A.L.; Weckhuysen, B.M. Transition Metal Catalyzed Oxidation of Alcell Lignin, Soda Lignin, and Lignin Model Compounds in Ionic Liquids. Green Chem. 2010, 12, 1225–1236. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Xie, X.; Yang, S.; Sun, L.; Li, T.; Chen, L.; Hua, D. Synthesis of Aromatic Monomers via Hydrogenolysis of Lignin over Nickel Catalyst Supported on Nitrogen-Doped Carbon Nanotubes. Fuel Process. Technol. 2023, 248, 107810. [Google Scholar] [CrossRef]
- Mushtaq, U.; Park, J.; Riaz, A.; Ranaware, V.; Khan, M.K.; Verma, D.; Kim, J. High-Yield Production of Deoxygenated Monomers from Kraft Lignin over ZnO-Co/N-CNTs in Water. ACS Sustain. Chem. Eng. 2021, 9, 3232–3245. [Google Scholar] [CrossRef]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z.; Ruan, T. Impact of Nitrogen Species and Content on the Catalytic Activity to C–O Bond Cleavage of Lignin over N-Doped Carbon Supported Ru-Based Catalyst. Fuel 2020, 278, 118324. [Google Scholar] [CrossRef]
- Jana, N.C.; Behera, S.; Maharana, S.K.; Behera, R.R.; Bagh, B. Selective Aerobic Oxidation of Biomass Model Compound Veratryl Alcohol Catalyzed by Air-Stable Copper(Ii) Complexes in Water. Catal. Sci. Technol. 2023, 13, 5422–5434. [Google Scholar] [CrossRef]
- Jana, N.C.; Sethi, S.; Saha, R.; Bagh, B. Aerobic Oxidation of Vanillyl Alcohol to Vanillin Catalyzed by Air-Stable and Recyclable Copper Complex and TEMPO under Base-Free Conditions. Green Chem. 2022, 24, 2542–2556. [Google Scholar] [CrossRef]
- Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S.S. Chemoselective Metal-Free Aerobic Alcohol Oxidation in Lignin. J. Am. Chem. Soc. 2013, 135, 6415–6418. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; Antonietti, M. Chemistry and Materials Options of Sustainable Carbon Materials Made by Hydrothermal Carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.-C. A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research. C 2018, 4, 54. [Google Scholar] [CrossRef]
- Mestl, G.; Maksimova, N.I.; Keller, N.; Roddatis, V.V.; Schlögl, R. Carbon Nanofilaments in Heterogeneous Catalysis: An Industrial Application for New Carbon Materials? Angew. Chem.—Int. Ed. 2001, 40, 2066–2068. [Google Scholar] [CrossRef]
- Su, D.S.; Wen, G.; Wu, S.; Peng, F.; Schlögl, R. Carbocatalysis in Liquid-Phase Reactions. Angew. Chem.—Int. Ed. 2017, 56, 936–964. [Google Scholar] [CrossRef]
- Zhang, J.; Su, D.S.; Blume, R.; Schlögl, R.; Wang, R.; Yang, X.; Gajović, A. Surface Chemistry and Catalytic Reactivity of a Nanodiamond in the Steam-Free Dehydrogenation of Ethylbenzene. Angew. Chem.—Int. Ed. 2010, 49, 8640–8644. [Google Scholar] [CrossRef]
- Barlocco, I.; Bellomi, S.; Tumiati, S.; Fumagalli, P.; Dimitratos, N.; Roldan, A.; Villa, A. Selective Decomposition of Hydrazine over Metal Free Carbonaceous Materials. Phys. Chem. Chem. Phys. 2022, 24, 3017–3029. [Google Scholar] [CrossRef]
- Barlocco, I.; Capelli, S.; Lu, X.; Tumiati, S.; Dimitratos, N.; Roldan, A.; Villa, A. Role of Defects in Carbon Materials during Metal-Free Formic Acid Dehydrogenation. Nanoscale 2020, 12, 22768–22777. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jia, H.P.; Bielawski, C.W. Graphene Oxide: A Convenient Carbocatalyst for Facilitating Oxidation and Hydration Reactions. Angew. Chem.—Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Luo, F.; Khoshi, M.R.; Rabie, E.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Garfunkel, E.; Szostak, M.; He, H. P-Doped Porous Carbon as Metal Free Catalysts for Selective Aerobic Oxidation with an Unexpected Mechanism. ACS Nano 2016, 10, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Sun, G.; Gao, Y.; Zhao, H.; Tang, P.; Tan, J.; Lu, A.H.; Ma, D. Direct Catalytic Oxidation of Benzene to Phenol over Metal-Free Graphene-Based Catalyst. Energy Environ. Sci. 2013, 6, 793–798. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, D.; Wang, C.; Guan, J.; Bao, X. Reduced Graphene Oxide as a Catalyst for Hydrogenation of Nitrobenzene at Room Temperature. Chem. Commun. 2011, 47, 2432–2434. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Su, D. Fabrication of Nitrogen-Modified Annealed Nanodiamond with Improved Catalytic Activity. ACS Nano 2014, 8, 7823–7833. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Gambarotti, C.; Carroccio, S.; Filippone, G.; Cicogna, F.; Guenzi, M. α-Tocopherol-Induced Radical Scavenging Activity in Carbon Nanotubes for Thermo-Oxidation Resistant Ultra-High Molecular Weight Polyethylene-Based Nanocomposites. Carbon N. Y. 2014, 74, 14–21. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree-Fock and Hybrid DFT Calculations. A “chain-of-Spheres” Algorithm for the Hartree-Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, Y.; Li, T.; Rong, N.; Jiang, H.; Shi, H.; Yang, W. Radical Induced Disproportionation of Alcohols Assisted by Iodide under Acidic Conditions. Green Chem. 2021, 23, 8108–8115. [Google Scholar] [CrossRef]
- Martínez-Morlanes, M.J.; Castell, P.; Alonso, P.J.; Martinez, M.T.; Puértolas, J.A. Multi-Walled Carbon Nanotubes Acting as Free Radical Scavengers in Gamma-Irradiated Ultrahigh Molecular Weight Polyethylene Composites. Carbon N. Y. 2012, 50, 2442–2452. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Z.; He, S.; Hao, F.; Yang, Y.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H. Nitrogen-Doped Carbon Nanotubes as a Highly Active Metal-Free Catalyst for Nitrobenzene Hydrogenation. Appl. Catal. B 2020, 260, 118105. [Google Scholar] [CrossRef]




| Catalyst | Composition (%) | N1s | |||||
|---|---|---|---|---|---|---|---|
| C | O | N | |||||
| CNT700 | 82.8 | 3.5 | 13.7 | 398.1 | 400.0 | 400.9 | 404.4 |
| contribution (%) | 45.8 | 21.2 | 28.6 | 4.3 | |||
| CNT800 | 94.3 | 3.3 | 2.3 | 397.2 | 399.5 | 400.9 | 403.3 |
| contribution (%) | 22.6 | 32.0 | 8.8 | 36.5 | |||
| CNTcomm | 95.7 | 4.3 | - | - | - | - | - |
| contribution (%) | |||||||
| Catalyst a | Activity b | Conversion (after 6 h) | Selectivity | ||
|---|---|---|---|---|---|
| Benzaldehyde | Benzoic Acid | Toluene | |||
| N-CNTs 700 | 186 | >99 | 76 | 1 | 21 |
| N-CNTs 800 | 54 | 59 | 67 | 1 | 28 |
| CNTs comm | 5 | 8 | 99 | - | - |
| Catalyst a | Activity b | Conversion (after 6 h) | Selectivity | ||
|---|---|---|---|---|---|
| Veretraldehyde | Veretric Acid | 4-Methyl Veratrol | |||
| N-CNTs 700 | 104 | 95 | 75 | 1 | 23 |
| NCNTs 800 | 37 | 42 | 63 | 2 | 32 |
| CNTs comm | 3 | 3 | 99 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Barlocco, I.; Khavryuchenko, O.; Villa, A. Metal-Free Catalytic Conversion of Veratryl and Benzyl Alcohols through Nitrogen-Enriched Carbon Nanotubes. C 2024, 10, 13. https://doi.org/10.3390/c10010013
Gupta N, Barlocco I, Khavryuchenko O, Villa A. Metal-Free Catalytic Conversion of Veratryl and Benzyl Alcohols through Nitrogen-Enriched Carbon Nanotubes. C. 2024; 10(1):13. https://doi.org/10.3390/c10010013
Chicago/Turabian StyleGupta, Neeraj, Ilaria Barlocco, Oleksiy Khavryuchenko, and Alberto Villa. 2024. "Metal-Free Catalytic Conversion of Veratryl and Benzyl Alcohols through Nitrogen-Enriched Carbon Nanotubes" C 10, no. 1: 13. https://doi.org/10.3390/c10010013