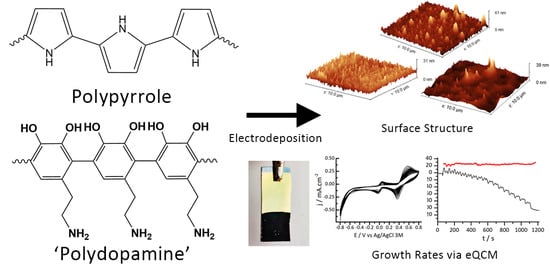
Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrochemical and Electrochemical Quartz Crystal Microbalance Studies
2.3. Preparation of PPY, PDA and PPY-PDA Films
2.4. Characterisation of PPY, PDA and PPY-PDA Films
2.5. Estimation of Film Thicknesses from eQCM Frequency Data
3. Results
3.1. Electrodeposition of Polydopamine-Polypyrrole Films by Cyclic Voltammetry
3.2. Electrochemical Quartz Crystal Microbalance (eQCM) Studies of PPY-PDA Deposition
3.3. Electrochemical Performance of PPY-PDA Coatings
3.4. Morphology and Roughness of PPY and PPY-PDA Films by AFM and Water Contact Angle Measurements
3.5. Spectroscopic Characterisation of PPY and PPY-PDA Films
3.6. Optoelectronic Properties of PPY and PPY-PDA Films
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asavapiriyanont, S.; Chandler, G.K.; Gunawardena, G.A.; Pletcher, D. The Electrodeposition of Polypyrrole Films from Aqueous Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1984, 177, 229–244. [Google Scholar] [CrossRef]
- Sabouraud, G.; Sadki, S.; Brodie, N. The Mechanisms of Pyrrole Electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Application of Polypyrrole for the Creation of Immunosensors. Crit. Rev. Anal. Chem. 2002, 32, 245–252. [Google Scholar] [CrossRef]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-Based Conducting Polymers and Interactions with Biological Tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a Flexible Electrode Material of Supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Choudhary, R.B.; Ansari, S.; Purty, B. Robust Electrochemical Performance of Polypyrrole (PPy) and Polyindole (PIn) Based Hybrid Electrode Materials for Supercapacitor Application: A Review. J. Energy Storage 2020, 29, 101302. [Google Scholar] [CrossRef]
- Fabregat, G.; Córdova-Mateo, E.; Armelin, E.; Bertran, O.; Alemán, C. Ultrathin Films of Polypyrrole Derivatives for Dopamine Detection. J. Phys. Chem. C 2011, 115, 14933–14941. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole Based next Generation Electrochemical Sensors and Biosensors: A Review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Andriukonis, E.; Reinikovaite, V.; Ramanavicius, A. Comparative Study of Polydopamine and Polypyrrole Modified Yeast Cells Applied in Biofuel Cell Design. Sustain. Energy Fuels 2022, 6, 4209–4217. [Google Scholar] [CrossRef]
- Yuan, X.; Ding, X.-L.; Wang, C.-Y.; Ma, Z.-F. Use of Polypyrrole in Catalysts for Low Temperature Fuel Cells. Energy Environ. Sci. 2013, 6, 1105–1124. [Google Scholar] [CrossRef]
- Kim, M.; Li, S.; Kong, D.S.; Song, Y.E.; Park, S.-Y.; Kim, H.; Jae, J.; Chung, I.; Kim, J.R. Polydopamine/Polypyrrole-Modified Graphite Felt Enhances Biocompatibility for Electroactive Bacteria and Power Density of Microbial Fuel Cell. Chemosphere 2023, 313, 137388. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical Deposition of Conductive and Adhesive Polypyrrole-Dopamine Films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef]
- Hemmatpour, H.; De Luca, O.; Crestani, D.; Stuart, M.C.A.; Lasorsa, A.; van der Wel, P.C.A.; Loos, K.; Giousis, T.; Haddadi-Asl, V.; Rudolf, P. New Insights in Polydopamine Formation via Surface Adsorption. Nat. Commun. 2023, 14, 664. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Park, J.; Lee, J.Y. Electrochemical Co-Deposition of Polydopamine/Hyaluronic Acid for Anti-Biofouling Bioelectrodes. Front. Chem. 2019, 7, 262. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.; Park, C.B. Polydopamine as a Biomimetic Electron Gate for Artificial Photosynthesis. Angew. Chem. Int. Ed. 2014, 53, 6364–6368. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-Step Modification of Superhydrophobic Surfaces by a Mussel-Inspired Polymer Coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Lupton, E.J.; Ma, Y.; Sun, R.; Grigsby, C.L.; Brachi, G.; Li, X.; Zhou, K.; Stuckey, D.J.; Stevens, M.M. Hybrid Polypyrrole and Polydopamine Nanosheets for Precise Raman/Photoacoustic Imaging and Photothermal Therapy. Adv. Healthc. Mater. 2023, 12, e2301148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, F.K.; Pan, Z.; Zhang, J.; Zhao, B. Bio-Inspired Dopamine Functionalization of Polypyrrole for Improved Adhesion and Conductivity. Macromol. Rapid Commun. 2014, 35, 350–354. [Google Scholar] [CrossRef]
- Behan, J.A.; Grajkowski, F.; Jayasundara, D.R.; Vilella-Arribas, L.; García-Melchor, M.; Colavita, P.E. Influence of Carbon Nanostructure and Oxygen Moieties on Dopamine Adsorption and Charge Transfer Kinetics at Glassy Carbon Surfaces. Electrochim. Acta 2019, 304, 221–230. [Google Scholar] [CrossRef]
- Ait-Itto, F.-Z.; Behan, J.A.; Martinez, M.; Barrière, F. Development of Bioanodes Rich in Exoelectrogenic Bacteria Using Iron-Rich Palaeomarine Sediment Inoculum. Bioelectrochemistry 2024, 156, 108618. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Q.; Pan, W.; Yao, Y. Advances in the Mass Sensitivity Distribution of Quartz Crystal Microbalances: A Review. Sensors 2022, 22, 5112. [Google Scholar] [CrossRef]
- Diaz, A.F.; Castillo, J.I.; Logan, J.A.; Lee, W.-Y. Electrochemistry of Conducting Polypyrrole Films. J. Electroanal. Chem. Interfacial Electrochem. 1981, 129, 115–132. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Ball, V.; Del Frari, D.; Michel, M.; Buehler, M.J.; Toniazzo, V.; Singh, M.K.; Gracio, J.; Ruch, D. Deposition Mechanism and Properties of Thin Polydopamine Films for High Added Value Applications in Surface Science at the Nanoscale. BioNanoScience 2012, 2, 16–34. [Google Scholar] [CrossRef]
- Tabačiarová, J.; Mičušík, M.; Fedorko, P.; Omastová, M. Study of Polypyrrole Aging by XPS, FTIR and Conductivity Measurements. Polym. Degrad. Stab. 2015, 120, 392–401. [Google Scholar] [CrossRef]
- Khadem, F.; Pishvaei, M.; Salami-Kalajahi, M.; Najafi, F. Morphology Control of Conducting Polypyrrole Nanostructures via Operational Conditions in the Emulsion Polymerization. J. Appl. Polym. Sci. 2017, 134, 44697. [Google Scholar] [CrossRef]
- Mallinson, D.; Mullen, A.B.; Lamprou, D.A. Probing Polydopamine Adhesion to Protein and Polymer Films: Microscopic and Spectroscopic Evaluation. J. Mater. Sci. 2018, 53, 3198–3209. [Google Scholar] [CrossRef]
- Nguyen Thi Le, H.; Bernard, M.C.; Garcia-Renaud, B.; Deslouis, C. Raman Spectroscopy Analysis of Polypyrrole Films as Protective Coatings on Iron. Synth. Met. 2004, 140, 287–293. [Google Scholar] [CrossRef]
- Morávková, Z.; Taboubi, O.; Minisy, I.M.; Bober, P. The Evolution of the Molecular Structure of Polypyrrole during Chemical Polymerization. Synth. Met. 2021, 271, 116608. [Google Scholar] [CrossRef]
- Rella, S.; Mazzotta, E.; Caroli, A.; De Luca, M.; Bucci, C.; Malitesta, C. Investigation of Polydopamine Coatings by X-Ray Photoelectron Spectroscopy as an Effective Tool for Improving Biomolecule Conjugation. Appl. Surf. Sci. 2018, 447, 31–39. [Google Scholar] [CrossRef]
- Idla, K.; Talo, A.; Niemi, H.E.-M.; Forsén, O.; Yläsaari, S. An XPS and AFM Study of Polypyrrole Coating on Mild Steel. Surf. Interface Anal. 1997, 25, 837–854. [Google Scholar] [CrossRef]
- Behan, J.A.; Stamatin, S.N.; Hoque, M.K.; Ciapetti, G.; Zen, F.; Esteban-Tejeda, L.; Colavita, P.E. Combined Optoelectronic and Electrochemical Study of Nitrogenated Carbon Electrodes. J. Phys. Chem. C 2017, 121, 6596–6604. [Google Scholar] [CrossRef]
- Zen, F.; Karanikolas, V.D.; Behan, J.A.; Andersson, J.; Ciapetti, G.; Bradley, A.L.; Colavita, P.E. Nanoplasmonic Sensing at the Carbon-Bio Interface: Study of Protein Adsorption at Graphitic and Hydrogenated Carbon Surfaces. Langmuir 2017, 33, 4198–4206. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Paiva, R.S.; Ramírez, A.M.R.; Mwanda, J.A.; Pereira, E.C.; Cuesta, A. Mapping the Electronic Structure of Polypyrrole with Image-Based Electrochemical Scanning Tunneling Spectroscopy. Electrochem. Sci. Adv. 2022, 2, e2100028. [Google Scholar] [CrossRef]
- Abdi, M.M.; Ekramul Mahmud, H.N.M.; Abdullah, L.C.; Kassim, A.; Zaki Ab. Rahman, M.; Chyi, J.L.Y. Optical Band Gap and Conductivity Measurements of Polypyrrole-Chitosan Composite Thin Films. Chin. J. Polym. Sci. 2012, 30, 93–100. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. Structural Basis of Polydopamine Film Formation: Probing 5,6-Dihydroxyindole-Based Eumelanin Type Units and the Porphyrin Issue. ACS Appl. Mater. Interfaces 2018, 10, 7670–7680. [Google Scholar] [CrossRef]








| Sample | Au | PPY | PDA | PPY-PDA |
|---|---|---|---|---|
| Roughness/nm | 1.7 ± 0.2 | 4.9 ± 0.9 | 2.4 ± 0.7 | 3.2 ± 0.1 |
| Water Contact Angle/° | >90 | 45 ± 1 | 54 ± 5 | 21.3 ± 0.9 |
| C 1s | N 1s | O 1s | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Contribution | Position | At.% | Contribution | Position | At.% | Contribution | Position | At.% |
| PDA | CHx | 284.8 | 43.81 | R3N/Ar-N | 397.8 | 10.86 | C=O | 531.36 | 12.19 |
| C-O/C-N | 285.9 | 35.65 | R2N-H | 400.2 | 77.06 | C-O | 533.09 | 87.81 | |
| C=O/C=N | 287.5 | 10.26 | RNH2 | 402.9 | 12.08 | ||||
| O-C=O | 288.6 | 6.9 | |||||||
| π-π* | 290.8 | 3.37 | |||||||
| PPY | Cβ | 283.9 | 24.05 | =N-C | 398.1 | 6.75 | SO42- | 530.79 | 22.11 |
| Cα | 284.9 | 29.65 | C-NH-C | 399.7 | 50.12 | C=O | 531.99 | 52 | |
| C-O and C-N | 285.8 | 24.67 | C-NH-C | 400.3 | 18.65 | C-O | 533.32 | 23.45 | |
| C=O and C=N | 287.4 | 11.56 | Polaron/Bipolaron | 401.2 | 21.66 | Oads | 536 | 2.44 | |
| O-C=O | 288.9 | 5.12 | 403.4 | 2.82 | |||||
| π-π* | 291.1 | 4.95 | |||||||
| PPY-PDA | CHx/Cβ | 283.9 | 15.61 | =N-C | 398 | 4.33 | SO42- | 530.84 | 29.16 |
| Cα | 285.1 | 51.19 | C-NH-C | 399.7 | 64.77 | C=O | 531.96 | 22.3 | |
| C-O/C-N | 286.4 | 20.4 | Polaron/Bipolaron | 401.2 | 25.13 | C-O | 533.14 | 44.26 | |
| C=O/C=N | 288.0 | 3.89 | 403.5 | 5.77 | Oads | 535.69 | 4.27 | ||
| O-C=O | 289.3 | 5.06 | |||||||
| π-π* | 291.2 | 3.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behan, J.A.; Barrière, F. Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films. C 2024, 10, 20. https://doi.org/10.3390/c10010020
Behan JA, Barrière F. Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films. C. 2024; 10(1):20. https://doi.org/10.3390/c10010020
Chicago/Turabian StyleBehan, James A., and Frédéric Barrière. 2024. "Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films" C 10, no. 1: 20. https://doi.org/10.3390/c10010020
APA StyleBehan, J. A., & Barrière, F. (2024). Effects of Polydopamine Incorporation on the Nanostructure and Electrochemical Performance of Electrodeposited Polypyrrole Films. C, 10(1), 20. https://doi.org/10.3390/c10010020