A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research
Abstract
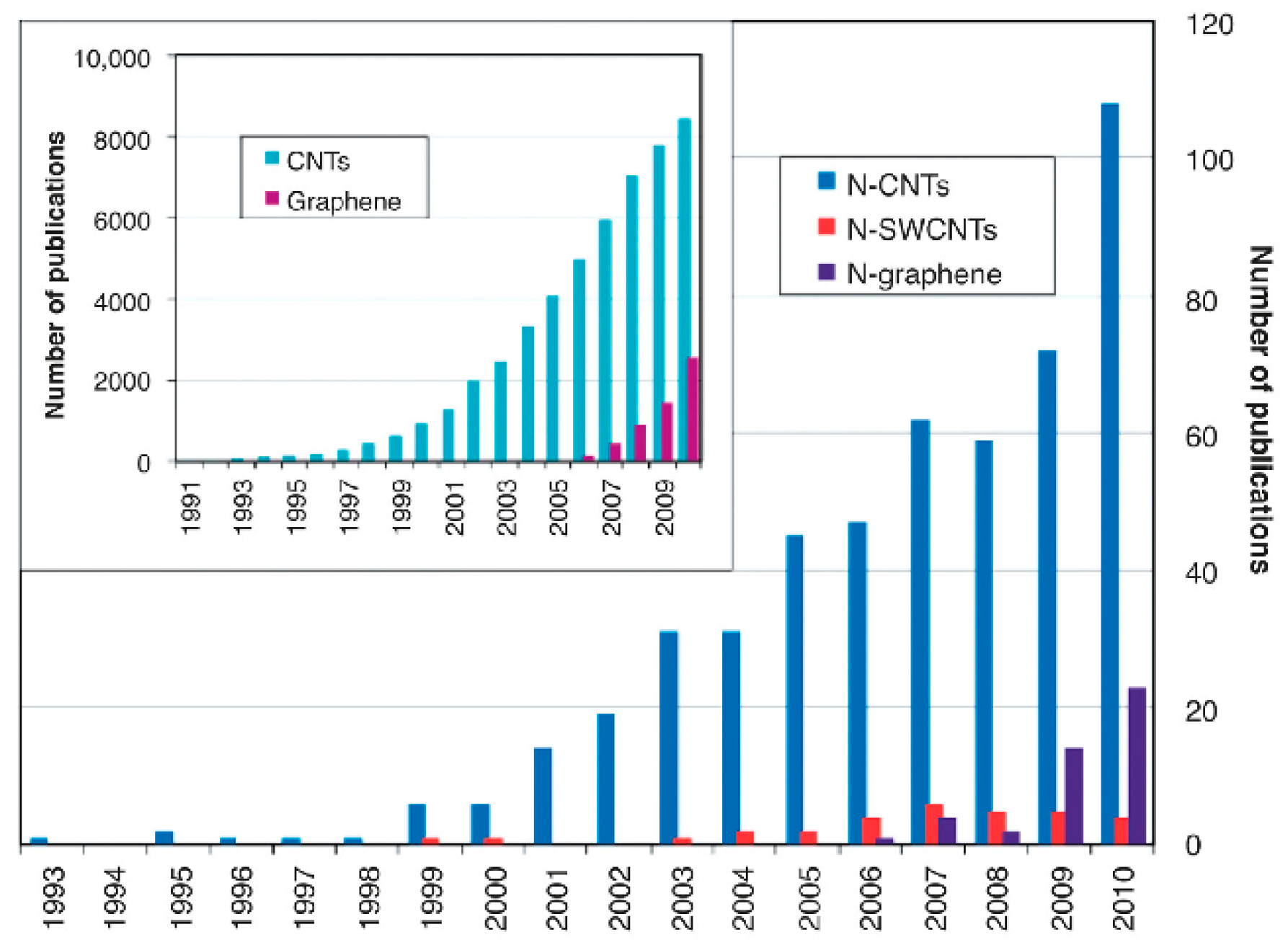
:1. Introduction
2. Graphene Nanocomposites for Catalysis
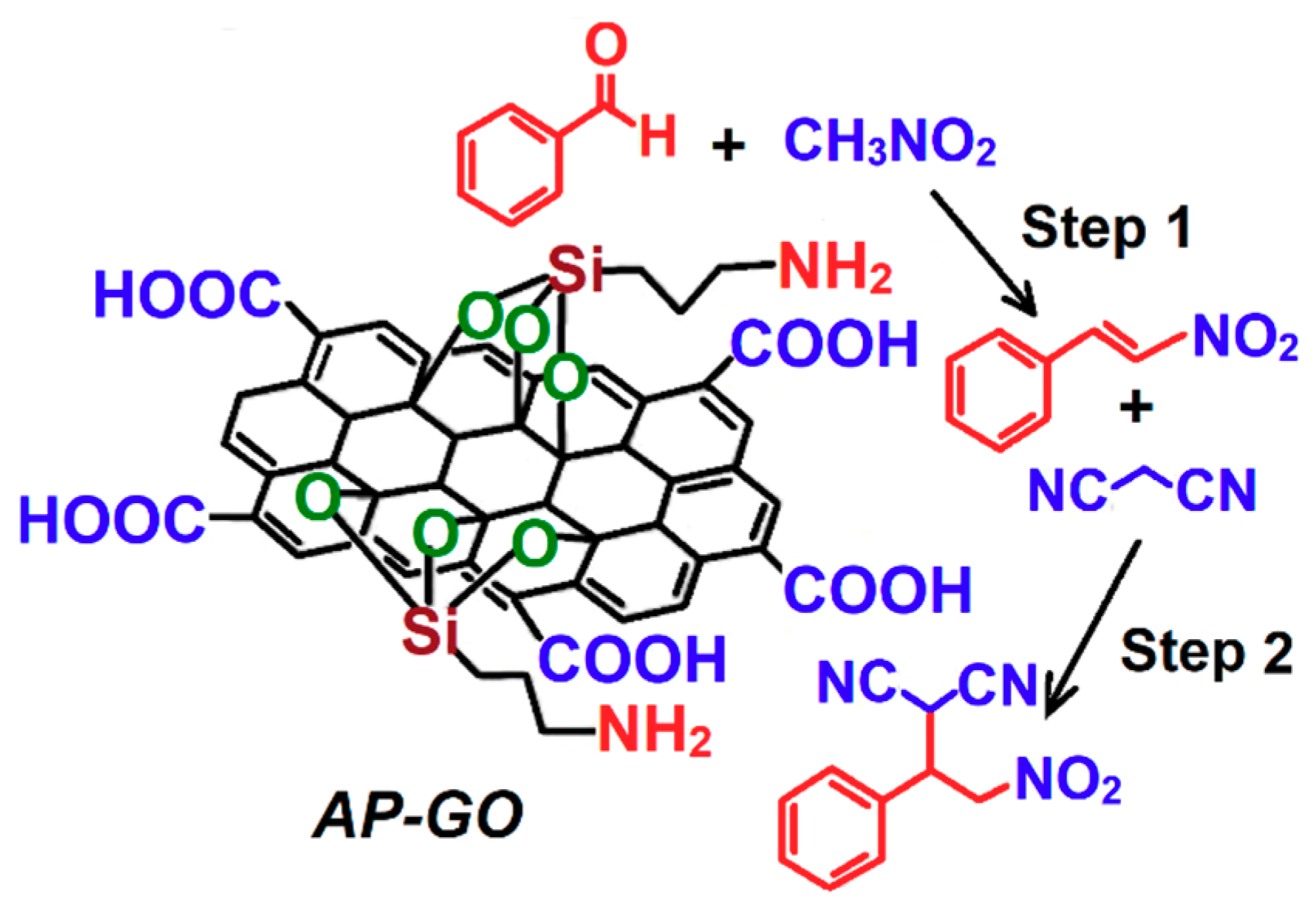
2.1. C–N Coupling Reaction
2.2. Dehydrogenative C–C Coupling Reaction
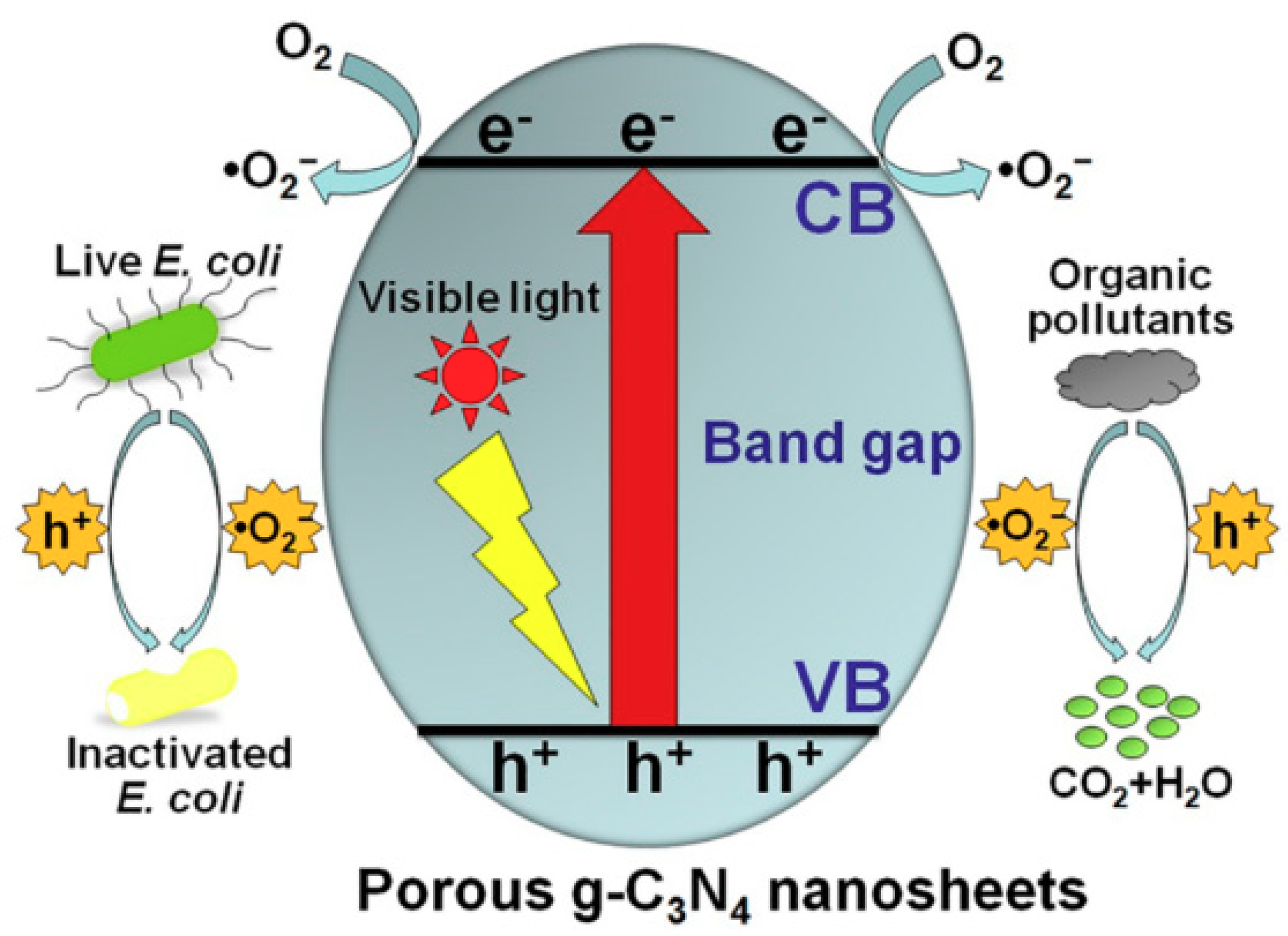
3. Graphitic Carbon Nitride Nanocomposites for Catalysis
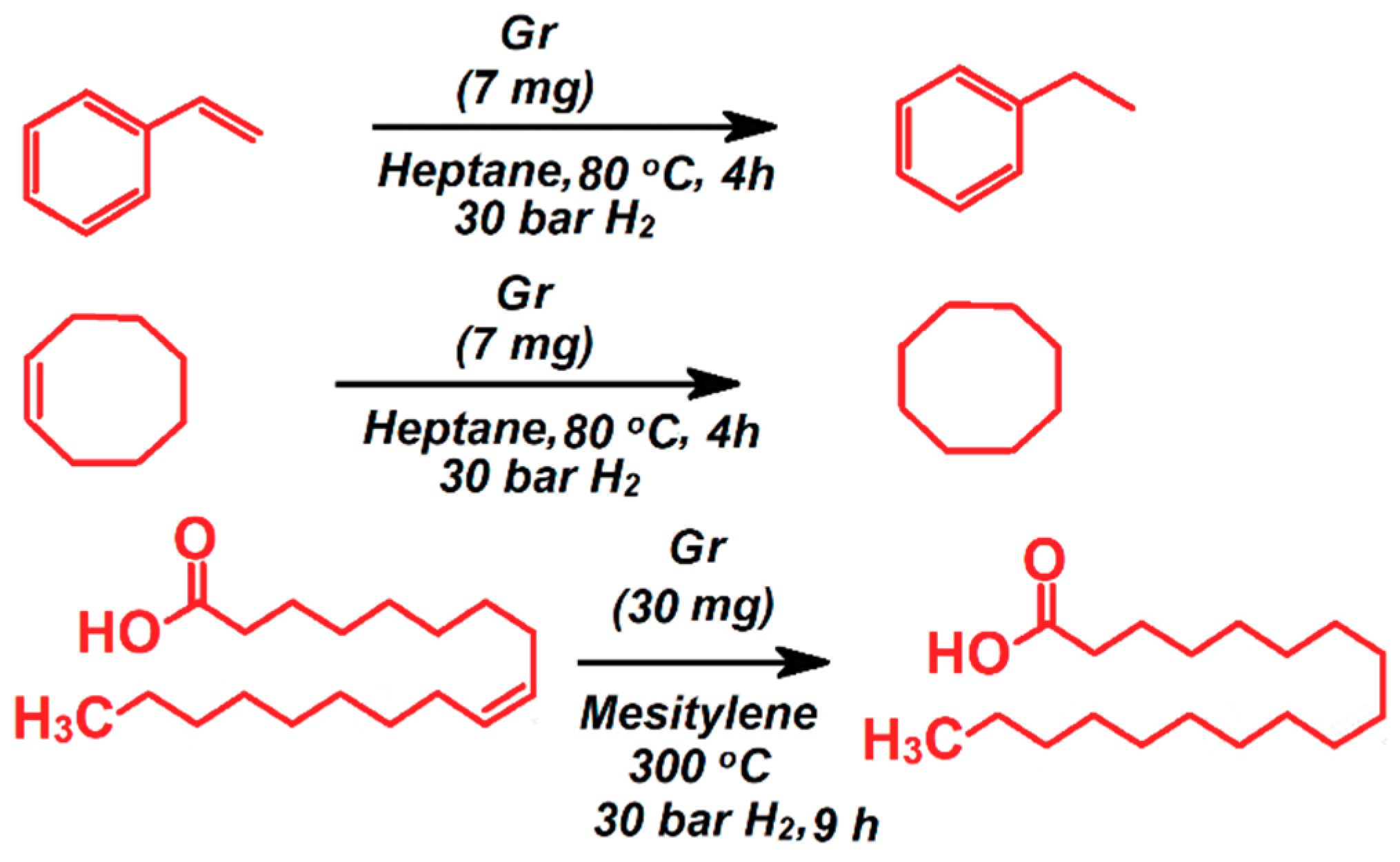
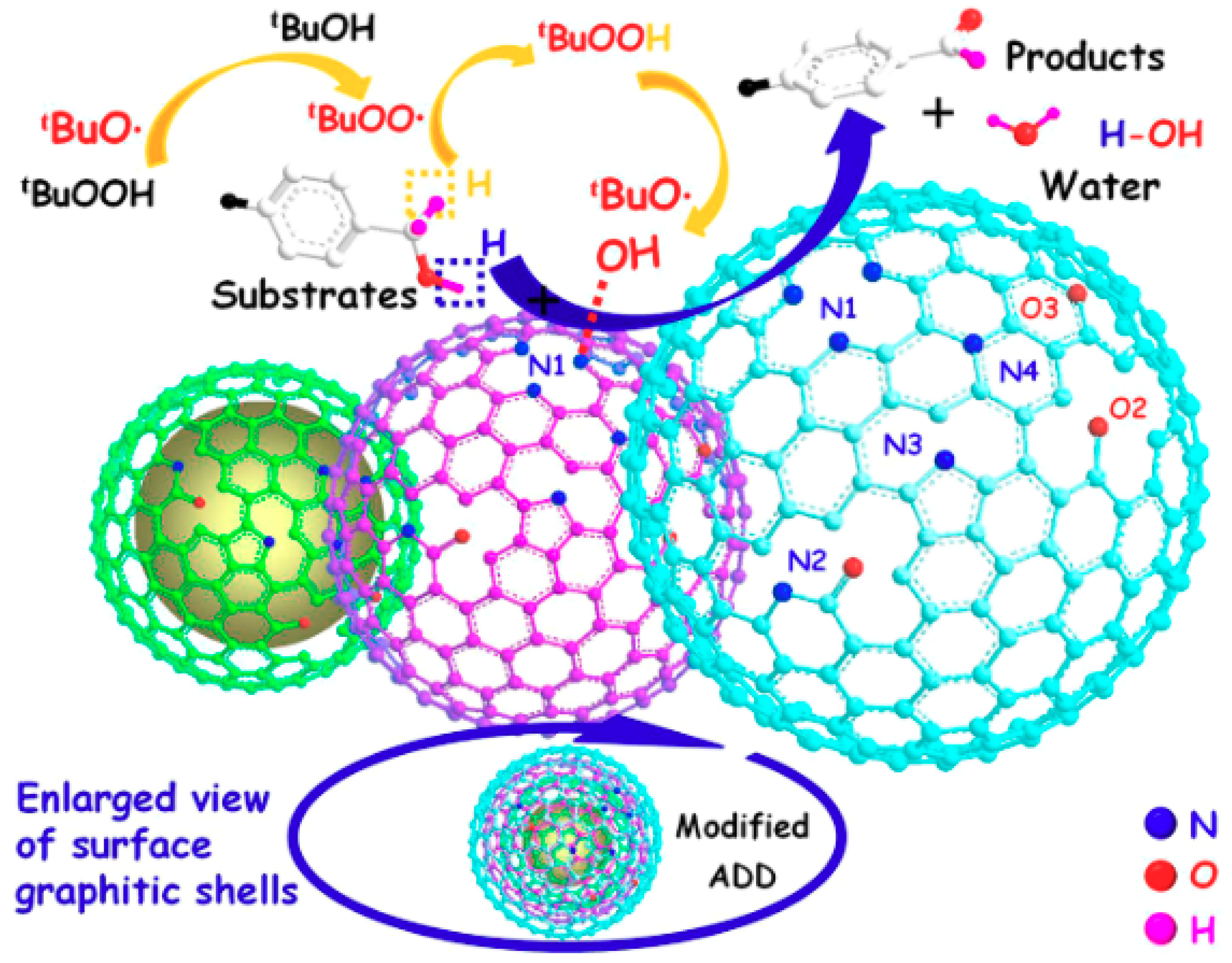
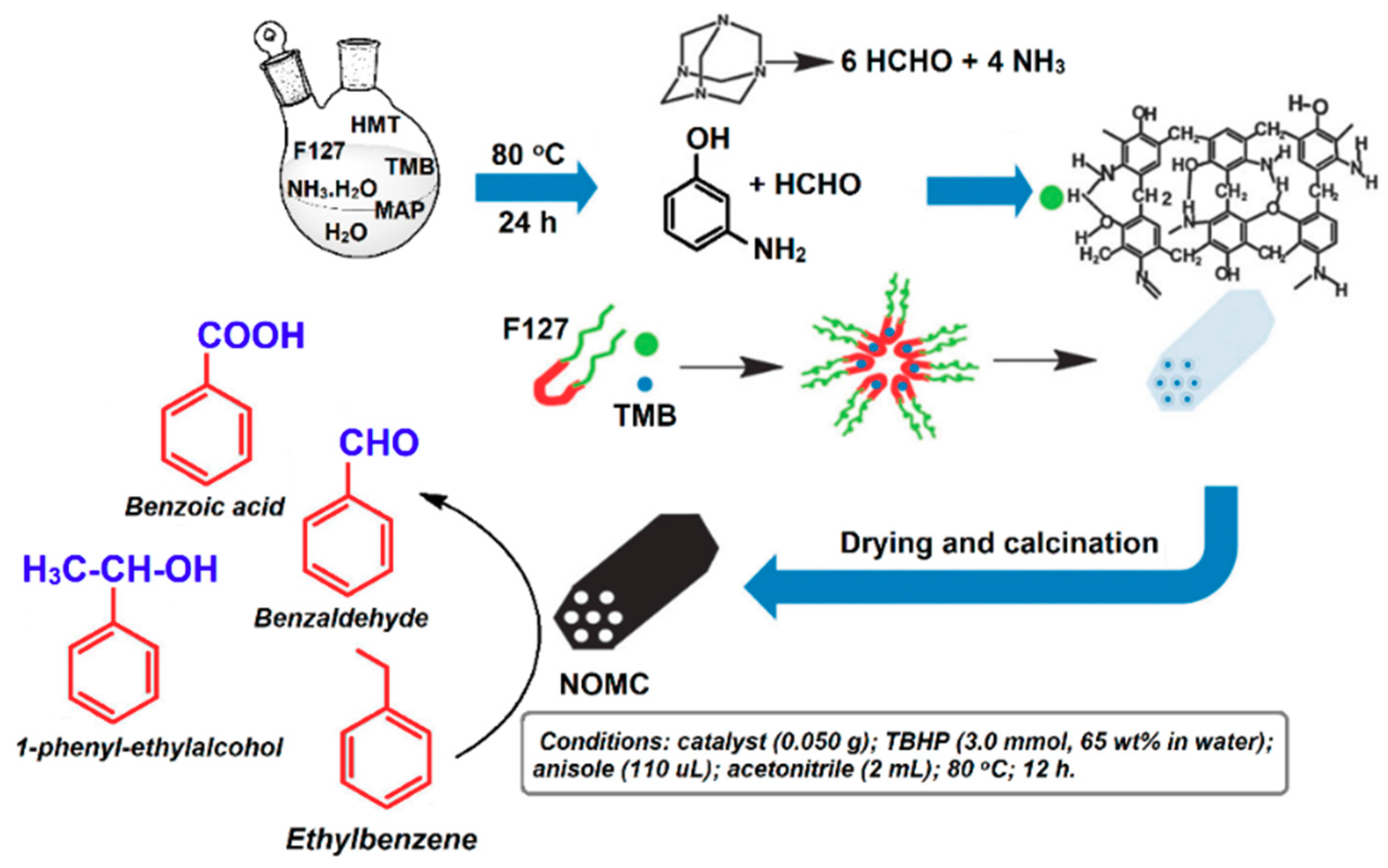
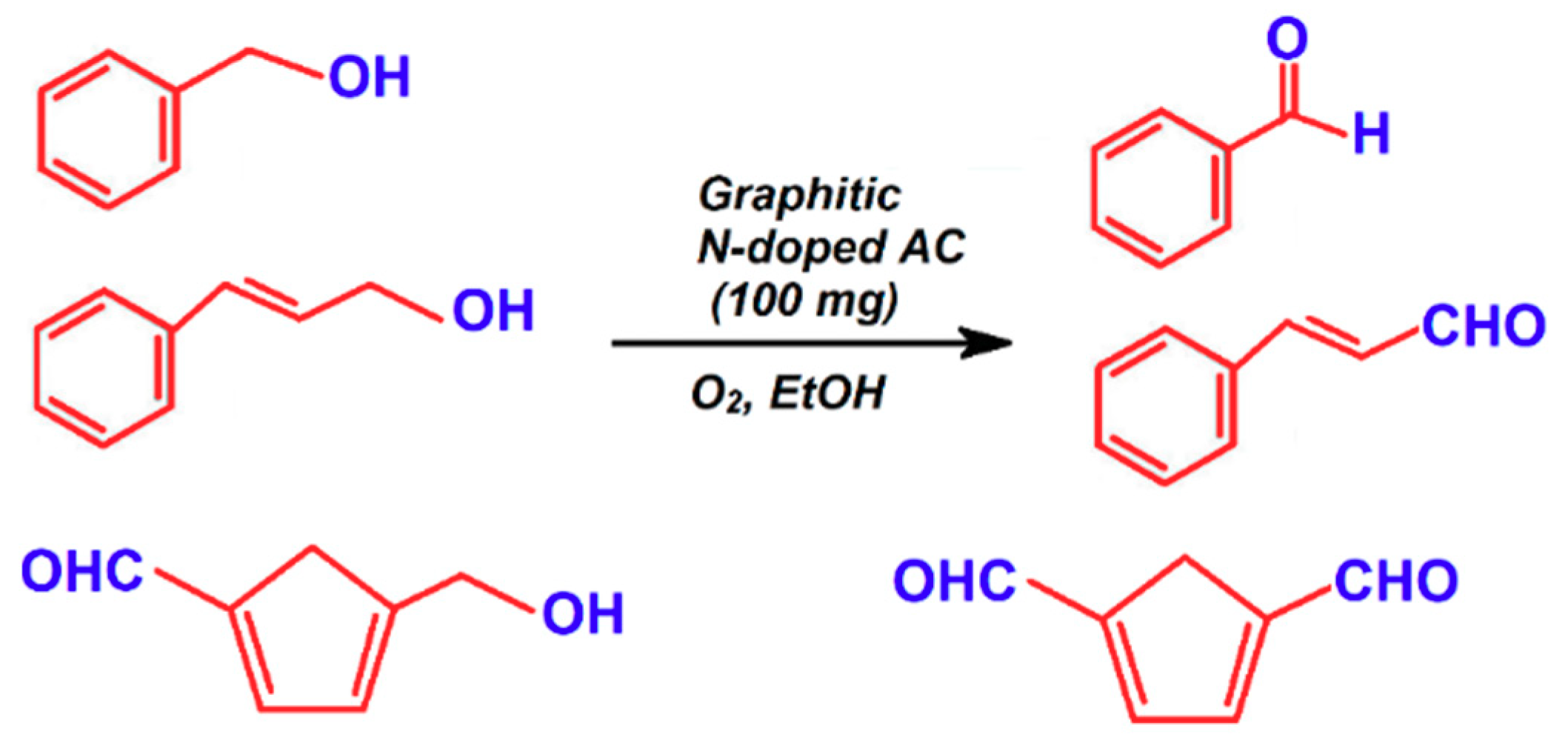
4. Porous Carbon Nanocomposites for Catalysis
5. Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| AC | Activated carbon |
| AP-GO | Amine functionalized graphene oxide |
| ATC | Ammonium thiocyanate |
| BCN | Bulk graphitic carbon nitride |
| CPMs | Carbon porous materials |
| CFs | Carbon fibers |
| CB | Carbon black |
| CNTs | Carbon nanotubes |
| CNDs | Carbon nanodots |
| CA | Cyanamide |
| DCA | Dicyandiamide |
| Gr | Graphene |
| GO | Graphene oxide |
| GCN | Graphitic carbon nitride |
| h-BN | Hexagonal-boron nitride |
| HMT | Hexamethylenetetramine |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| MA | Melamine |
| MB | Methylene blue |
| MO | Methyl orange |
| ND | Nanodiamond |
| NPC | N-doped porous carbon |
| N–RGO | N-doped reduced graphene oxide |
| OMCs | Ordered mesoporous carbon |
| PCNS | Porous graphitic carbon nitride nanosheets |
| RhB | Rhodamine B |
| TC | Tetracycline |
| TU | Thiourea |
| UV | Ultra-violet |
| U | Urea |
References
- Zhang, P.; Zhu, H.; Dai, S. Porous carbon supports: Recent advances with various morphologies and compositions. ChemCatChem 2015, 7, 2788–2805. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. High hydrogen storage capacity of porous carbons prepared by using activated carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Veerakumar, P.; Madhu, R.; Chen, S.-M.; Hung, C.-T.; Tang, P.-H.; Wang, C.-B.; Liu, S.-B. Porous carbon-modified electrodes as highly selective and sensitive sensors for detection of dopamine. Analyst 2014, 139, 4994–5000. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Hu, G.; Li, M.; Ma, D. Graphene-based metal-free catalysts for catalytic reactions in the liquid phase. ACS Catal. 2016, 6, 6948–6958. [Google Scholar] [CrossRef]
- Serp, P.; Machado, B. Nanostructured Carbon Materials for Catalysis; The Royal Society of Chemistry: Cambridge, UK, 2015; ISSN 1757–6725. [Google Scholar]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Xiaoyan, S.; Rui, W.; Dangsheng, S. Research progress in metal-free carbon-based catalysts. Chin. J. Catal. 2013, 34, 508–523. [Google Scholar]
- Nakajima, K.; Hara, M. Amorphous carbon with SO3H groups as a solid brønsted acid catalyst. ACS Catal. 2012, 2, 1296–1304. [Google Scholar] [CrossRef]
- Dutta, S.; Bohre, A.; Zheng, W.; Jenness, G.R.; Núñez, M.; Saha, B.; Vlachos, D.G. Solventless C–C coupling of low carbon furanics to high carbon fuel precursors using an improved graphene oxide carbocatalyst. ACS Catal. 2017, 7, 3905–3915. [Google Scholar] [CrossRef]
- Haag, D.; Kung, H.H. Metal free graphene based catalysts: A review. Top. Catal. 2014, 57, 762–773. [Google Scholar] [CrossRef]
- Shearer, C.J.; Cherevan, A.; Eder, D. Application and future challenges of functional nanocarbon hybrids. Adv. Mater. 2014, 26, 2295–2318. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.K.; Chen, C.L.; Chen, Q.W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2014, 43, 2841–2857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Blume, R.; Zhang, A.; Schlögl, R.; Su, D.S. Surface-modified carbon nanotubes catalyze oxidative dehydrogenation of n-butane. Science 2008, 322, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.; Morassutto, M.; Schomaecker, R.; Schloegl, R.; Su, D.S. Oxidative dehydrogenation of ethane over multiwalled carbon nanotubes. ChemCatChem 2010, 2, 644–648. [Google Scholar] [CrossRef]
- McGregor, J.; Huang, Z.; Parrott, E.P.J.; Zeitler, J.A.; Nguyen, K.L.; Rawson, J.M.; Carley, A.; Hansen, T.W.; Tessonnier, J.-P.; Su, D.S.; et al. Active coke: Carbonaceous materials as catalysts for alkane dehydrogenation. J. Catal. 2010, 269, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, A.; Zhang, J.; Frank, B.; Su, D.S.; Hamid, S.B.A.; Schlçgl, R. Oxidative purification of carbon nanotubes and its impact on catalytic performance in oxidative dehydrogenation reactions. ChemSusChem 2010, 3, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Jia, H.P.; Bielawski, C.W. Graphene oxide: A convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew. Chem. Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Li, H.; Chen, Z.; Wang, Y. Solvent-free aerobic oxidation of hydrocarbons and alcohols with Pd@N-doped carbon from glucose. Nat. Commun. 2013, 4, 1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhang, J.; Zhang, M.; Wang, X. Molecular and textural engineering of conjugated carbon nitride catalysts for selective oxidation of alcohols with visible light. Chem. Sci. 2013, 4, 3244–3248. [Google Scholar] [CrossRef]
- Huang, H.; Huang, J.; Liu, Y.-M.; He, H.-Y.; Cao, Y.; Fan, K.-N. Graphite oxide as an efficient and durable metal-free catalyst for aerobic oxidative coupling of amines to imines. Green Chem. 2012, 14, 930–934. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jia, H.-P.; Todd, A.D.; Geng, J.; Bielawski, C.W. Graphite oxide: A selective and highly efficient oxidant of thiols and sulfides. Org. Biomol. Chem. 2011, 9, 7292–7295. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, M.; Wang, Y.; Zhai, L.; Cui, Y.; Li, Y.; Li, Y.; Zhou, H.; Hong, X.; Denga, Z. Metal-free direct amidation of peptidyl thiol esters with α-amino acid esters. Green Chem. 2011, 13, 2723–2726. [Google Scholar] [CrossRef]
- Jia, H.-P.; Dreyer, D.R.; Bielawski, C.W. C–H oxidation using graphite oxide. Tetrahedron 2011, 67, 4431–4464. [Google Scholar] [CrossRef]
- Ahmad, H.; Fana, M.; Hui, D. Graphene oxide incorporated functional materials: A review. Composites B 2018, 145, 270–280. [Google Scholar] [CrossRef]
- Boukhvalov, D.V.; Dreyer, D.R.; Bielawski, C.W.; Soon, Y.-W. A computational investigation of the catalytic properties of graphene oxide: Exploring mechanisms by using DFT methods. ChemCatChem 2012, 4, 1844–1849. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; He, J.; Franconetti, A.; Asiri, A.M.; Primo, A.; Garcia, H. Defective graphene as a metal-free catalyst for chemoselective olefin hydrogenation by hydrazine. Catal. Sci. Technol. 2018, 8, 1589–1598. [Google Scholar] [CrossRef]
- Vijay, K.A.; Rao, K.R. Recyclable graphite oxide catalyzed Friedel-Crafts addition of indoles to α,β-unsaturated ketones. Tetrahedron Lett. 2011, 52, 5188–5191. [Google Scholar] [CrossRef]
- Favaretto, L.; An, J.; Sambo, M.; Nisi, A.D.; Bettini, C.; Melucci, M.; Kovtun, A.; Liscio, A.; Palermo, V.; Bottoni, A.; et al. Graphene oxide promotes site-selective allylic alkylation of thiophenes with alcohols. Org. Lett. 2018, 20, 3705–3709. [Google Scholar] [CrossRef] [PubMed]
- Acocella, M.R.; Mauro, M.; Falivene, L.; Cavallo, L.; Guerra, G. Inverting the diastereoselectivity of the Mukaiyama-Michael addition with graphite-based catalysts. ACS Catal. 2014, 4, 492–496. [Google Scholar] [CrossRef]
- Acocella, M.R.; Pascale, M.D.; Maggio, M.; Guerra, G. Graphite oxide as catalyst for diastereoselective Mukaiyama aldol reaction of 2-(trimethylsilyloxy) furan in solvent free conditions. J. Mol. Catal. A 2015, 408, 237–241. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jarvis, K.A.; Ferriera, P.J.; Bielawski, C.W. Graphite oxide as a dehydrative polymerization catalyst: A one-step synthesis of carbon-reinforced poly (phenylene methylene) composites. Macromolecules 2011, 44, 7659–7667. [Google Scholar] [CrossRef]
- Mauro, M.; Acocella, M.R.; Corcione, C.E.; Maffezzoli, A.; Guerra, G. Catalytic activity of graphite-based nanofillers on cure reaction of epoxy resins. Polymer 2014, 55, 5612–5615. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Concepcion, P.; Fornes, V.; Garcia, H. Graphene oxide as an acid catalyst for the room temperature ring opening of epoxides. Chem. Commun. 2012, 48, 5443–5445. [Google Scholar] [CrossRef] [PubMed]
- Acocella, M.R.; Mauro, M.; Guerra, G. Regio- and Enantioselective Friedel-Crafts reactions of indoles to epoxides catalyzed by graphene oxide: A green approach. ChemSusChem 2014, 7, 3279–3283. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Ghosh, S.; Ghosh, P.; Basu, B. Graphene oxide (GO) or reduced graphene oxide (rGO): Efficient catalysts for one-pot metal-free synthesis of quinoxalines from 2-nitroaniline. Tetrahedron Lett. 2015, 56, 6762–6767. [Google Scholar] [CrossRef]
- Kundu, S.; Basu, B. Graphene oxide (GO)-catalyzed multi-component reactions: Green synthesis of library of pharmacophore 3-sulfenylimidazo[1,2-a]pyridines. RSC Adv. 2015, 5, 50178–50185. [Google Scholar] [CrossRef]
- Furimsky, E. Carbon materials for catalysis. J. Am. Chem. Soc. 2009, 131, 9856–9857. [Google Scholar] [CrossRef]
- Sengupta, A.; Su, C.L.; Bao, C.L.; Nai, C.T.; Loh, K.P. Graphene oxide and its functionalized derivatives as carbocatalysts in the multicomponent strecker reaction of ketones. ChemCatChem 2014, 6, 2507–2511. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; Wang, S. Metal-free carbocatalysis in advanced oxidation reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Loh, K.P. Carbocatalysts: Graphene oxide and its derivatives. Acc. Chem. Res. 2013, 46, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Jamatia, R.; Gupta, A.; Dam, B.; Saha, M.; Pal, A.K. Graphite oxide: A metal free highly efficient carbocatalyst for the synthesis of 1,5-benzodiazepines under room temperature and solvent free heating conditions. Green Chem. 2017, 19, 1576–1585. [Google Scholar] [CrossRef]
- Mohammadi, O.; Golestanzadeh, M.; Abdouss, M. Recent advances in organic reactions catalyzed by graphene oxide and sulfonated graphene as heterogeneous nanocatalysts: A review. New J. Chem. 2017, 41, 11471–11497. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and LEDs. Nano-Micro Lett. 2017, 9, 47. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Nikokavoura, A.; Trapalis, C. Graphene and g-C3N4 based photocatalysts for NOx removal: A review. Appl. Surf. Sci. 2018, 430, 18–52. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Bahuguna, A.; Sharma, V.; Krishnan, V. Two-dimensional carbon-based nanocomposites for photocatalytic energy generation and environmental remediation applications. Beilstein J. Nanotechnol. 2017, 8, 1571–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Jeong, D.-Y.; Lim, J.-H.; Singh, P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018. [Google Scholar] [CrossRef]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons: New Jersey, NJ, USA, 2009; ISBN 978-0-470-17885-0. [Google Scholar]
- Su, D.S.; Wen, G.; Wu, S.; Peng, F.; Schlögl, R. Carbocatalysis in liquid-phase reactions. Angew. Chem. Int. Ed. 2017, 56, 936–964. [Google Scholar] [CrossRef] [PubMed]
- Malaika, A.; Wower, K.; Kozłowski, M. Chemically modified activated carbons as catalysts of oxidative dehydrogenation of n-butane. Acta Phys. Pol. A 2010, 118, 459–464. [Google Scholar] [CrossRef]
- Liu, R.; Li, F.; Chen, C.; Song, Q.; Zhao, N.; Xiao, F. Nitrogen-functionalized reduced graphene oxide as carbocatalysts with enhanced activity for polyaromatic hydrocarbon hydrogenation. Catal. Sci. Technol. 2017, 7, 1217–1226. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Carrasco-Marín, F.; Parejo-Pérez, C.; López, R.M. Dehydration of methanol to dimethyl ether catalyzed by oxidized activated carbons with varying surface acidic character. Carbon 2001, 39, 869–875. [Google Scholar] [CrossRef]
- Szymański, G.S.; Grzybek, T.; Papp, H. Influence of nitrogen surface functionalities on the catalytic activity of activated carbon in low temperature SCR of NOx with NH3. Catal. Today 2004, 90, 51–59. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Linares-Solano, A. The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibers. Carbon 2003, 41, 1925–1932. [Google Scholar] [CrossRef]
- Li, K.; Ling, L.; Lu, C.; Qiao, W.; Liu, Z.; Liu, L.; Mochida, I. Catalytic removal of SO2 over ammonia-activated carbon fibers. Carbon 2001, 39, 1803–1808. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereir, M.F.R. A ozone decomposition in water catalyzed by activated carbon: Influence of chemical and textural properties. Ind. Eng. Chem. Res. 2006, 45, 2715–2721. [Google Scholar] [CrossRef]
- Gomes, H.T.; Machado, B.F.; Ribeiro, A.; Moreira, I.; Rosário, M.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L. Catalytic properties of carbon materials for wet oxidation of aniline. J. Hazard. Mater. 2008, 159, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Yustos, P.; Rodriguez, S.; Garcia-Ochoa, F. Wet oxidation of phenol, cresols and nitrophenols catalyzed by activated carbon in acid and basic media. Appl. Catal. B Environ. 2006, 65, 269–281. [Google Scholar] [CrossRef]
- Zhou, H.; Lou, H.; Lu, W. Improving the catalytic efficiency of carbon-based active sites by trace oxide promoters for highly productive olefin synthesis. Catal. Sci. Technol. 2017, 7, 802–806. [Google Scholar] [CrossRef]
- Espinosa, J.C.; Navalon, S.; Alvaro, M.; Dhakshinamoorthy, A.; Garcia, H. Reduction of C=C double bonds by hydrazine using active carbons as metal-free catalysts. ACS Sustain. Chem. Eng. 2018, 6, 5607–5614. [Google Scholar] [CrossRef]
- Li, M.; Xu, F.; Li, H.; Wang, Y. Nitrogen-doped porous carbon materials: Promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation. Catal. Sci. Technol. 2016, 6, 3670–3693. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Wang, X. Graphene-based nanomaterials for catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by graphene-based materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cui, X.; Dai, X.; Deng, Y.; Shi, F. Carbon-catalysed reductive hydrogen atom transfer reactions. Nat. Commun. 2015, 6, 6478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navalón, S.; Herance, J.R.; Álvaro, M.; García, H. General aspects in the use of graphenes in catalysis. Mater. Horiz. 2018, 5, 363–378. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Primo, A.; Concepcion, P.; Alvaro, M.; Garcia, H. Doped graphene as a metal-free carbocatalyst for the selective aerobic oxidation of benzylic hydrocarbons, cyclooctane and styrene. Chem. Eur. J. 2013, 19, 7547–7554. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Chen, K.; Wang, Y.; Kang, L.; Zhu, M. Boron and nitrogen doping in graphene for the catalysis of acetylene hydrochlorination. ACS Catal. 2015, 5, 2541–2547. [Google Scholar] [CrossRef]
- Zhang, S.; Hong, B.; Fan, Z.; Lu, J.; Xu, Y.; Pera-Titus, M. Aquivion-carbon composites with tunable amphiphilicity for pickering interfacial catalysis. ACS Appl. Mater. Interfaces 2018, 10, 26795–26804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, H.; Wu, X.; Mao, Z.; Li, H. Organoamine-functionalized graphene oxide as a bifunctional carbocatalyst with remarkable acceleration in a one-pot multistep reaction. ACS Appl. Mater. Interfaces 2015, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Shekhar, A.; Mungse, H.P.; Khatri, O.P.; Pathak, D.D. Metal-free one-pot synthesis of amides using graphene oxide as an efficient catalyst. RSC Adv. 2014, 4, 41690–41695. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, H.; Li, X.; Wu, X.; Li, H. Amine-functionalized GO as an active and reusable acid-base bifunctional catalyst for one-pot cascade reactions. ACS Catal. 2014, 4, 394–401. [Google Scholar] [CrossRef]
- Majumdar, B.; Sarma, D.; Bhattacharya, T.; Sarma, T.K. Graphene oxide as metal-free catalyst in oxidative dehydrogenative C–N coupling leading to α-ketoamides: Importance of dual catalytic activity. ACS Sustain. Chem. Eng. 2017, 5, 9286–9294. [Google Scholar] [CrossRef]
- Fang, J.; Peng, Z.; Yang, Y.; Wang, J.; Guo, J.; Gong, H. Graphene-oxide-promoted direct dehydrogenative coupling reaction of aromatics. Asian J. Org. Chem. 2018, 7, 355–358. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, J.; Huang, C.; Gu, Q.; Zeng, Y.; Xu, W.; Meng, X.; Fu, W.; Sun, Y. Selective etching of N-doped graphene meshes as metal free catalyst with tunable kinetics, high activity and the origin of new catalytic behaviors. Part. Part. Syst. Charact. 2018, 35, 1700395. [Google Scholar] [CrossRef]
- Dai, Y.; Chai, Y.; Sun, Y.; Fu, W.; Wang, X.; Gu, Q.; Zeng, T.H.; Sun, Y. New versatile Pt supports composed of graphene sheets decorated by Fe2O3 nanorods and N-dopants with high activity based on improved metal/support interactions. J. Mater. Chem. A 2015, 3, 125–130. [Google Scholar] [CrossRef]
- Liu, J.; Yan, X.; Wang, L.; Kong, L.; Jian, P. Three-dimensional nitrogen-doped graphene foam as metal-free catalyst for the hydrogenation reduction of p-nitrophenol. J. Colloid Interface Sci. 2017, 497, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kang, S.W.; Lee, Y.W.; Park, Y.; Kim, J.H.; Han, S.W. Regulating the catalytic function of reduced graphene oxides using capping agents for metal-free catalysis. ACS Appl. Mater. Interfaces 2017, 9, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Sun, Z.; Chen, M.; Chen, C.; Chen, Q.W. Metal-free catalytic reduction of 4-nitrophenol to 4-aminophenol by N-doped graphene. Energy Environ. Sci. 2013, 6, 3260–3266. [Google Scholar] [CrossRef]
- Primo, A.; Neatu, F.; Florea, M.; Parvulescu, V.; Garcia, H. Graphenes in the absence of metals as carbocatalysts for selective acetylene hydrogenation and alkene hydrogenation. Nat. Commun. 2014, 5, 5291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, S.; Deng, G.-J.; Gong, H. Metal-free, oxidant-free, and controllable graphene oxide catalyzed direct iodination of arenes and ketones. ChemCatChem 2018, 10, 376–380. [Google Scholar] [CrossRef]
- Kim, Y.; Some, S.; Lee, H. Graphene oxide as a recyclable phase transfer catalyst. Chem. Commun. 2013, 49, 5702–5704. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tang, P.; Zhou, H.; Zhang, W.; Yang, H.; Yan, N.; Hu, G.; Mei, D.; Wang, J.; Ma, D. Graphene oxide catalyzed C–H bond activation: The importance of oxygen functional groups for biaryl construction. Angew. Chem. Int. Ed. 2016, 55, 3124–3128. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Alvaro, M.; Puche, M.; Fornes, V.; Garcia, H. Graphene oxide as catalyst for the acetalization of aldehydes at room temperature. ChemCatChem 2012, 4, 2026–2030. [Google Scholar] [CrossRef]
- Basu, B.; Kundu, S.; Sengupta, D. Graphene oxide as a carbocatalyst: The first example of a one-pot sequential dehydration-hydrothiolation of secondary aryl alcohols. RSC Adv. 2013, 3, 22130–22134. [Google Scholar] [CrossRef]
- Nagai, M.; Isoe, R.; Ishiguro, K.; Tominaga, H.; Shimizu, M. Graphite and graphene oxides catalyze bromination or alkylation in reaction of phenol with t-butylbromide. Chem. Eng. J. 2012, 207, 938–942. [Google Scholar] [CrossRef]
- Goncalves, G.A.B.; Pires, S.M.G.; Simoes, M.M.Q.; Neves, M.G.P.M.S.; Marques, P.A.A.P. Three-dimensional graphene oxide: A promising green and sustainable catalyst for oxidation reactions at room temperature. Chem. Commun. 2014, 50, 7673–7676. [Google Scholar] [CrossRef] [PubMed]
- Sedrpoushan, A.; Heidari, M.; Akhavan, O. Nanoscale graphene oxide sheets as highly efficient carbocatalysts in green oxidation of benzylic alcohols and aromatic aldehydes. Chin. J. Catal. 2017, 38, 745–757. [Google Scholar] [CrossRef]
- Mungse, H.P.; Bhakuni, N.; Tripathi, D.; Sharma, O.P.; Sain, B.; Khatri, O.P. Fractional distribution of graphene oxide and its potential as an efficient and reusable solid catalyst for esterification reactions. J. Phys. Org. Chem. 2014, 27, 944–951. [Google Scholar] [CrossRef]
- Ghiaci, M.; Ghazaie, M. Modification of a heterogeneous catalyst: Sulfonated graphene oxide coated by SiO2 as an efficient catalyst for Beckmann rearrangement. Catal. Commun. 2016, 87, 70–73. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Tavana, M.M.; Boukherroub, R. Sulfonated reduced graphene oxide as a highly efficient catalyst for direct amidation of carboxylic acids with amines using ultrasonic irradiation. Ultrason. Sonochem. 2016, 29, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, G.; Chen, H.; Wang, S.; Zhang, G.; Zhang, F.; Fan, X. Sulfonated graphene as water-tolerant solid acid catalyst. Chem. Sci. 2011, 2, 484–487. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Menendez, J.A.; Arenillas, A.; MartinezPalou, R.; Romero, A.A.; Luque, R. Selectivity matters: Graphene oxide-mediated oxidative coupling of benzylamine to N-benzylidine-1-phenylmethanamine under microwave irradiation. J. Mol. Catal. A Chem. 2015, 406, 19–22. [Google Scholar] [CrossRef]
- Acocella, M.R.; D’Urso, L.; Maggio, M.; Guerra, G. Green region- and enantioselective aminolysis catalyzed by graphite and graphene oxide under solvent-free conditions. ChemCatChem 2016, 8, 1915–1920. [Google Scholar] [CrossRef]
- Verma, S.; Mungse, H.P.; Kumar, N.; Choudhary, S.; Jain, S.L.; Sain, B.; Khatri, O.P. Graphene oxide: An efficient and reusable carbocatalyst for aza-Michael addition of amines to activated alkenes. Chem. Commun. 2011, 47, 12673–12675. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Sun, L.; Zhu, W.; Chen, G.; Wang, X.; Sun, W. P-Doped carbons derived from cellulose as highly efficient metal-free catalysts for aerobic oxidation of benzyl alcohol in water under an air atmosphere. Chem. Commun. 2018, 54, 8991–8994. [Google Scholar] [CrossRef] [PubMed]
- Su, F.Z.; Mathew, S.C.; Mçhlmann, L.; Antonietti, M.; Wang, X.C.; Blechert, S. Aerobic oxidative coupling of amines by carbon nitride photocatalysis with visible light. Angew. Chem. Int. Ed. 2011, 50, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef] [PubMed]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Carbon-based metal-free catalysts for electrocatalysis beyond the ORR. Angew. Chem. Int. Ed. 2016, 55, 11736–11758. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Balasubramanian, V.V.; Selvan, S.T.; Sawant, D.P.; Chari, M.A.; Lu, G.Q.; Vinu, A. Highly ordered mesoporous carbon nitride nanoparticles with high nitrogen content: A metal-free basic catalyst. Angew. Chem. Int. Ed. 2009, 48, 7884–7887. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zuo, X.; Tang, H.; Li, G.; Zhou, Z. Origin of photoactivity in graphitic carbon nitride and strategies for enhancement of photocatalytic efficiency: Insights from first principles computations. Phys. Chem. Chem. Phys. 2015, 17, 6280–6288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.C.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic self-assembly of nanosized carbon nitride nanosheet onto a zirconium metal-organic framework for enhanced photocatalytic CO2 reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Su, F.Z.; Mathew, S.C.; Lipner, G.; Fu, X.Z.; Antonietti, M.; Blechert, S.; Wang, X.C. mpg-C3N4-catalyzed selective oxidation of alcohols using O2 and visible light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Zhang, J.S.; Fu, X.Z.; Antonietti, M.; Wang, X.C. Fe-gC3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light. J. Am. Chem. Soc. 2009, 131, 11658–11659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.R.; Yao, J.; Wang, X.C.; Antonietti, M. Synthesis of boron doped polymeric carbon nitride solids and their use as metal-free catalysts for aliphatic C–H bond oxidation. Chem. Sci. 2011, 2, 446–450. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Wang, B.; Wang, X. Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Int. Ed. 2015, 54, 12868–12884. [Google Scholar]
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for Friedel-Crafts reaction of benzene. Angew. Chem. Int. Ed. 2006, 45, 4467–4471. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, H.; Lu, J.; Zeng, S.; Wang, M.; Luo, N.; Xu, S.; Wang, F. Photocatalytic cleavage of C–C bond in lignin models under visible light on mesoporous graphitic carbon nitride through π–π stacking interaction. ACS Catal. 2018, 8, 4761–4771. [Google Scholar] [CrossRef]
- Hu, C.; Hung, W.-Z.; Wang, M.-S.; Lu, P.-J. Phosphorus and sulfur codoped g-C3N4 as an efficient metal-free photocatalyst. Carbon 2018, 127, 374–383. [Google Scholar] [CrossRef]
- Lakhi, K.S.; Park, D.-H.; Al-Bahily, K.; Cha, W.; Viswanathan, B.; Choy, J.-H.; Vinu, A. Mesoporous carbon nitrides: Synthesis, functionalization, and applications. Chem. Soc. Rev. 2017, 46, 72–101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, W.; Sun, Y.; Chu, Y.; Cen, W.; Dong, F. Directional electron delivery via a vertical channel between g-C3N4 layers promotes photocatalytic efficiency. J. Mater. Chem. A 2017, 5, 9358–9364. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Huang, X.; Chen, N.; Qu, L. Three-dimensional graphitic carbon nitride functionalized graphene-based high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 6761–6766. [Google Scholar]
- Zhu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Mesoporous phosphorus-doped g-C3N4 nanostructured flowers with superior photocatalytic hydrogen evolution performance. ACS Appl. Mater. Interfaces 2015, 7, 16850–16856. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Qin, Z.; Wang, J.; Fan, W. Synthesis of two-dimensional mesoporous carbon nitride under different carbonization temperatures and investigation of its catalytic properties in knoevenagel condensations. RSC Adv. 2015, 5, 22838–22846. [Google Scholar] [CrossRef]
- Xu, J.; Shang, J.-K.; Jiang, Q.; Wang, Y.; Li, Y.-X. Facile alkali-assisted synthesis of g-C3N4 materials and their high-performance catalytic application in solvent-free cycloaddition of CO2 to epoxides. RSC Adv. 2016, 6, 55382–55392. [Google Scholar] [CrossRef]
- Tian, C.; Lu, C.; Wang, B.; Xie, X.; Miao, Y.; Li, X. Mesoporous carbon nitride as a basic catalyst in dehydrochlorination of 1,1,2-trichloroethane into 1,1-dichloroethene. RSC Adv. 2015, 5, 103829–103833. [Google Scholar] [CrossRef]
- Xu, J.; Long, K.-Z.; Chen, T.; Xue, B.; Li, Y.-X.; Cao, Y. Mesostructured graphitic carbon nitride as a new base catalyst for the efficient synthesis of dimethyl carbonate by transesterification. Catal. Sci. Technol. 2013, 3, 3192–3199. [Google Scholar] [CrossRef]
- Min, B.-H.; Ansari, M.B.; Mo, Y.-H.; Park, S.-E. Mesoporous carbon nitride synthesized by nanocasting with urea/formaldehyde and metal-free catalytic oxidation of cyclic olefins. Catal. Today 2013, 204, 156–163. [Google Scholar] [CrossRef]
- Fang, S.; Lv, K.; Li, Q.; Ye, H.; Du, D.; Li, M. Effect of acid on the photocatalytic degradation of rhodamine B over g-C3N4. Appl. Surf. Sci. 2015, 358, 336–342. [Google Scholar] [CrossRef]
- Veerakumar, P.; Chen, S.-M.; Madhu, R.; Veeramani, V.; Hung, C.-T.; Liu, S.-B. Nickel nanoparticle-decorated porous carbons for highly active catalytic reduction of organic dyes and sensitive detection of Hg(II) ions. ACS Appl. Mater. Interfaces 2015, 7, 24810–24821. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, A.; Janik, M.J.; Li, K.; Song, C.; Guo, X. Facile fabrication of ordered mesoporous graphitic carbon nitride for RhB photocatalytic degradation. Appl. Surf. Sci. 2017, 396, 78–84. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, A.; Janik, M.J.; Li, K.; Song, C.; Guo, X. Inorganic salt-assisted fabrication of graphitic carbon nitride with enhanced photocatalytic degradation of Rhodamine, B. Mater. Lett. 2017, 188, 130–133. [Google Scholar] [CrossRef]
- Chai, B.; Yan, J.; Wang, C.; Ren, Z.; Zhu, Y. Enhanced visible light photocatalytic degradation of Rhodamine B over phosphorus doped graphitic carbon nitride. Appl. Surf. Sci. 2017, 391, 376–383. [Google Scholar] [CrossRef]
- Zhao, R.; Gao, J.; Mei, S.; Wu, Y.; Wang, X.; Zhai, X.; Yang, J.; Hao, C.; Yan, J. Facile synthesis of graphitic C3N4 nanoporous tube with high enhancement of visible-light photocatalytic activity. Nanotechnology 2017, 28, 495710. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, W.; Zhao, Y.; Zhu, J.; Zhu, Y.; Wang, L. Metal-free mesoporous carbon nitride catalyze the Friedel-Crafts reaction by activation of benzene. RSC Adv. 2015, 5, 54978–54984. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Shang, J.-K.; Jiang, Q.; Li, Y.-X. Synthesis of mesoporous carbon nitride via a novel detemplation method and its superior performance in base-catalyzed reactions. Catal. Sci. Technol. 2016, 6, 4192–4200. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, Y.; Lin, J.; Wang, G. Highly-ordered mesoporous carbon nitride with ultrahigh surface area and pore volume as a superior dehydrogenation catalyst. Chem. Mater. 2014, 26, 3151–3161. [Google Scholar] [CrossRef]
- Ma, X.; Lv, Y.; Xu, J.; Liu, Y.; Zhang, R.; Zhu, Y. A strategy of enhancing the photoactivity of g-C3N4 via doping of nonmetal elements: A first-principles study. J. Phys. Chem. C 2012, 116, 23485–23493. [Google Scholar] [CrossRef]
- Zhu, J.; Diao, T.; Wang, W.; Xu, X.; Sun, X.; Carabineiro, S.A.C.; Zhao, Z. Boron doped graphitic carbon nitride with acid-base duality for cycloaddition of carbon dioxide to epoxide under solvent-free condition. Appl. Catal. B: Environ. 2017, 219, 92–100. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, M.; Li, G.; Dai, P.; Yu, X.; Bai1, Z.; Chen, P. Photocatalytic degradation of methylene blue over boron-doped g-C3N4 together with nitrogen vacancies under visible light irradiation. Reac. Kinet. Mech. Cat. 2018, 1–12. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Wu, Z.; Liang, J.; Chen, X.; Leng, L.; Wang, H.; Wang, H. Metal-free efficient photocatalyst for stable visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 221, 715–725. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ma, L.; Xie, Y.; Li, F.; Fan, Z.; Wang, F.; Wang, Q.; Wang, Y.; Kang, X.; Wu, G. Hydrothermal synthesis of oxygen functionalized S–P codoped g-C3N4 nanorods with outstanding visible light activity under anoxic conditions. Dalton Trans. 2015, 44, 20889–20897. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Zhang, X.; Zhang, W.; Dong, X.; Wang, G.; Ma, C.; Zhang, X.; Ma, H.; Xue, M. Ultra-thin C3N4 nanosheets for rapid charge transfer in the core-shell heterojunction of α-sulfur@C3N4 for superior metal-free photocatalysis under visible light. RSC Adv. 2015, 5, 15052–15058. [Google Scholar] [CrossRef]
- Liao, G.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Graphene oxide modified g-C3N4 hybrid with enhanced photocatalytic capability under visible light irradiation. J. Mater. Chem. 2012, 22, 2721–2726. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhao, H.; Wang, H. Atomic single layer graphitic-C3N4: Fabrication and its high photocatalytic performance under visible light irradiation. RSC Adv. 2014, 4, 624–628. [Google Scholar] [CrossRef]
- Zou, H.; Yan, X.; Ren, J.; Wu, X.; Dai, Y.; Sha, D.; Pan, J.; Liu, J. Photocatalytic activity enhancement of modified g-C3N4 by ionothermal copolymerization. J. Materiomics 2015, 1, 340–347. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Z.; Zhu, Y. Enhanced visible-light-driven photocatalytic disinfection performance and organic pollutant degradation activity of porous g-C3N4 nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 27727–27735. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. A simple fabrication for sulfur doped graphitic carbon nitride porous rods with excellent photocatalytic activity degrading RhB dye. Appl. Surf. Sci. 2017, 391, 360–368. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Mohan, M.; Nair, B.N.; Mohamed, A.A.P.; Warrier, K.G.; Hareesh, U.S. Photoregenerable, bifunctional granules of carbon-doped g-C3N4 as adsorptive photocatalyst for the efficient removal of tetracycline antibiotic. ACS Sustain. Chem. Eng. 2017, 5, 1610–1618. [Google Scholar] [CrossRef]
- You, R.; Dou, H.; Chen, L.; Zheng, S.; Zhang, Y. Graphitic carbon nitride with S and O codoping for enhanced visible light photocatalytic performance. RSC Adv. 2017, 7, 15842–15850. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.Y.; Zhou, C.; Jin, Y.R.; Jing, Q.Y.; Yang, Y.L.; Shen, X.; Tang, Q.; Mu, Y.H.; Du, A.K. Facile synthesis of 3D porous thermally exfoliated g-C3N4 nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interfaces Sci. 2016, 468, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Wang, T.; Zhang, P.; Li, A.; Gong, J. Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J. Mater. Chem. A 2014, 2, 2885–2890. [Google Scholar] [CrossRef]
- Tahir, M.; Cao, C.; Mahmood, N.; Butt, F.K.; Mahmood, A.; Idrees, F.; Hussain, S.; Tanveer, M.; Ali, Z.; Aslam, I. Multifunctional g-C3N4 nanofibers: A template-free fabrication and enhanced optical, electrochemical, and photocatalyst properties. ACS Appl. Mater. Interfaces 2014, 6, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Cao, C.; Butt, F.K.; Idrees, F.; Mahmood, N.; Ali, Z.; Aslam, I.; Tanveer, M.; Rizwan, M.; Mahmood, T. Tubular graphitic-C3N4: A prospective material for energy storage and green photocatalysis. J. Mater. Chem. A 2013, 1, 13949–13955. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Zhu, Y. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. J. Phys. Chem. C 2013, 117, 9952–9961. [Google Scholar] [CrossRef]
- Justicia, I.; Ordejon, P.; Canto, G.; Mozos, J.L.; Fraxedas, J.; Battiston, G.A.; Gerbasi, R.; Figueras, A. Designed self-doped titanium oxide thin films for efficient visible-light photocatalysis. Adv. Mater. 2002, 14, 1399–1402. [Google Scholar] [CrossRef]
- Heymann, L.; Schiller, B.; Noei, H.; Stierle, A.; Klin, C. A new synthesis approach for carbon nitrides: Poly (triazine imide) and its photocatalytic properties. ACS Omega 2018, 3, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Z.Y.; Nursam, N.M.; Xia, F.; Sani, M.-A.; Li, W.; Wang, X.; Caruso, R.A. High-performance coral reef-like carbon nitrides: Synthesis and application in photocatalysis and heavy metal ion adsorption. ACS Appl. Mater. Interfaces 2017, 9, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Hou, Z.; Chen, X. Graphitic-C3N4 nanosheets: Synergistic effects of hydrogenation and n/n junctions for enhanced photocatalytic activities. Dalton Trans. 2017, 46, 10641–10649. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Mao, Z.; An, N.; Wang, D.; Fahlman, B.D. Ultrathin g-C3N4 nanosheets with an extended visible-light-responsive range for significant enhancement of photocatalysis. RSC Adv. 2017, 7, 2333–2341. [Google Scholar] [CrossRef]
- Sun, C.; Chen, C.; Ma, W.; Zhao, J. Photocatalytic debromination of decabromodiphenyl ether by graphitic carbon nitride. Sci. China Chem. 2012, 55, 2532–2536. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, Y.-P.; Su, M.; Yuan, Z.-Y. Metal-free carbonaceous materials as promising heterogeneous catalysts. ChemCatChem 2015, 7, 2765–2787. [Google Scholar] [CrossRef]
- Francisco, R.R. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Liu, J.; Wang, L.-Q.; Nie, Z.; Samuels, W.D.; Fryxell, G.E.; Exarhos, G.J. Ordered hierarchical porous materials: Towards tunable size- and shape-selective microcavities in nanoporous channels. Angew. Chem. 2000, 112, 2814–2819. [Google Scholar] [CrossRef]
- Garcia, H. Allotropic Carbon nanoforms as advanced metal-free catalysts or as supports. Adv. Chem. 2014, 2014, 906781. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Ba, H.; Truong-Phuoc, L.; Liu, Y.; Tessonnier, J.-P.; Nhut, J.-M.; Granger, P.; Pham-Huu, C. Nitrogen-doped carbon composites as metal-free catalysts. New Materials for Catalytic Applications; Parvulescu, V.I., Kemnitz, E., Eds.; Elsevier: New York, NY, USA, 2016; pp. 273–311. [Google Scholar]
- Fujita, S.-I.; Yoshida, H.; Arai, M. Nitrogen-doped activated carbon as metal-free catalysts having various functions. J. Carbon Res. 2017, 3, 31. [Google Scholar] [CrossRef]
- Matos, I.; Neves, P.D.; Castanheiro, J.E.; Perez-Mayoral, E.; Martin-Aranda, R.; Duran-Valle, C.; Vital, J.; Botelho do Rego, A.M.; Fonseca, I.M. Mesoporous carbon as an efficient catalyst for alcoholysis and aminolysis of epoxides. Appl. Catal. A Gen. 2012, 439, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.-B.; Zong, Z.-M.; Kou, J.-H.; Zhang, L.-F.; Ni, Z.-H.; Yu, G.-Y.; Chen, H.; Wei, X.-Y. Activated carbon-catalyzed hydrogenation of polycyclic arenes. Energy Fuels 2004, 18, 1500–1504. [Google Scholar] [CrossRef]
- Sun, L.-B.; Wei, X.-Y.; Liu, X.-Q.; Zong, Z.-M.; Li, W.; Kou, J.-H. Selective hydrogen transfer to anthracene and its derivatives over an activated carbon. Energy Fuels 2009, 23, 4877–4882. [Google Scholar] [CrossRef]
- Borchardt, L.; Zhu, Q.-L.; Casco, M.E.; Berg, R.; Zhuang, X.; Kaskel, S.; Feng, X.; Xu, Q. Toward a molecular design of porous carbon materials. Mat. Today 2017, 20, 592–610. [Google Scholar] [CrossRef]
- Jong, K.P.D.; Geus, J.W. Carbon nanofibers: Catalytic synthesis and applications. Catal. Rev. Sci. Eng. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Antolini, E. Nitrogen-doped carbons by sustainable N- and C-containing natural resources as nonprecious catalysts and catalyst supports for low temperature fuel cells. Renew. Sustain. Energy. Rev. 2016, 58, 34–51. [Google Scholar] [CrossRef]
- Murray, A.T.; Surendranath, Y. Reversing the native aerobic oxidation reactivity of graphitic carbon: Heterogeneous metal-free alkene hydrogenation. ACS Catal. 2017, 7, 3307–3312. [Google Scholar] [CrossRef]
- Gupta, N.K.; Peng, B.; Haller, G.L.; Ember, E.E.; Lercher, J.A. Nitrogen modified carbon nano-materials as stable catalysts for phosgene synthesis. ACS Catal. 2016, 6, 5843–5855. [Google Scholar] [CrossRef]
- Lin, Y.; Su, D. Fabrication of nitrogen-modified annealed nanodiamond with improved catalytic activity. ACS Nano 2014, 8, 7823–7833. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Gu, X.; Wang, H.; Su, D.S. Synthesis of nitrogen-containing ordered mesoporous carbon as a metal-free catalyst for selective oxidation of ethylbenzene. Chem. Commun. 2014, 50, 9182–9184. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Asano, S.; Fujita, S.-I.; Yoshida, H.; Arai, M. Nitrogen-doped, metal-free activated carbon catalysts for aerobic oxidation of alcohols. ACS Catal. 2015, 5, 2886–2894. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, D. Graphene oxide-facilitated reduction of nitrobenzene in sulfide containing aqueous solutions. Environ. Sci. Technol. 2013, 47, 4204–4210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, R.; Wang, D.; Shi, J.; Wang, J.-X.; Pu, Y.; Chen, J.-F. Sulfurized graphene as efficient metal-free catalysts for reduction of 4-nitrophenol to 4-aminophenol. Ind. Eng. Chem. Res. 2017, 56, 13610–13617. [Google Scholar] [CrossRef]
- Acocella, M.R.; Maggio, M.; Ambrosio, C.; Aprea, N.; Guerra, G. Oxidized carbon black as an activator of transesterification reactions under solvent-free conditions. ACS Omega 2017, 2, 7862–7867. [Google Scholar] [CrossRef]
- Shaikh, M.; Sahu, A.; Kumar, A.K.; Sahu, M.; Singha, S.K.; Ranganath, K.V.S. Metal-free carbon as a catalyst for oxidative coupling: Solvent-enhanced poly-coupling with regioselectivity. Green Chem. 2017, 19, 4533–4537. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Wang, A.; Zhang, T. Synthesis of nitrogen-doped ordered mesoporous carbons for catalytic dehydrochlorination of 1,2-dichloroethane. Carbon 2014, 80, 610–616. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Antonietti, M.; García, H. Active sites on graphene-based materials as metal-free catalysts. Chem. Soc. Rev. 2017, 46, 4501–4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Nagelli, E.; Du, F.; Dai, L. Metal-free carbon nanomaterials become more active than metal catalysts and last longer. J. Phys. Chem. Lett. 2010, 1, 2165–2173. [Google Scholar] [CrossRef]





















| Name of Reaction | Reaction Condition | Yield (%) | Time | Examples/Recycle | Ref. |
|---|---|---|---|---|---|
| Hydrogenation |  | NR a | 36 min | 1/NR | Song et al. [83] |
| Michael addition |  | 69–83 | 10–30 min | 7/9 | Kim et al. [87] |
| C–H coupling |  | 0–92.4 | 24 h | 15/5 | Gao et al. [88] |
| Acetalization | 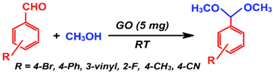 | 2–95 | 1–24 h | 14/3 | Dhakshinamoorthy et al. [89] |
| Synthesis of thioethers | 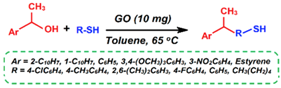 | 68–82 | 1.5–24 h | 20/5 | Basu et al. [90] |
| Bromination |  | NR | 5 h | NR/NR | Nagai et al. [91] |
| Sulfoxidation | 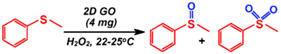 | 60.5–80.9/19.4–39.5 | 48 | 1/4 | Goncalves et al. [92] |
| Sulfoxidation | 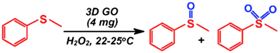 | 74.8–93.1/6.9–25.2 | 48 | 1/4 | Goncalves et al. [92] |
| Oxidation of benzylic alcohol |  | 99 | 3–12 | 6/NR | Sedrpoushan et al. [93] |
| Oxidation of aromatic aldehydes | 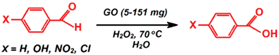 | 99 | 2–3 | 4/NR | Sedrpoushan et al. [93] |
| Esterification of carboxylic acids | 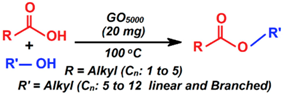 | 45–98 b | 0.5–12 h | 11/6 | Mungese et al. [94] |
| Beckmann rearrangement | 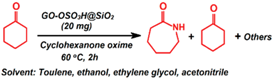 | 3–91.3 b | 2 h | 1/6 | Ghiaci et al. [95] |
| Synthesis of amides | 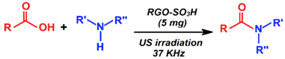 | 56–95 | 20–40 min | 17/7 | Mirza-Aghayn et al. [96] |
| Hydrolysis | 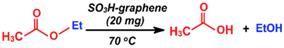 | NR | 6 h | 1/5 | Ji et al. [97] |
| Oxidative coupling |  | 99 b | 30 min | 1/NR | Bermudez et al. [98] |
| Ring opening of epoxide | 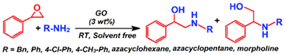 | 82–97 | 2–48 h | 9/5 | Acocella et al. [99] |
| Aza-Michael addition | 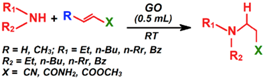 | 65–97 | 5–35 min | 26/9 | Verma et al. [100] |
| Oxidation of benzyl alcohol |  | 99.7 | 5 h | 1/5 | Long et al. [101] |
| Photocatalyst | Precursor | Dye | Experimental Condition | Comments | Ref. |
|---|---|---|---|---|---|
| PTCNs a | MA + Liq.NH3 | RhB | 5 mg of catalyst, 20 mL of dye solution (70 mg L−1) and visible light (450 W, Hg lamp) by a cutoff filter (λ > 380 nm) | SBET:107.4 m2 g−1, BG: 2.63 eV, kRhB: 0.04491 min−1); Four times recycled. | Zhao et al. [134] |
| B-Nv b-GCN | BA + MA | MB | 100 g of catalyst, 100 mL of dye solution (20 mg L−1) and visible light (metal halide lamp, 300 W). | SBET: 27.0 m2 g−1, BG: 2.63 eV; kMB: 0.03 min−1, Three times recycled. | Mao et al. [140] |
| h-BN c/GCN | DCA | RhB | 50 mg of catalyst, 100 mL of dye solution (20 mg L−1) and visible light (300 W Xe lamp) with a 420 nm cut-off filter. | SBET: 34.69 m2 g−1, BG: 2.56 eV, kRhB: 0.13097 min−1, Five times recycled. | Jiang et al. [141] |
| B d-doped GCN | MA + BO o | RhB and MO | 200 mg of catalyst, 100 mL of dye solution (4 mg L−1) visible light (300 W Xe lamp) with cutoff filter L42 and a water filter. | SBET: 30 m2 g−1, BG: 2.66 eV, Three times recycled. | Yan et al. [142] |
| S,P e-doped GCN nanorods | TU p+ (NH4)2HPO4 | RhB | 50 mg of catalyst, 200 mL of dye solution (10 ppm) and visible light (250 W), high-pressure Na lamp with a 0.5 M NaNO2 solution filter. | SBET:13.6 m2 g−1, BG: 2.53 eV, kRhB: 0.026 min−1, Three times recycled. | Hu et al. [143] |
| α-S@GCN core-shell | MA + Na2S2O3.2H2O | RhB | 80 mg of catalyst, 80 mL of dye solution (5 mg L−1) and visible light (300 W, Xe lamp). Light intensity = 30 mW cm−2. | SBET: 13.6 m2 g−1, BG: 3.03 eV, kRhB: 0.0382 min−1, Three times recycled. | Dang et al. [144] |
| SL f-GCN | MA | RhB | 10 mg of catalyst, 50 mL of dye solution (10 mg L−1) and visible light (Xe lamp, 100 mW cm2, λ > 400 nm. | SBET: 13.6 m2 g−1, BG: 2.9 eV, k: 1.96 h−1, Ten times recycled. | Zhao et al. [146] |
| BBA g-GCN | DCA + BBA | RhB | 100 g of catalyst, 100 mL of dye solution (5 mg L−1) and visible light (125 W, Xe lamp) with a 420 nm cut-off filter. | SBET:175.38 m2 g−1, kRhB: 0.02031 min−1); Four times recycled. | Zou et al. [147] |
| GCN | MA | MO | 300 mg of catalyst, 100 mL of dye solution (0.4 mg L−1), and visible light lamp (300 W, Xe lamp) with cutoff filter L42 and a water filter. | SBET: 8 m2 g−1, BG: 2.75 eV, Three times recycled. | Yan et al. [148] |
| PCNS gh | MA | MB | 10 mg of catalyst, 1.2 × 10−5 M of dye solution, and visible light (500 W, Xe lamp) equipped with UV cut-off filter (>420 nm). | SBET:190.1 m2 g−1, BG: 2.82 eV, kMB: 0.551 h−1, Five times recycled. | Xu et al. [149] |
| S-pg-GCN i | MA + TTCA q | RhB | 100 mg of catalyst, 100 mL of dye solution (10 mg L−1), visible light (500 W Xe lamp) with a 400 nm cutoff filter | SBET: 20–52 m2 g−1, BG: 2.56 eV, kRhB: 0.0176 min−1, Four times recycled. | Fan et al. [150] |
| C j-doped GCN | U + TU | TC s | 40 mg of catalyst, 80 mL of 10−4 M TC, Sunlight (07/10/2015, Trivandrum, India, between 11 p.m. and 1 p.m., 78,000–80,000 lux). | SBET: 151 m2 g−1, BG: 2.54 eV, Five times recycled. | Panneri et al. [151] |
| S,O k co-doped GCN | DCA | RhB | 50 mg of catalyst, 50 mL of dye solution (10 ppm), visible light (500 W, Xe lamp) with a 420 nm cut-off filter. | BG: 2.3 eV, Four times recycled. | You et al. [152] |
| TE l-GCN | MA | RhB | 50 mg of catalyst, 50 mL dye solution, visible light (500 W UV lamp). | SBET:54.3 m2 g−1, BG: 2.79 eV, kRhB: 0.0358 min−1, Eleven times recycled. | Yuan et al. [153] |
| GCN Nanotubes | MA | MB | 50 mg of catalyst, 100 mL of dye solution (10 mg L−1), visible light (300 W, Xe lamp) with a 420 nm cut-off filter. | SBET:10 m2 g−1, BG: 2.64 eV, kRhB: (0.01 min−1), Not recycled. | Wang et al. [154] |
| GCN Nanofibers | MA | RhB | 100 mg of catalyst, 0.4 L of dye solution (0.01 M), visible light (500 W, Xe lamp). | SBET:165 m2 g−1, BG: 2.80 eV, kRhB: 0.0412 min−1, Three times recycled | Tahir et al. [155] |
| GCN Tubulars | MA | MO and MB | 100 mg of catalyst, 40 mL of dye solution (10 mg L−1), visible light (500 W Xe lamp) with a 420 nm cut-off filter. | SBET: 182.61 m2 g−1, BG: 2.85 eV, kMB: 0.02116 min−1, kMO: 0.0067 min−1, Three times recycled. | Tahir et al. [156] |
| GCN Nanorods | DCA | MB | 25 mg of catalyst, 50 mL of dye solution (0.03 mM), visible light (500 W Xe lamp) with a 420 nm cut-off filter. | SBET: 182.61 m2 g−1, BG: 2.66 eV, kMB: 0.14812 h−1, Three times recycled. | Bai et al. [157] |
| PTI m | MA | MB | 50 mg of catalyst, 100 mL of dye solution (10 mg L−1), pH 7, visible light (300 W Xe lamp) with a water filter. | SBET: 96 m2 g−1, BG: 3.28 eV, kMB: 1.5 × 10−3 min−1, Three times recycled. | Heymann et al. [159] |
| CNx | LiNO3 and C3N3Cl3 | MB | Catalyst (1.0 mg L−1), dye solution (10 mg L−1), visible light (500 W Hg-Xe lamp) with a filter. | SBET: 24.5 m2 g−1, BG: 2.4 eV, kMB: 0.0098 min−1, Four times recycled. | Tan et al. [160] |
| HM-CN n | U + TU | MB | 10 mg of catalyst, 50 mL of dye solution (10 mg·L−1) and visible light (150 W Xe lamp) | SBET:119.53 m2 g−1, BG: 2.11 eV, kMB: 0.125 min−1, Not recycled. | Zhou et al. [161] |
| Ultrathin GCN NSs | U r | RhB | 100 mg of catalyst, 100 mL of dye solution (20 mg L−1), visible light (300 W Xe lamp), cut-off filter > 420 nm. | SBET:131.2 m2 g−1, BG: 2.80 eV, kRhB: 0.07346 min−1, Four times recycled. | Yang et al. [162] |
| GCN | DCA | BDE209 | 20 mg of catalyst, 20 mL of 1 × 10−3 mol L−1 BDE209 solution, visible light (300 W, Xe lamp), cut-off filter > 360 nm. | BG: 2.7 eV, kBDE209: 0.057 ± 0.01 min−1, Not recycled. | Sun et al. [163] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.-C. A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research. C 2018, 4, 54. https://doi.org/10.3390/c4040054
Veerakumar P, Thanasekaran P, Subburaj T, Lin K-C. A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research. C. 2018; 4(4):54. https://doi.org/10.3390/c4040054
Chicago/Turabian StyleVeerakumar, Pitchaimani, Pounraj Thanasekaran, Thiruvengadam Subburaj, and King-Chuen Lin. 2018. "A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research" C 4, no. 4: 54. https://doi.org/10.3390/c4040054