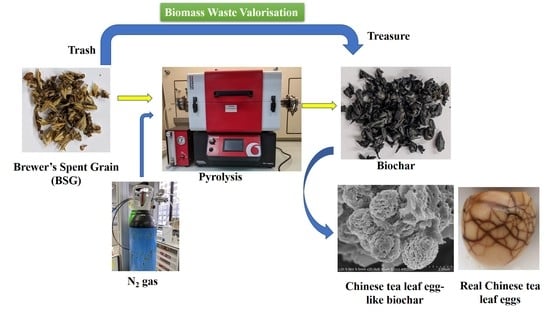
Brewer’s Spent Grain Biochar: Grinding Method Matters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatuses
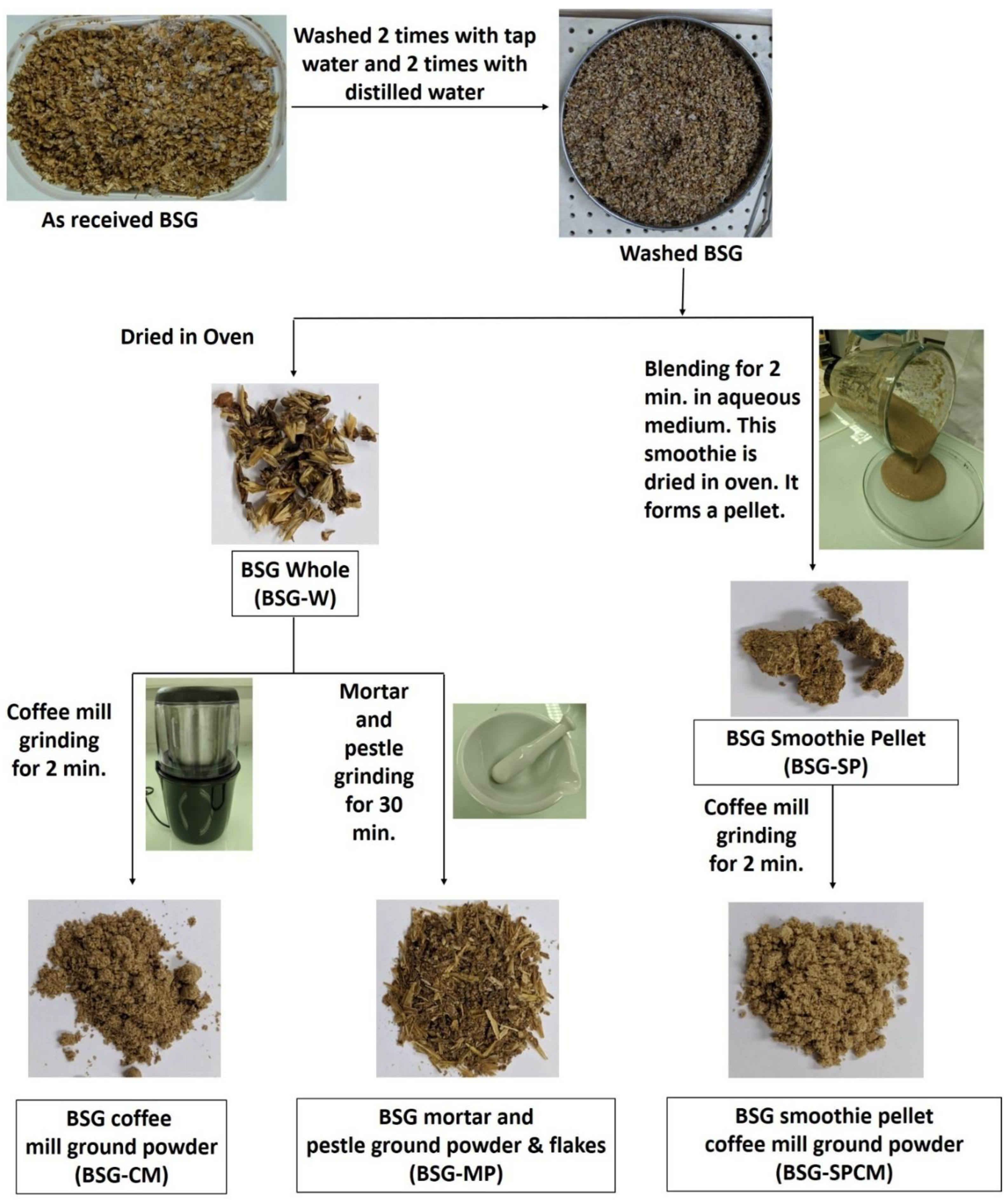
2.3. Synthesis of Brewer’s Spent Grain Biochar
3. Results and Discussions
3.1. Surface Chemical Composition of Biochar
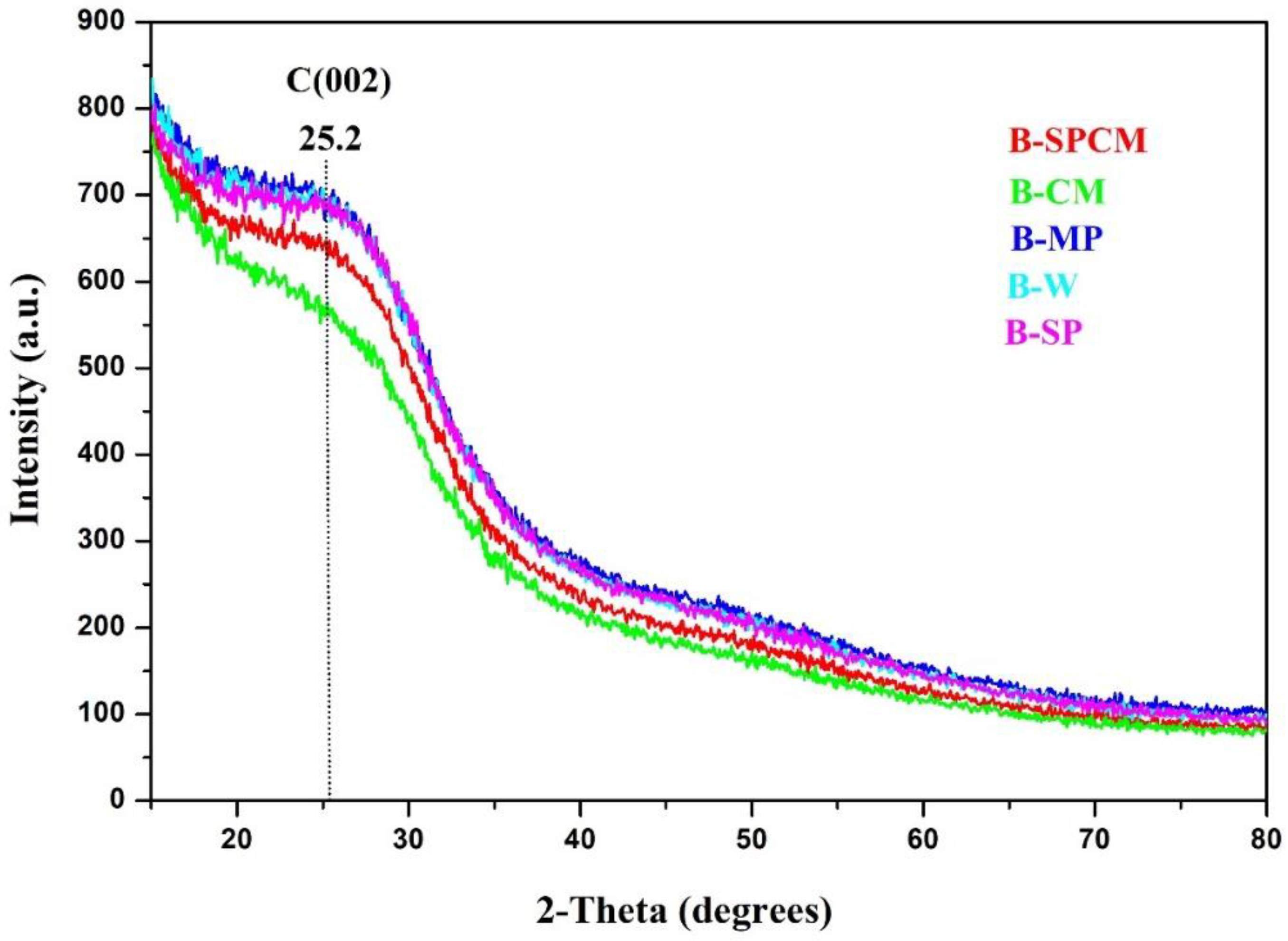
3.2. Crystallinity of Biochar
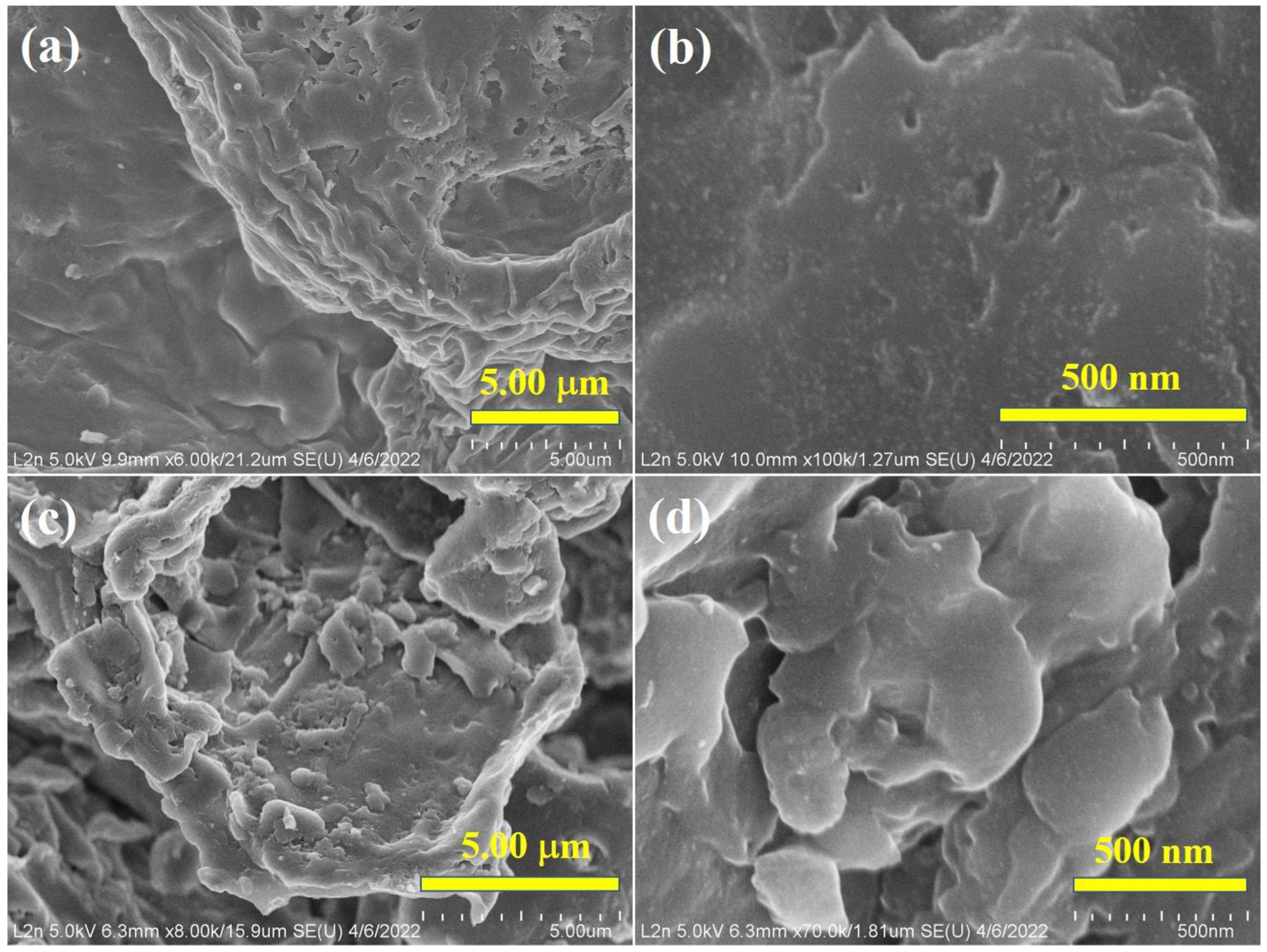
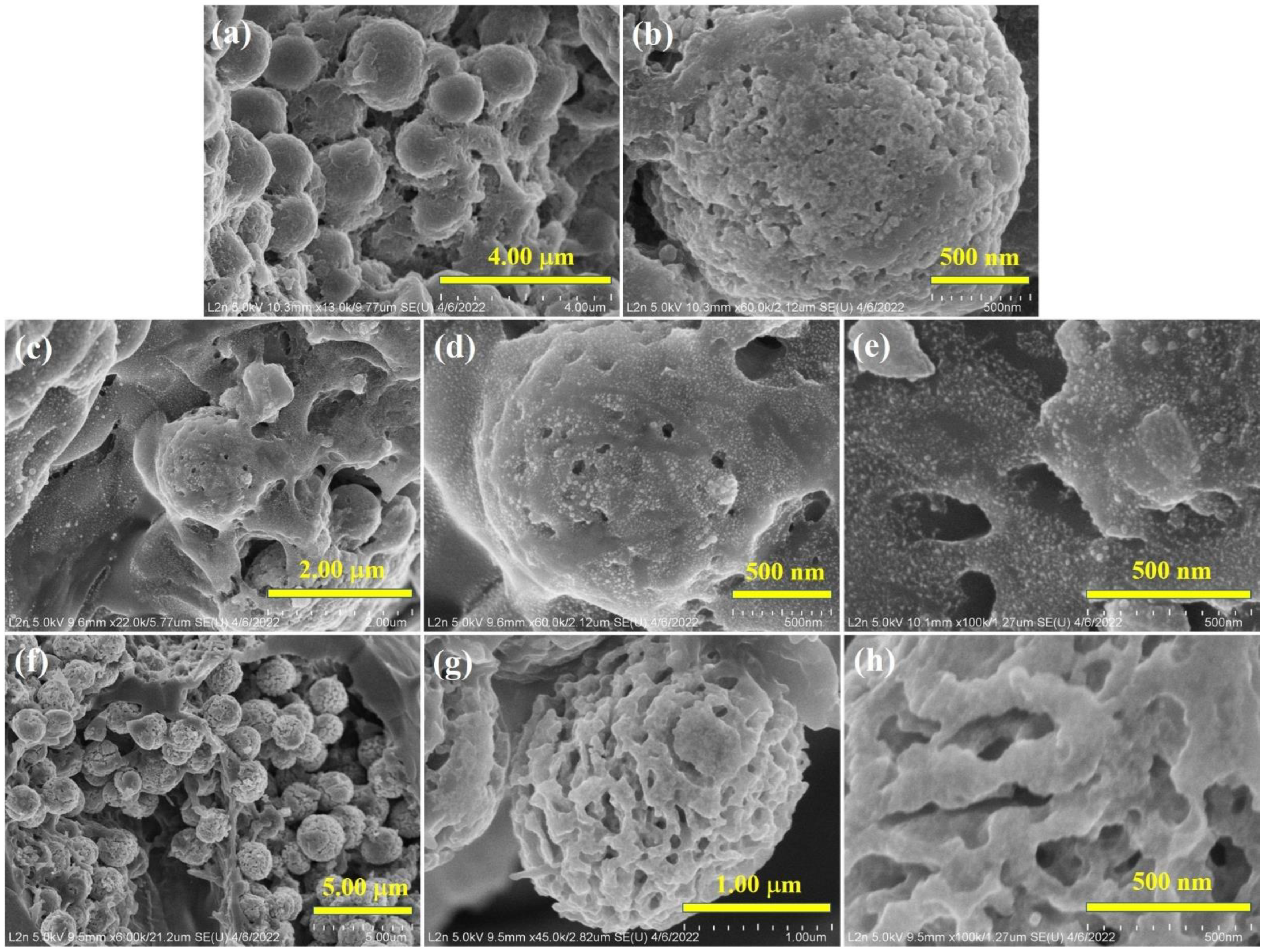
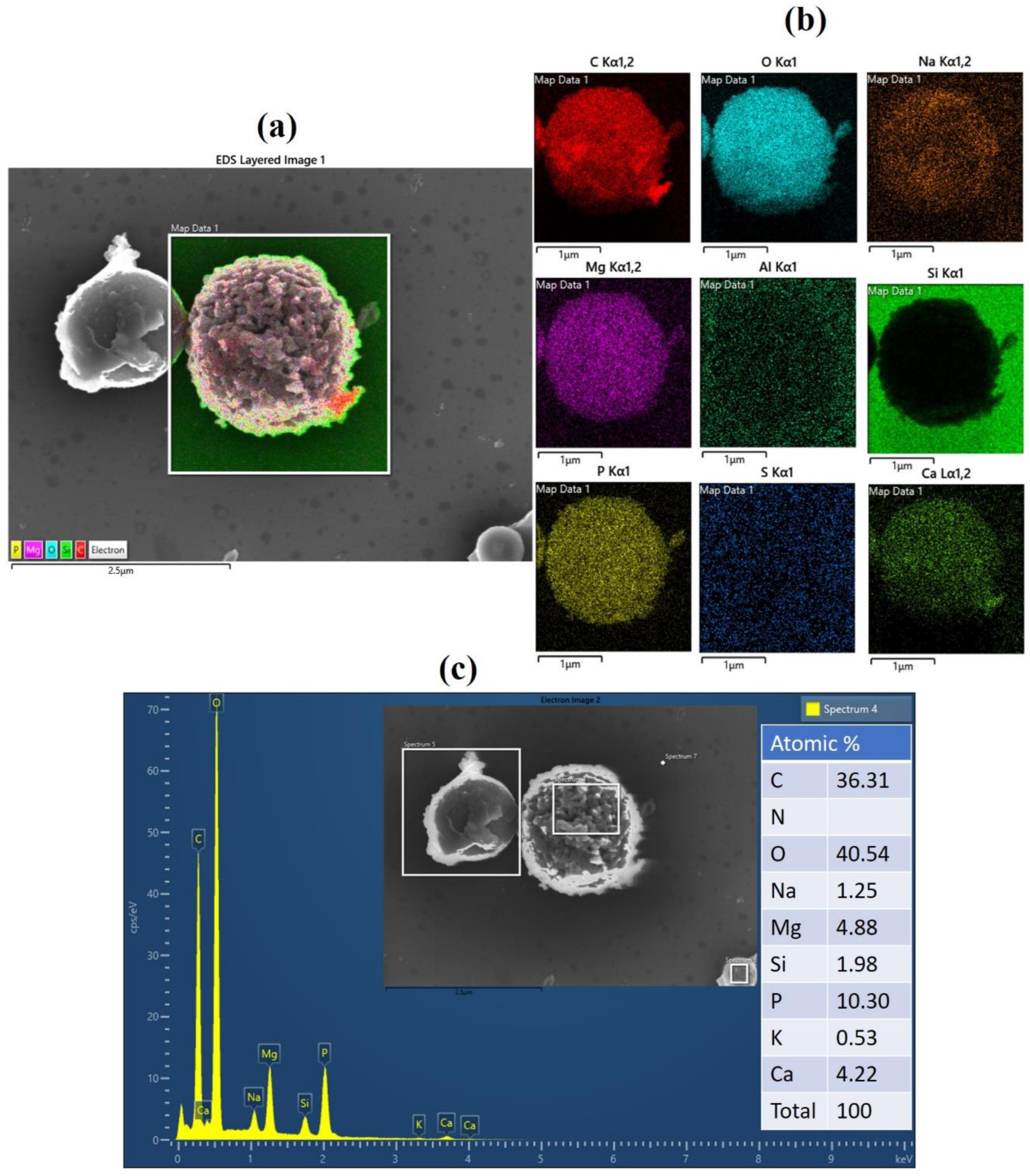
3.3. Morphology of Biochar and Bulk Composition
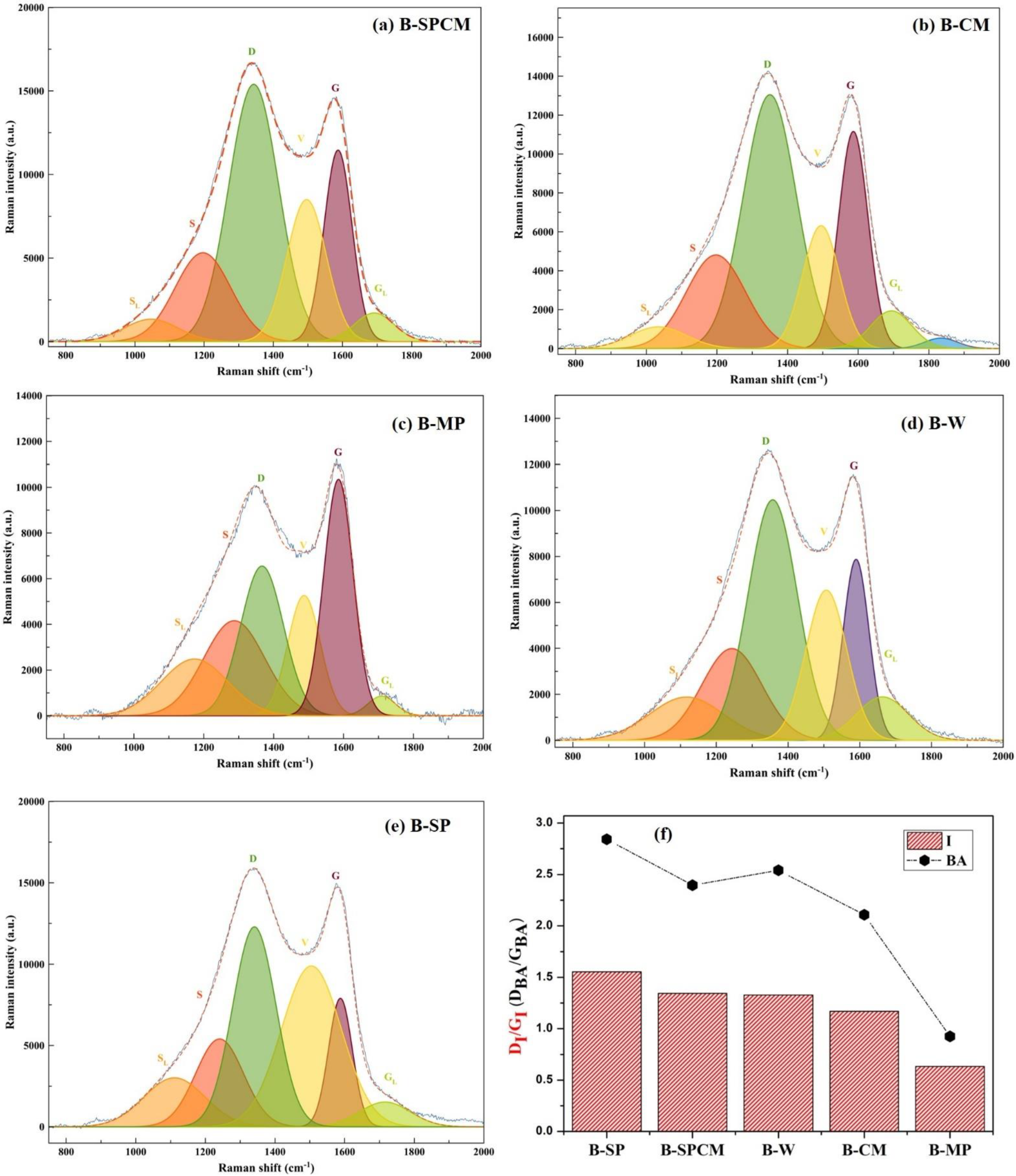
3.4. Carbon Skeleton Structure of Biochar
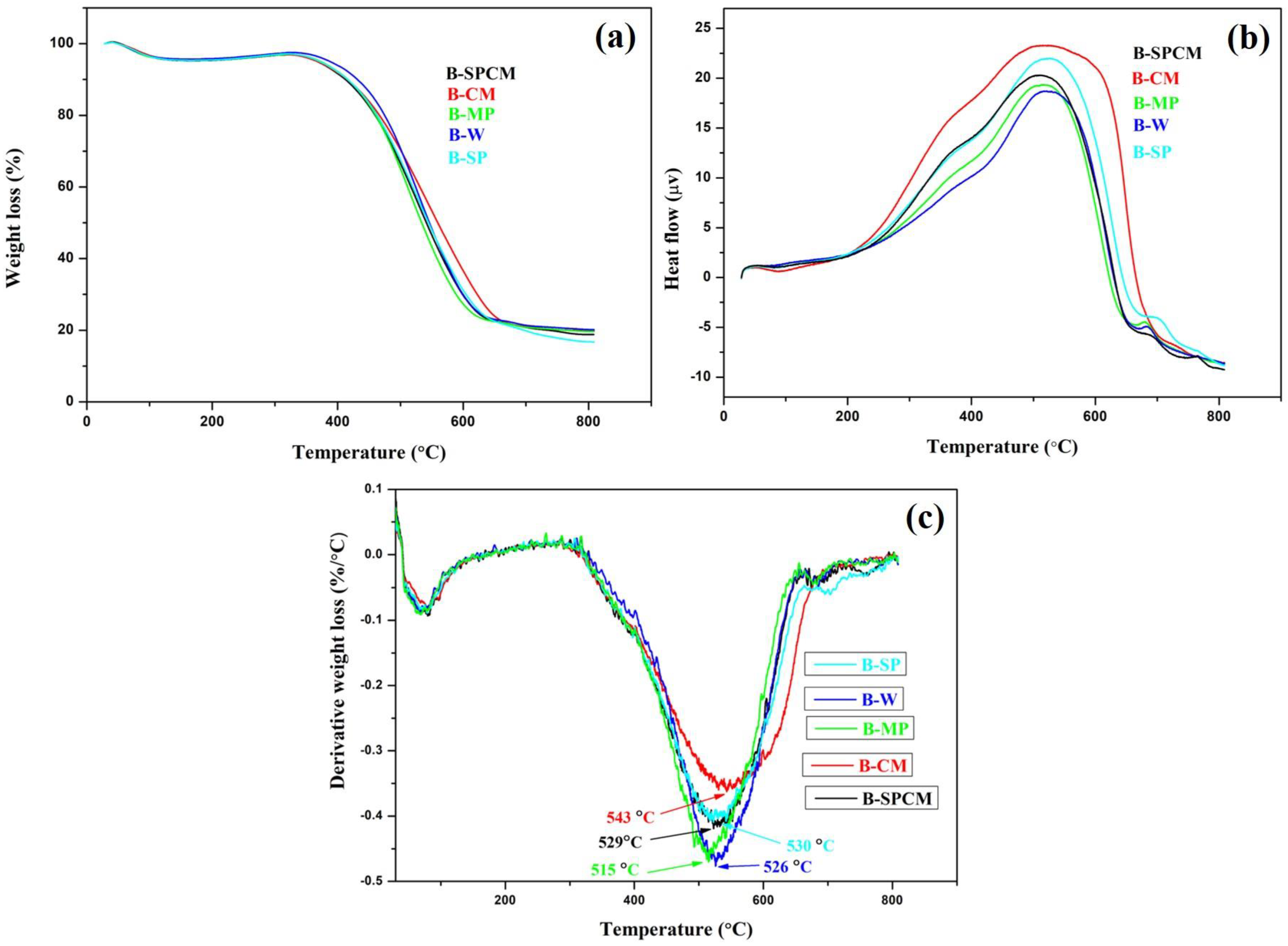
3.5. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meunier, V.; Ania, C.; Bianco, A.; Chen, Y.; Choi, G.B.; Kim, Y.A.; Koratkar, N.; Liu, C.; Tascon, J.M.; Terrones, M. Carbon science perspective in 2022: Current research and future challenges. Carbon 2022, 195, 272–291. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Dou, S.; Ke, X.; Shao, Z.; Zhong, L.; Zhao, Q.; Zheng, Y.-M. Fish scale-based biochar with defined pore size and ultrahigh specific surface area for highly efficient adsorption of ciprofloxacin. Chemosphere 2022, 287, 131962. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Ok, Y.S.; Dissanayake, P.D.; Tsang, D.C.W.; Kim, S.; Kua, H.W.; Shah, K.W. Preparation and thermal conductivity enhancement of a paraffin wax-based composite phase change material doped with garlic stem biochar microparticles. Sci. Total Environ. 2022, 827, 154341. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Mukherjee, S.; Sarkar, B. A perspective on biochar for repairing damages in the soil—plant system caused by climate change—driven extreme weather events. Biochar 2022, 4, 1–23. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen—doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- de Gomes, S.C.; Zhou, J.L.; Zeng, X.; Long, G. Water treatment sludge conversion to biochar as cementitious material in cement composite. J. Environ. Manage. 2022, 306, 114463. [Google Scholar] [CrossRef]
- Dewangan, S.; Bhatia, A.K.; Singh, A.K.; Carabineiro, S.A.C. Removal of Hydrophobic Contaminants from the Soil by Adsorption onto Carbon Materials and Microbial Degradation. J. Carbon Res. 2021, 7, 83. [Google Scholar] [CrossRef]
- Peterson, S.C.; Kim, S.; Adkins, J. Surface Charge Effects on Adsorption of Solutes by Poplar and Elm Biochars. J. Carbon Res. 2021, 7, 11. [Google Scholar] [CrossRef]
- Binda, G.; Faccini, D.; Zava, M.; Pozzi, A.; Dossi, C.; Monticelli, D.; Spanu, D. Exploring the Adsorption of Pb on Microalgae-Derived Biochar: A Versatile Material for Environmental Remediation and Electroanalytical Applications. Chemosensors 2022, 10, 168. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; L-Shwaiman, H.A.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Qin, F.; Li, J.; Zhang, C.; Zeng, G.; Huang, D.; Tan, X.; Qin, D.; Tan, H. Biochar in the 21st century: A data-driven visualization of collaboration, frontier identifi cation, and future trend. Sci. Total Environ. 2022, 818, 151774. [Google Scholar] [CrossRef] [PubMed]
- EAzzi, S.; Karltun, E.; Sundberg, C. Life cycle assessment of urban uses of biochar and case study in Uppsala, Sweden. Biochar 2022, 4, 1–17. [Google Scholar] [CrossRef]
- Vieira, M.C.; Brandelli, A.; Thys, R.C.S. Evaluation of the technological functional properties and antioxidant activity of protein hydrolysate obtained from brewers’ spent grain. J. Food Process. Preserv. 2022, 46, e16638. [Google Scholar] [CrossRef]
- Allegretti, C.; Bellinetto, E.; Arrigo, P.D.; Griffini, G.; Marzorati, S.; Rossato, L.A.M.; Ruffini, E.; Schiavi, L.; Serra, S.; Strini, A.; et al. Towards a Complete Exploitation of Brewers’ Spent Grain from a Circular Economy Perspective. Fermentation 2022, 8, 151. [Google Scholar] [CrossRef]
- Jackowski, M.; Niedzwiecki, Ł.; Jagiełło, K.; Uchanska, O.; Trusek, A. Brewer ’s Spent Grains—Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef]
- Mugoronji, M.; Manyuchi, M.M.; Sukdeo, N.; Stinner, W. Techno-economic assessment for bio coal production from brewers spent grain. South African J. Chem. Eng. 2022, 40, 1–9. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Zhao, B.; Connor, D.O.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987. [Google Scholar] [CrossRef]
- Suman, S.; Panwar, D.S.; Gautam, S. Surface morphology properties of biochars obtained from different biomass waste. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1007–1012. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Guy, M.; Mathieu, M.; Jebrane, M.; Lima, E.C.; Thyrel, M.; Dotto, G.L.; Larsson, S.H. A comparative study of chemical treatment by MgCl2, ZnSO4, ZnCl2, and KOH on physicochemical properties and acetaminophen adsorption performance of biobased porous materials from tree bark residues. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128626. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Tang, M.; Snoussi, Y.; Bhakta, A.K.; el Garah, M.; Khalil, A.M.; Ammar, S.; Chehimi, M.M. Unusual, hierarchically structured composite of sugarcane pulp bagasse biochar loaded with Cu/Ni bimetallic nanoparticles. Chemrxiv 2022. [Google Scholar] [CrossRef]
- Meng, F.; Wang, D. Effects of vacuum freeze drying pretreatment on biomass and biochar properties. Renew. Energy. 2020, 155, 1–9. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Singhal, A.; Tolvanen, H.; Valtonen, K.; Joronen, T.; Konttinen, J. Effect of pretreatment and biomass blending on bio-oil and biochar quality from two-step slow pyrolysis of rice straw. Waste Manag. 2022, 138, 298–307. [Google Scholar] [CrossRef]
- Maliutina, K.; Tahmasebi, A.; Yu, J. Effects of pressure on morphology and structure of bio-char from pressurized entrained- flow pyrolysis of microalgae. Data Br. 2018, 18, 422–431. [Google Scholar] [CrossRef]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Daniel, C.; Tsang, W. Recent advances in mechanochemical production of chemicals and carbon materials from sustainable biomass resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Ban, S.; Lee, E.; Lim, D.; Kim, I.; Lee, J. Evaluation of sulfuric acid-pretreated biomass-derived biochar characteristics and its diazinon adsorption mechanism. Bioresour. Technol. 2022, 348, 126828. [Google Scholar] [CrossRef]
- Alayont, S.; Kayan, D.B.; Durak, H.; Alayont, E.K.; Genel, S. The role of acidic, alkaline and hydrothermal pretreatment on pyrolysis of wild mustard (Sinapis arvensis) on the properties of bio-oil and bio-char. Bioresour. Technol. Reports. 2022, 17, 100980. [Google Scholar] [CrossRef]
- Garrido, R.A.; Reckamp, J.; Bastian, P.; Hammer, N.H.; Coe, C.G.; Satrio, J.A. Influences of zinc chloride on fast pyrolysis of pinewood. IOP Conf. Ser. Earth Environ. Sci. 2022, 1034, 012042. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Gurav, R.; Bhatia, R.K.; Muthusamy, G.; Tyagi, V.K.; Kumar, V.; Pugazendhi, A.; Banu, R.J.; et al. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Bioresour. Technol. 2022, 358, 127437. [Google Scholar] [CrossRef]
- Hadiya, V.; Popat, K.; Vyas, S.; Varjani, S.; Vithanage, M.; Gupta, V.K.; Delgado, A.N.; Zhou, Y.; Show, P.L.; Bilal, M.; et al. Biochar production with amelioration of microwave-assisted pyrolysis: Current scenario, drawbacks and perspectives. Bioresour. Technol. 2022, 355, 127303. [Google Scholar] [CrossRef] [PubMed]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; van Gerven, T. Ball milling as an important pretreatment technique in lignocellulose biorefineries: A review. Biomass Convers. Biorefinery 2021, 1–24. [Google Scholar] [CrossRef]
- Hung, C.; Chen, C.; Huang, C.; Yang, Y.; Dong, C. Suppression of polycyclic aromatic hydrocarbon formation during pyrolytic production of lignin-based biochar via nitrogen and boron co-doping. Bioresour. Technol. 2022, 355, 127246. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Meng, H.; Shen, Y.; Ding, J.; Zhou, H.; Cong, H.; Li, L. A comprehensive assessment on bioavailability, leaching characteristics and potential risk of polycyclic aromatic hydrocarbons in biochars produced by a continuous pyrolysis system. Chemosphere 2022, 287, 132116. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, K.; Jin, J.; Han, L.; Sun, H.; Zhao, Y.; Xia, X.; Wu, F.; Xing, B. Metal/metalloid elements and polycyclic aromatic hydrocarbon in various biochars: The effect of feedstock, temperature, minerals, and properties. Environ. Pollut. 2015, 206, 298–305. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Nan, H.; Yang, F.; Qiu, H.; Xu, X.; Cao, X. Suppressed formation of polycyclic aromatic hydrocarbons (PAHs) during pyrolytic production of Fe-enriched composite biochar. J. Hazard. Mater. 2020, 382, 121033. [Google Scholar] [CrossRef]
- Wang, J.; Odinga, E.S.; Zhang, W.; Zhou, X.; Yang, B.; Waigi, M.G.; Gao, Y. Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: A synthesis study. Environ. Int. 2019, 130, 104899. [Google Scholar] [CrossRef]
- EBC. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar. Switzerland Version 8E of 1st January 2019; European Biochar Foundation (EBC): Arbaz, Switzerland, 2017. [Google Scholar] [CrossRef]
- Chen, W.; Feng, J.; Liu, S.; Zhang, J.; Cai, Y.; Lv, Z.; Fang, M.; Tan, X. A green and economical MgO/biochar composite for the removal of U (VI) from aqueous solutions. Chem. Eng. Res. Des. 2022, 180, 391–401. [Google Scholar] [CrossRef]
- Jin, Z.; Lan, Y.; Ohm, J.; Gillespie, J.; Schwarz, P.; Chen, B. Physicochemical composition, fermentable sugars, free amino acids, phenolics, and minerals in brewers’ spent grains obtained from craft brewing operations. J. Cereal Sci. 2022, 104, 103413. [Google Scholar] [CrossRef]
- Endres, F.; Prowald, A.; Elisabeth, U.; Fittschen, A.; Hampel, S.; Oppermann, S.; Jacob, F.; Hutzler, M.; Laus, A.; Methner, Y.; et al. Constant temperature mashing at 72 °C for the production of beers with a reduced alcohol content in micro brewing systems. Eur. Food Res. Technol. 2022, 248, 1457–1468. [Google Scholar] [CrossRef]
- Peterson, C.A.; Hornbuckle, M.K.; Brown, R.C. Biomass pyrolysis devolatilization kinetics of herbaceous and woody feedstocks. Fuel Process. Technol. 2022, 226, 107068. [Google Scholar] [CrossRef]
- Agar, D.A.; Kwapinska, M.; Leahy, J.J. Pyrolysis of wastewater sludge and composted organic fines from municipal solid waste: Laboratory reactor characterisation and product distribution. Sustain. WASTE Manag. 2018, 25, 35874–35882. [Google Scholar] [CrossRef]
- Pecha, M.B.; Thornburg, N.E.; Peterson, C.A.; Crowley, M.F.; Gao, X.; Lu, L.; Wiggins, G.; Brown, R.C.; Ciesielski, P.N. Impacts of Anisotropic Porosity on Heat Transfer and Off-Gassing during Biomass Pyrolysis. Energy Fuels. 2021, 35, 20131–20141. [Google Scholar] [CrossRef]
- Gibson, L.T. Archaeometry and Antique Analysis|Metallic and Ceramic Objects. Encycl. Anal. Sci. Second Ed. 2005, 117–123. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Chen, B. H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci. Rep. 2016, 6, 22644. [Google Scholar] [CrossRef]
- Tran, H.N.; Tomul, F.; Ha, N.T.H.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.-T.; Masindi, V.; Woo, S.H. Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J. Hazard. Mater. 2020, 394, 122255. [Google Scholar] [CrossRef]
- Mcdonald-wharry, J. 2013–2014 Survey of Chars Using Raman Spectroscopy. J. Carbon Res. 2021, 7, 63. [Google Scholar] [CrossRef]
- Inoue, J.; Yoshie, A.; Tanaka, T.; Onji, T.; Inoue, Y. Disappearance and alteration process of charcoal fragments in cumulative soils studied using Raman spectroscopy. Geoderma 2017, 285, 164–172. [Google Scholar] [CrossRef]
- Pusceddu, E.; Montanaro, A.; Fioravanti, G.; Santilli, S.F.; Foscolo, P.U.; Criscuoli, I.; Raschi, A.; Miglietta, F. Comparison between Ancient and Fresh Biochar Samples, A Study on The Recalcitrance of Carbonaceous Structures During Soil Incubation. Int. J. New Technol. Res. 2017, 3, 39–46. [Google Scholar]
- Feng, D.; Zhao, Y.; Zhang, Y.; Sun, S.; Gao, J. Steam Gasification of Sawdust Biochar Influenced by Chemical Speciation of Alkali and Alkaline Earth Metallic Species. Energies 2018, 11, 205. [Google Scholar] [CrossRef]
- Wang, W.; Bai, J.; Lu, Q.; Zhang, G.; Wang, D.; Jia, J.; Guan, Y.; Yu, L. Pyrolysis temperature and feedstock alter the functional groups and carbon sequestration potential of Phragmites australis—and Spartina alterniflora-derived biochars. GCB Bioenergy 2021, 13, 493–506. [Google Scholar] [CrossRef]
- Cui, B.; Chen, Z.; Guo, D.; Liu, Y. Investigations on the pyrolysis of microalgal-bacterial granular sludge: Products, kinetics, and potential mechanisms. Bioresour. Technol. 2022, 349, 126328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, Z.; Ding, H.; Wan, Y.; Tang, Z.; Gao, J. Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method. Int. J. Mol. Sci. 2018, 19, 3055. [Google Scholar] [CrossRef] [PubMed]
- Riva, L.; Cardarelli, A.; Andersen, G.J.; Buø, T.V.; Barbanera, M.; Bartocci, P.; Fantozzi, F.; Nielsen, H.K. On the self-heating behavior of upgraded biochar pellets blended with pyrolysis oil: Effects of process parameters. Fuel 2020, 278, 118395. [Google Scholar] [CrossRef]



| Biomass | Weight of Biomass | Biochar | Weight of Biochar | % Yield |
|---|---|---|---|---|
| BSG-SPCM | 5.8096 | B-SPCM | 1.6226 | 27.9% |
| BSG-CM | 3.7843 | B-CM | 1.0893 | 28.8% |
| BSG-MP | 3.2917 | B-MP | 0.9337 | 28.4% |
| BSG-W | 2.0717 | B-W | 0.6126 | 29.6% |
| BSG-SP | 2.8120 | B-SP | 0.8026 | 28.5% |
| Sample | C | O | N | P | Si | Ca |
|---|---|---|---|---|---|---|
| B-SPCM | 80.1 | 12.1 | 4.58 | 1.82 | 1.25 | 0.14 |
| B-CM | 78.2 | 13.2 | 3.74 | 2.39 | 1.35 | 1.16 |
| B-MP | 82.1 | 11.3 | 3.83 | 1.25 | 1.56 | - |
| B-W | 78.5 | 13.3 | 3.76 | 2.16 | 1.39 | 0.93 |
| B-SP | 78.3 | 13.5 | 3.80 | 1.46 | 2.27 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhakta, A.K.; Snoussi, Y.; Garah, M.E.; Ammar, S.; Chehimi, M.M. Brewer’s Spent Grain Biochar: Grinding Method Matters. C 2022, 8, 46. https://doi.org/10.3390/c8030046
Bhakta AK, Snoussi Y, Garah ME, Ammar S, Chehimi MM. Brewer’s Spent Grain Biochar: Grinding Method Matters. C. 2022; 8(3):46. https://doi.org/10.3390/c8030046
Chicago/Turabian StyleBhakta, Arvind K., Youssef Snoussi, Mohamed El Garah, Souad Ammar, and Mohamed M. Chehimi. 2022. "Brewer’s Spent Grain Biochar: Grinding Method Matters" C 8, no. 3: 46. https://doi.org/10.3390/c8030046