Autochthonous Ingredients for Craft Beer Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Beer Ingredients
2.1.1. Water
2.1.2. Barley Malt
- Steeping phase: Grains were soaked with tap water at 18 °C for 7 h, followed by a water-free rest period of 17 h at 18 °C. This phase was carried out over 48 h with a final rest period of 48 h, by which time the first rootlets had appeared.
- The germination phase was carried out for 48–72 h at 18 °C, maintaining humidification and oxygenation during the entire process. This phase was finished once the roots and plumule had reached the same length, with the final water content of the barley grains being 40%.
- The kilning phase was conducted in forced-air ovens at a temperature of 52 °C for 24 h, followed by 2 h at 80 °C. In addition, part of the malt obtained was also kilned at 150 °C for 1 h. The final water content was less than 5%.
2.1.3. Hops
2.1.4. Yeast Strains
- S. cerevisiae GENL 520: The beers brewed with this yeast were mainly characterised by a fruity profile, with a banana aroma and flavour, as well as being phenolic. The beers showed medium bitterness, high body, and persistence. The balance between aroma and flavour was high.
- S. cerevisiae GENL 354: The beers brewed with this strain were noted for their sweet, fruity taste and accentuated bitterness. They showed a medium body, as well as low persistence, which makes the beer more drinkable. The beers were balanced in aroma and flavour.
2.2. Recipes, Wort Obtention, and Fermentation 16 L
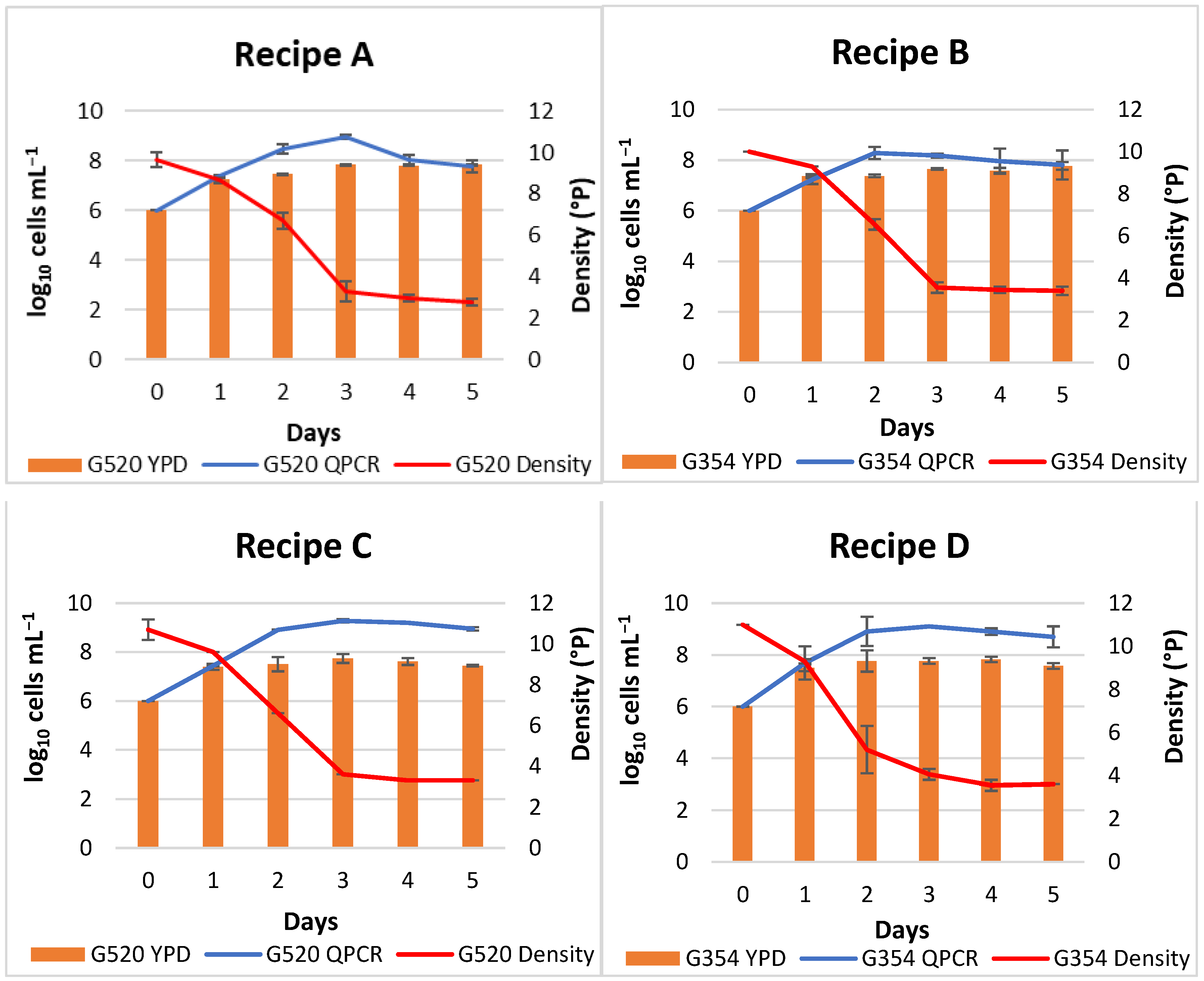
2.3. Fermentation Control and Determination of Yeast Concentration during the Process
DNA Extraction and Real-Time qPCR Assays
2.4. Beer Analyses
2.4.1. CDR FoodLab
2.4.2. Volatile Compounds
2.4.3. Residual Sugars, Glycerol, and Ethanol Determination by HPLC
2.4.4. Antioxidant Capacity
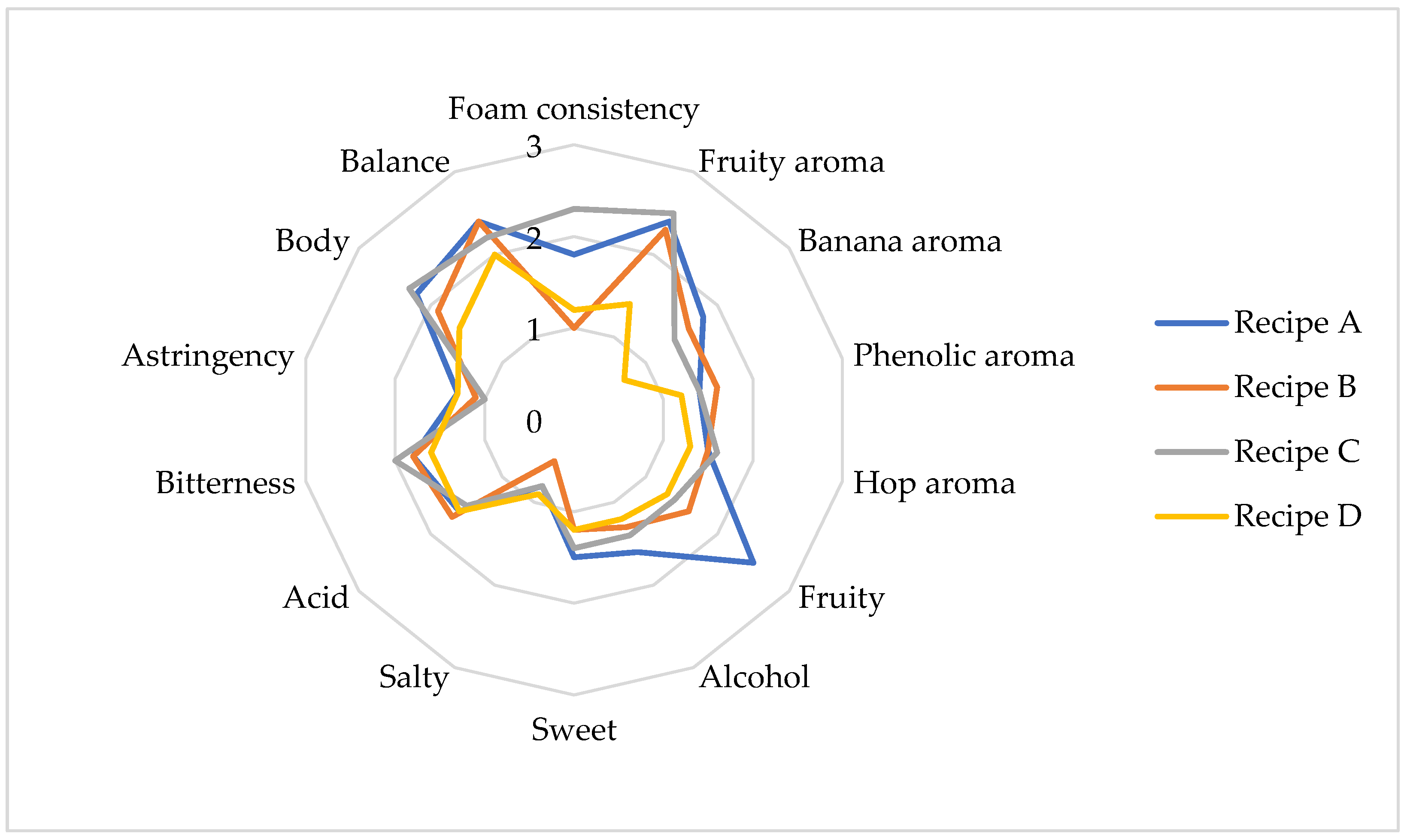
2.5. Organoleptic Profile
2.6. Consumer Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Ingredient Characteristics
3.1.1. Barley Malt
3.1.2. Hops
3.2. Study of Yeast Inoculated Population during Beer Fermentation
3.3. Physicochemical Characteristics of the Obtained Beers
3.3.1. CDR Beer Analysis
3.3.2. Residual Sugars, Glycerol, and Ethanol Determination Using HPLC
3.4. Main Volatile Compound Production
3.5. Antioxidant Activity
3.6. Principal Component Analysis (PCA)
3.7. Sensory Analysis
3.8. Consumer Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer Choice and Consumption Determinants When Craft Beers Are Tasted: An Exploratory Study of Consumer Preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could Non-Saccharomyces Yeasts Contribute on Innovative Brewing Fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Skoglund, W.; Rennemo, Ø. Craft Breweries and the Corona Crisis–Exploring the Scandinavian Context. In INTERNATIONAL SYMPOSIUM: New Metropolitan Perspectives; Springer International Publishing: Cham, Switzerland, 2022; pp. 246–256. [Google Scholar] [CrossRef]
- Schroeder, S. Craft Entrepreneurship during a Disruptive Global Pandemic Crisis: An Ethnography of Craft Breweries in Berlin. In Cultural Entrepreneurship: New Societal Trends; Springer Nature: Singapore, 2022; pp. 153–175. [Google Scholar] [CrossRef]
- Cabras, I.; Shakina, E.; Waehning, N.; Sohns, F.; Bosworth, G. Brewing at the Time of COVID: The Impact of the Pandemic Crisis on UK Craft Breweries and Its Implications for the Sector and Local Economies. Reg. Stud. 2023, 57, 1937–1953. [Google Scholar] [CrossRef]
- Parker, D.K. Beer: Production, Sensory Characteristics and Sensory Analysis. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Alcoholic Beverages; Piggot, J., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 133–158. [Google Scholar]
- Briggs, D.; Boulton, C.; Brookes, P.; Stevens, R. Brewing; CRC Press: Boca Raton, FL, USA, 2004; Volume 1, ISBN 978-0-8493-2547-2. [Google Scholar]
- Omari, I.O.; Charnock, H.M.; Fugina, A.L.; Thomson, E.L.; McIndoe, J.S. Magnesium-Accelerated Maillard Reactions Drive Differences in Adjunct and All-Malt Brewing. J. Am. Soc. Brew. Chem. 2021, 79, 145–155. [Google Scholar] [CrossRef]
- Bastgen, N.; Becher, T.; Titze, J. Influencing Factors on Hop Isomerization Beyond the Conventional Range. J. Am. Soc. Brew. Chem. 2019, 77, 126–133. [Google Scholar] [CrossRef]
- Mallett, J. Malt: A Practical Guide from Field to Brewhouse; Brewers Publications: Boulder, CO, USA, 2014; Volume 4, ISBN 978-1-938469-12-1. [Google Scholar]
- Capozzi, V.; Russo, P.; Spano, G. Microbial Information Regimen in EU Geographical Indications. World Pat. Inf. 2012, 34, 229–231. [Google Scholar] [CrossRef]
- Petruzzi, L.; Rosaria Corbo, M.; Sinigaglia, M.; Bevilacqua, A. Brewer’s Yeast in Controlled and Uncontrolled Fermentations, with a Focus on Novel, Nonconventional, and Superior Strains. Food Rev. Int. 2016, 32, 341–363. [Google Scholar] [CrossRef]
- UNE-EN ISO 9001:2015; Quality Management Systems. Spanish Association for Standardisation and Certification: Madrid, Spain, 2015.
- European Brewery Convention Analytica—EBC. Section 4 Extract of Malt: Congress Mash Method 4.5.1. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2004. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 4 Extract Difference of Malt: Congress Mash Method 4.5.2. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 4 Colour of Malt. Spectrophotometric Method (RM) Method 4.7.1. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2000. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 4 High Molecular Weight β-Glucan Content of Malt Wort: Spectrophotometric Method Method 4.16.3. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2005. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 4 Soluble Nitrogen of Malt: Kjeldahl Method Method 4.9.1. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 4 Total Nitrogen of Malt: Kjeldahl Method (IM) Method 4.3.1. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2004. [Google Scholar]
- Mitteleuropäische Brautechnische Analysen kommission (MEBAK®) e.V., Freising, BY, G. MEBAK Online. Method R-205.12.999. Ratio of Soluble Nitrogen to Total Nitrogen (Kolbach Index) in Congress Wort. Available online: https://www.mebak.org/en/methode/r-205-12-999/ratio-of-soluble-nitrogen-to-total-nitrogen-kolbach-index-in-congress-wort/2703 (accessed on 25 January 2024).
- European Brewery Convention Analytica—EBC. Section 4 Diastatic Power of Malt by Spectrophotometry (Manual Method) Method 4.12.1. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2018. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 7 Hops and Hop Products: Hop Oil Content of Hops and Hop Products Method 7.10. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2002. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 7 Hops and Hop Products: Total Polyphenols in Hops and Hop Pellets Method 7.14. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2015. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 7 Hops and Hop Products: α- and β-Acids in Hops and Hop Products by HPLC Method 7.7. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2012. [Google Scholar]
- Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation 2021, 7, 290. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Yeast Monitoring of Wine Mixed or Sequential Fermentations Made by Native Strains from D.O. “Vinos de Madrid” Using Real-Time Quantitative PCR. Front. Microbiol. 2017, 8, 2520. [Google Scholar] [CrossRef]
- Postigo, V.; Sanz, P.; García, M.; Arroyo, T. Impact of Non-Saccharomyces Wine Yeast Strains on Improving Healthy Characteristics and the Sensory Profile of Beer in Sequential Fermentation. Foods 2022, 11, 2029. [Google Scholar] [CrossRef]
- Zott, K.; Claisse, O.; Lucas, P.; Coulon, J.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Characterization of the Yeast Ecosystem in Grape Must and Wine Using Real-Time PCR. Food Microbiol. 2010, 27, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Fockler, C.; Dollinger, G.; Watson, R. Kinetic PCR Analysis: Real-Time Monitoring of DNA Amplification Reactions. Nat. Biotechnol. 1993, 11, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.; López, R.; Cacho, J.; Ferreira, V. Fast Analysis of Important Wine Volatile Compounds—Development and Validation of a New Method Based on Gas Chromatographic-Flame Ionisation Detection Analysis of Dichloromethane Microextracts. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.-M.; Porumb, R.; Epure, P. Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.10. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- Varela, P.; Ares, G. Sensory Profiling, the Blurred Line between Sensory and Consumer Science. A Review of Novel Methods for Product Characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Choi, E.J.; Lee, S.J.; Kwon, Y.A.; Hong, K.W.; Kim, W.J. Multivariate Analysis for Feasibility of Korean Six-Row Barleys for Beer Brewing. J. Inst. Brew. 2014, 120, 371–378. [Google Scholar] [CrossRef]
- Fox, G.P.; Panozzo, J.F.; Li, C.D.; Lance, R.C.M.; Inkerman, P.A.; Henry, R.J. Molecular Basis of Barley Quality. Aust. J. Agric. Res. 2003, 54, 1081. [Google Scholar] [CrossRef]
- Rani, H.; Bhardwaj, R.D. Quality Attributes for Barley Malt: “The Backbone of Beer”. J. Food Sci. 2021, 86, 3322–3340. [Google Scholar] [CrossRef]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of Its By-Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef]
- Fox, G.P. Chemical Composition in Barley Grains and Malt Quality. In Genetics and Improvement of Barley Malt Quality; Spinger: Berlin/Heidelberg, Germany, 2009; pp. 63–98. [Google Scholar] [CrossRef]
- Howard, K.A.; Gayler, K.R.; Eagles, H.A.; Halloran, G.M. The Relationship Between D Hordein and Malting Quality in Barley. J. Cereal Sci. 1996, 24, 47–53. [Google Scholar] [CrossRef]
- Deme, G.D.; Gari, M.T.; Tessema, B. Evaluation of Malting Potential of Different Barley Varieties. J. Water Pollut. Purif. Res. 2019, 6, 24–35. [Google Scholar]
- Grujić, O. Application of a Commercial Enzyme Preparation in the Barley Malting Process. J. Inst. Brew. 1998, 104, 249–253. [Google Scholar] [CrossRef]
- Kefale, B.; Abushu, Y. Malt Quality Profile of Malt Barley Varieties Malt Quality Profile of Malt Barley Varieties Grown in the Central Highlands of Ethiopia. Acad. Res. J. Agric. Sci. Res. 2022, 5, 128–133. [Google Scholar]
- Jones, B.L.; Budde, A.D. How Various Malt Endoproteinase Classes Affect Wort Soluble Protein Levels. J. Cereal Sci. 2005, 41, 95–106. [Google Scholar] [CrossRef]
- Guerrero, B.G. Effects of Amino Acid Profile, Endoprotease Activities and Wort Quality on Fermentability under Different Malting and Brewing Conditions; University of Manitoba: Winnipeg, MB, Canada, 2009. [Google Scholar]
- Sulaiman, A.F.H. The Effect of Barley (Hordeum vulgare L.) Water Extract as a Chemolytic Agents in Cholelithiasis (Gallbladder Stone)—An In-Vitro Study. Master’s Thesis, USM-Health Campus, Universiti Sains Malaysia, Kota Bharu, Malaysia, 2017. [Google Scholar]
- Brazil, C.; Oliveira, D.F.; Duarte, R.A.; Galo, J.M.; Lucchetta, L.; Santos, E.d.C.d.; Hashimoto, E.H. β-Glucanase Addition in Brewing Malt Produced by Reduced Time of Germination. Braz. Arch. Biol. Technol. 2019, 62, 1–15. [Google Scholar] [CrossRef]
- Allosio-Ouarnier, N.; Quemener, B.; Bertrand, D.; Boivin, P. Application of High Performance Anion Exchange Chromatography to the Study of Carbohydrate Changes in Barley During Malting. J. Inst. Brew. 2000, 106, 45–52. [Google Scholar] [CrossRef]
- Psota, V. Free Amino Nitrogen in Sweet Wort Made from Barley Varieties Tested in the Czech Republic. Kvas. Prum. 2019, 65, 142–148. [Google Scholar] [CrossRef]
- Bravi, E.; Marconi, O.; Perretti, G.; Fantozzi, P. Influence of Barley Variety and Malting Process on Lipid Content of Malt. Food Chem. 2012, 135, 1112–1117. [Google Scholar] [CrossRef]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Mirzan, D. Comparative Evaluation of the Physico-Chemical Characteristics of Buckwheat Malt and Barley Malt. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Vienna, Austria, 6–8 December 2022; Volume 21, pp. 97–104. [Google Scholar]
- Kunze, W.; Manger, H.-J.; Pratt, S. Technology Brewing & Malting, 3rd ed.; Die Deustche Bibliothek: Berlin, Germany, 2014; p. 968. [Google Scholar]
- Evans, D.E.; Collins, H.; Eglinton, J.; Wilhelmson, A. Assessing the Impact of the Level of Diastatic Power Enzymes and Their Thermostability on the Hydrolysis of Starch during Wort Production to Predict Malt Fermentability. J. Am. Soc. Brew. Chem. 2005, 63, 185–198. [Google Scholar] [CrossRef]
- Sakamoto, K.; Konings, W.N. Beer Spoilage Bacteria and Hop Resistance. Int. J. Food Microbiol. 2003, 89, 105–124. [Google Scholar] [CrossRef]
- Buiatti, S. Beer Composition: An Overview. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 213–225. [Google Scholar] [CrossRef]
- Eßlinger, H.M.; Narziß, L. Beer. In Ullmann’s Enzyclopedia of Industrial Chemistry, 6th ed.; Wiley—VCH Verlag GmbH: Weinheim, Germany, 2002; Volume 4, ISBN 9783527306732. [Google Scholar]
- Van Opstaele, F.; De Rouck, G.; De Clippeleer, J.; Aerts, G.; De Cooman, L. Analytical and Sensory Assessment of Hoppy Aroma and Bitterness of Conventionally Hopped and Advanced Hopped Pilsner Beers. Cerevisia 2011, 36, 47–59. [Google Scholar] [CrossRef]
- Habschied, K.; Košir, I.J.; Krstanović, V.; Kumrić, G.; Mastanjević, K. Beer Polyphenols—Bitterness, Astringency, and Off-Flavors. Beverages 2021, 7, 38. [Google Scholar] [CrossRef]
- Rodolfi, M.; Chiancone, B.; Liberatore, C.M.; Fabbri, A.; Cirlini, M.; Ganino, T. Changes in Chemical Profile of Cascade Hop Cones According to the Growing Area. J. Sci. Food Agric. 2019, 99, 6011–6019. [Google Scholar] [CrossRef] [PubMed]
- Česlová, L.; Holčapek, M.; Fidler, M.; Drštičková, J.; Lísa, M. Characterization of Prenylflavonoids and Hop Bitter Acids in Various Classes of Czech Beers and Hop Extracts Using High-Performance Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 2009, 1216, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, V.; Callemien, D.; Collin, S. Determination of Stilbenes in Hop Pellets from Different Cultivars. J. Agric. Food Chem. 2005, 53, 4202–4206. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Gahr, A. On the Fate of Certain Hop Substances during Dry Hopping. Brew. Sci. 2013, 66, 93–103. [Google Scholar]
- Juvonen, R.; Koivula, T.; Haikara, A. Group-Specific PCR-RFLP and Real-Time PCR Methods for Detection and Tentative Discrimination of Strictly Anaerobic Beer-Spoilage Bacteria of the Class Clostridia. Int. J. Food Microbiol. 2008, 125, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Schneiderbanger, J.; Grammer, M.; Jacob, F.; Hutzler, M. Statistical Evaluation of Beer Spoilage Bacteria by Real-Time PCR Analyses from 2010 to 2016. J. Inst. Brew. 2018, 124, 173–181. [Google Scholar] [CrossRef]
- Oldham, R.C.; Held, M.A. Methods for Detection and Identification of Beer-Spoilage Microbes. Front. Microbiol. 2023, 14, 1217704. [Google Scholar] [CrossRef]
- Marongiu, A.; Zara, G.; Legras, J.-L.; Del Caro, A.; Mascia, I.; Fadda, C.; Budroni, M. Novel Starters for Old Processes: Use of Saccharomyces cerevisiae Strains Isolated from Artisanal Sourdough for Craft Beer Production at a Brewery Scale. J. Ind. Microbiol. Biotechnol. 2015, 42, 85–92. [Google Scholar] [CrossRef]
- Rossi, S.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Evaluation of Saccharomyces cerevisiae Strains Isolated from Non-Brewing Environments in Beer Production. J. Inst. Brew. 2018, 124, 381–388. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Gibson, B.; Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J. Bioprospecting for Brewers: Exploiting Natural Diversity for Naturally Diverse Beers. Yeast 2019, 36, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Hierro, N.; Esteve-Zarzoso, B.; Mas, A.; Guillamón, J.M. Monitoring of Saccharomyces and Hanseniaspora Populations during Alcoholic Fermentation by Real-Time Quantitative PCR. FEMS Yeast Res. 2007, 7, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Hierro, N.; Esteve-Zarzoso, B.; González, A.; Mas, A.; Guillamón, J.M. Real-Time Quantitative PCR (QPCR) and Reverse Transcription-QPCR for Detection and Enumeration of Total Yeasts in Wine. Appl. Environ. Microbiol. 2006, 72, 7148–7155. [Google Scholar] [CrossRef] [PubMed]
- Permyakova, L.V.; Pomozova, V.A.; Antipova, L.V. Improvement of Brewer’s Yeast Viability by Adjusting Wort Composition. Foods Raw Mater. 2017, 5, 94–104. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Z.; Sun, W.; Luan, Y.; Piao, M.; Deng, Y. Characterization and Formation Mechanisms of Viable, but Putatively Non-Culturable Brewer’s Yeast Induced by Isomerized Hop Extract. LWT 2022, 155, 112974. [Google Scholar] [CrossRef]
- Einfalt, D. Barley-Sorghum Craft Beer Production with Saccharomyces cerevisiae, Torulaspora delbrueckii and Metschnikowia pulcherrima Yeast Strains. Eur. Food Res. Technol. 2021, 247, 385–393. [Google Scholar] [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Impact of Static Dry-hopping Rate on the Sensory and Analytical Profiles of Beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological Traits of Lachancea thermotolerans Show Signs of Domestication and Allopatric Differentiation. Sci. Rep. 2018, 8, 14812. [Google Scholar] [CrossRef] [PubMed]
- Engan, S. Organoleptic Threshold Values of Some Organic Acids in Beer. J. Inst. Brew. 1974, 80, 162–163. [Google Scholar] [CrossRef]
- Clapperton, J.F.; Brown, D.G.W. Caprylic Flavour as a Feature of Beer Flavour. J. Inst. Brew. 1978, 84, 90–92. [Google Scholar] [CrossRef]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The Chemical Profiling of Fatty Acids during the Brewing Process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Popescu, V.; Soceanu, A.; Dobrinas, S.; Stanciu, G. A Study of Beer Bitterness Loss during the Various Stages of the Romanian Beer Production Process. J. Inst. Brew. 2013, 119, 111–115. [Google Scholar] [CrossRef]
- Bamforth, C.W. Beer and Health. In Beer; Elsevier: Amsterdam, The Netherlands, 2009; pp. 229–253. ISBN 9780126692013. [Google Scholar]
- Laws, D.R.J.; McGuinness, J.D.; Rennie, H. The Losses Of Bitter Substances During Fermentation. J. Inst. Brew. 1972, 78, 314–321. [Google Scholar] [CrossRef]
- Meilgaard, M.; Elizondo, A.; Moya, E. A Study of Carbonyl Compounds in Beer, Part II. Flavor and Flavor Thresholds of Aldehydes and Ketones Added to Beer. Tec. Q. Master Brew. Assoc. Am. 1970, 7, 143–149. [Google Scholar]
- Krogerus, K.; Gibson, B.R. 125 Th Anniversary Review: Diacetyl and Its Control during Brewery Fermentation. J. Inst. Brew. 2013, 119, 86–97. [Google Scholar] [CrossRef]
- Pickerell, A.T.W.; Hwang, A.; Axcell, B.C. Impact of Yeast-Handling Procedures on Beer Flavor Development during Fermentation. J. Am. Soc. Brew. Chem. 1991, 49, 87–92. [Google Scholar] [CrossRef]
- Bamforth, C. Beer Flavour: Sulphur Substances. Brew. Guard. 2001, 130, 20–23. [Google Scholar]
- Van Haecht, J.L.; Dufour, J.P. The Production of Sulfur Compounds by Brewing Yeast: A Review. Cerevisia Biotechnol. 1995, 20, 51–64. [Google Scholar]
- Duan, W.; Roddick, F.A.; Higgins, V.J.; Rogers, P.J. A Parallel Analysis of H2S and SO2 Formation by Brewing Yeast in Response to Sulfur-Containing Amino Acids and Ammonium Ions. J. Am. Soc. Brew. Chem. 2004, 62, 35–41. [Google Scholar] [CrossRef]
- Kaneda, H.; Kano, Y.; Sekine, T.; Ishii, S.; Takahashi, K.; Koshino, S. Effect of Pitching Yeast and Wort Preparation on Flavor Stability of Beer. J. Ferment. Bioeng. 1992, 73, 456–460. [Google Scholar] [CrossRef]
- Lugasi, A. Polyphenol Content and Antioxidant Properties of Beer. Acta Aliment. 2003, 32, 181–192. [Google Scholar] [CrossRef]
- Tefera, M.; Ayele, D. Investigation of Total Polyphenol, Antioxidant Activity, and Levels of Metals in Ethiopian Commercial Beers. Cogent Chem. 2020, 6, 1824336. [Google Scholar] [CrossRef]
- Nardini, M.; Foddai, M.S. Phenolics Profile and Antioxidant Activity of Special Beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.S.; Baxter, E.D. Beer: Quality, Safety and Nutritional Aspects; Royal Society of Chemistry: Cambridge, UK, 2001; ISBN 0854045880. [Google Scholar]
- Langstaff, S.A.; Lewis, M.J. The Mouthfeel of Beer—A Review. J. Inst. Brew. 1993, 99, 31–37. [Google Scholar] [CrossRef]
- Parker, W.E.; Richardson, P.J. The Quantitative Determination Of Glycerol In Beer By Gas-Liquid Chromatography. J. Inst. Brew. 1970, 76, 191–198. [Google Scholar] [CrossRef]
- Langstaff, S.A.; Guinard, J.-X.; Lewis, M.J. Instrumental Evaluation of the Mouthfeel of Beer and Correlation With Sensory Evaluation. J. Inst. Brew. 1991, 97, 427–433. [Google Scholar] [CrossRef]
- Tan, Y.; Siebert, K.J. Quantitative Structure-Activity Relationship Modeling of Alcohol, Ester, Aldehyde, and Ketone Flavor Thresholds in Beer from Molecular Features. J. Agric. Food Chem. 2004, 52, 3057–3064. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Daenen, L.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Furfuryl Ethyl Ether: Important Aging Flavor and a New Marker for the Storage Conditions of Beer. J. Agric. Food Chem. 2004, 52, 1661–1668. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Z.; Xiong, B. Preparation of Novel Solid-Phase Microextraction Fibers by Sol-Gel Technology for Headspace Solid-Phase Microextraction-Gas Chromatographic Analysis of Aroma Compounds in Beer. J. Chromatogr. A 2005, 1065, 287–299. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Wlazły, K.; Wa̧sowicz, E.; Kamiński, E. Solid-Phase Microextraction for the Analysis of Some Alcohols and Esters in Beer: Comparison with Static Headspace Method. J. Agric. Food Chem. 1998, 46, 1469–1473. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verstrepen, K.J.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G. Influence of the Brewing Process on Furfuryl Ethyl Ether Formation during Beer Aging. J. Agric. Food Chem. 2004, 52, 6755–6764. [Google Scholar] [CrossRef] [PubMed]
- Guido, L. The Impact of the Physiological Condition of the Pitching Yeast on Beer Flavour Stability: An Industrial Approach. Food Chem. 2004, 87, 187–193. [Google Scholar] [CrossRef]
- Budroni, M.; Zara, G.; Ciani, M.; Comitini, F. Saccharomyces and Non-Saccharomyces Starter Yeasts. In Brewing Technology; InTech: Rijeka, Croatia, 2017; pp. 81–100. [Google Scholar]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer Volatile Compounds and Their Application to Low-Malt Beer Fermentation. J. Biosci. Bioeng. 2008, 106, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R. Higher Alcohol and Acetic Acid Production by Apiculate Wine Yeasts. J. Appl. Bacteriol. 1992, 73, 126–130. [Google Scholar] [CrossRef]
- Piddocke, M.P.; Kreisz, S.; Heldt-Hansen, H.P.; Nielsen, K.F.; Olsson, L. Physiological Characterization of Brewer’s Yeast in High-Gravity Beer Fermentations with Glucose or Maltose Syrups as Adjuncts. Appl. Microbiol. Biotechnol. 2009, 84, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.C. Flavor Chemistry in Beer: Part I: Flavor Interaction between Principal Volatiles. Master Brew. Assoc. Am. Tech. Q. 1975, 12, 107–117. [Google Scholar]
- Bamforth, C.W. Standards of Brewing: Formulas for Consistency and Excellence; Brewers Publications: Boulder, CO, USA, 2002; p. 210. [Google Scholar]
- Meilgaard, M.C. Prediction of Flavor Differences between Beers from Their Chemical Composition. J. Agric. Food Chem. 1982, 30, 1009–1017. [Google Scholar] [CrossRef]
- Humia, B.V.; Santos, K.S.; Barbosa, A.M.; Sawata, M.; Mendonça, M.D.C.; Padilha, F.F. Beer Molecules and Its Sensory and Biological Properties: A Review. Molecules 2019, 24, 1568. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. Phenolic Compounds in Beer Inhibit Formation of Polycyclic Aromatic Hydrocarbons from Charcoal-Grilled Chicken Wings. Food Chem. 2019, 294, 578–586. [Google Scholar] [CrossRef]
- Callemien, D.; Jerkovic, V.; Rozenberg, R.; Collin, S. Hop as an Interesting Source of Resveratrol for Brewers: Optimization of the Extraction and Quantitative Study by Liquid Chromatography/Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Goupy, P.; Hugues, M.; Boivin, P.; Amiot, M.J. Antioxidant Composition and Activity of Barley (Hordeum vulgare) and Malt Extracts and of Isolated Phenolic Compounds. J. Sci. Food Agric. 1999, 79, 1625–1634. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae Var. Boulardii in Co-Fermentations with S. cerevisiae for the Production of Craft Beers with Potential Healthy Value-Added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Branco, G.F.; Faria, J.D.A.F.; Cruz, A.G. Characterization of Brazilian Lager and Brown Ale Beers Based on Color, Phenolic Compounds, and Antioxidant Activity Using Chemometrics. J. Sci. Food Agric. 2011, 91, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Aliani, M.; Eskin, M.N.A. (Eds.) Bitterness; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; ISBN 9781118590263. [Google Scholar]
- da Costa Jardim, C.; de Souza, D.; Cristina Kasper Machado, I.; Massochin Nunes Pinto, L.; de Souza Ramos, R.; Garavaglia, J. Sensory Profile, Consumer Preference and Chemical Composition of Craft Beers from Brazil. Beverages 2018, 4, 106. [Google Scholar] [CrossRef]
- Eßlinger, H.M. Handbook of Brewing Processes Technology Markets; Wiley: Weinheim, Germany, 2009; ISBN 9783527314065. [Google Scholar]
- Calvo-Porral, C.; Rivaroli, S.; Orosa-Gonzalez, J. How Consumer Involvement Influences Beer Flavour Preferences. Int. J. Wine Bus. Res. 2020, 32, 537–554. [Google Scholar] [CrossRef]



| Bicarbonates | Calcium | Zinc | Magnesium | Potassium | Sulphate | Chlorides | pH |
|---|---|---|---|---|---|---|---|
| 49.50 ± 0.50 | 24.50 ± 2.40 | 0.06 ± 0.01 | 4.70 ± 0.00 | <50 | <10 | <50 | 8.21 ± 0.38 |
| Parameters | Barley Malt |
|---|---|
| Congress Mash: | |
| Saccharification rate (min) | 10.00 ± 0.00 |
| Speed of filtration (h) | <1.00 ± 0.00 |
| Odour | normal |
| Fine–coarse difference (%) | 2.60 ± 0.28 |
| pH of wort | 5.96 ± 0.06 |
| Colour (EBC) | 4.50 ± 1.13 |
| β-glucans (mg L−1) | 217.15 ± 40.31 |
| Soluble nitrogen content (%) | 5.85 ± 1.34 |
| Total nitrogen (%) | 14.35 ± 0.92 |
| Kolbach Index | 40.50 ± 6.36 |
| Diastatic power (° WK) | 399.00 ± 24.04 |
| Parameters | Cascade | Rayo Verde Tajuña | Torreta Tajuña |
|---|---|---|---|
| Essential oils (mL) | 0.51 ± 0.03 a | 0.05 ± 0.00 b | 0.05 ± 0.00 b |
| Polyphenols (%) | 1.00 ± 0.10 a | 0.59 ± 0.09 b | 0.90 ± 0.10 a |
| α-acids: | |||
| Cohumulone (%) | 2.06 ± 0.16 a | 0.25 ± 0.05 b | 0.21 ± 0.02 b |
| Adhumulone (%) | 5.83 ± 0.13 a | 1.03 ± 0.05 b | 0.80 ± 0.10 b |
| β-acids: | |||
| Colupulone (%) | 2.18 ± 0.18 a | 1.29 ± 0.09 b | 1.03 ± 0.03 b |
| Adlupulone (%) | 2.48 ± 0.18 a | 2.02 ± 0.02 b | 1.63 ± 0.13 c |
| Yeast Strain | Slope | Intersection | R2 | Efficiency |
|---|---|---|---|---|
| S. cerevisiae G 520 | −3.17 ± 0.05 | 38.39 ± 0.32 | 0.986 ± 0.02 | 106.83 ± 2.31 |
| S. cerevisiae G 354 | −3.36 ± 0.08 | 40.03 ± 0.47 | 0.997 ± 0.00 | 98.37 ± 3.15 |
| Recipes | Lactic Acid mg L−1 | Colour EBC | Bitterness IBU | VDKs mg L−1 | SO2 mg L−1 | Polyphenols mg L−1 |
|---|---|---|---|---|---|---|
| A | 273.50 ± 10.50 a | 17.5 ± 0.50 a | 11.80 ± 1.10 a | 0.29 ± 0.05 a | 3.80 ± 0.20 c | 269.5 ± 6.50 a |
| B | 294.00 ± 29.00 a | 16.00 ± 0.00 ab | 11.15 ± 0.55 a | 0.17 ± 0.01 bc | 12.45 ± 0.95 a | 299.5 ± 12.50 a |
| C | 209.5 ± 21.50 b | 15.5 ± 0.50 bc | 10.80 ± 0.80 a | 0.23 ± 0.03 ab | 4.60 ± 0.90 bc | 267.5 ± 41.50 a |
| D | 282.5 ± 18.50 a | 14.0 ± 1.00 c | 10.70 ± 0.70 a | 0.10 ± 0.02 c | 6.70 ± 1.60 b | 327.5 ± 59.50 a |
| Recipes | Residual Fermentable Sugars (g L−1) | Apparent Attenuation (%) | Ethanol % (v/v) | Glycerol (g L−1) | |
|---|---|---|---|---|---|
| Maltotriose | Maltose | ||||
| A | 8.40 ± 0.44 a | 2.39 ±0.02 b | 69.5 ± 0.50 a | 3.25 ± 0.06 a | 1.72 ± 0.05 a |
| B | 8.83 ± 0.07 a | 2.50 ±0.03 a | 67.5 ± 0.50 bc | 3.21 ± 0.03 a | 1.68 ± 0.00 ab |
| C | 9.24 ± 0.66 a | 2.15 ± 0.03 c | 69.0 ± 1.00 ab | 3.33 ± 0.04 a | 1.59 ± 0.02 b |
| D | 8.90 ± 0.01 a | 2.04 ± 0.06 d | 67.0 ± 0.00 c | 3.05 ± 0.07 b | 1.47 ± 0.07 c |
| Recipes | A | B | C | D |
|---|---|---|---|---|
| Higher alcohols | ||||
| Isobutanol | 33.72 ± 0.94 a | 13.17 ± 1.76 b | 35.24 ± 3.43 a | 12.17 ± 0.65 b |
| 1-butanol | 0.14 ± 0.00 a | 0.13 ± 0.00 b | nd | nd |
| Isoamyl alcohol | 107.80 ± 0.36 b | 82.07 ± 2.90 c | 116.85 ± 4.91 a | 76.43 ± 3.32 c |
| Methionol | 0.47 ± 0.13 a | 0.32 ± 0.00 a | 0.35 ± 0.04 a | 0.33 ± 0.01 a |
| Benzyl alcohol | 0.05 ± 0.00 b | 0.07 ± 0.01 a | 0.04 ± 0.00 b | 0.04 ± 0.00 b |
| β-phenylethanol | 26.69 ± 1.44 a | 11.04 ± 0.07 b | 32.92 ± 1.41 a | 15.23 ± 4.40 b |
| Esters | ||||
| Isoamyl acetate | 2.92 ± 0.27 a | 1.32± 0.02 b | 2.38 ± 0.11 a | 0.78 ± 0.36 b |
| Ethyl butyrate | 0.03 ± 0.03 b | 0.18 ± 0.00 a | 0.11 ± 0.00 ab | 0.09 ± 0.09 ab |
| Ethyl isovalerate | 0.15 ± 0.00 a | 0.14 ± 0.01 a | 0.14 ± 0.00 a | 0.14 ± 0.01 a |
| Ethyl hexanoate | 0.32 ± 0.00 a | 0.33 ± 0.01 a | 0.29 ± 0.00 ab | 0.20 ± 0.08 b |
| Ethyl octanoate | 0.08 ± 0.01 a | 0.10 ± 0.01 a | 0.11 ± 0.00 a | 0.09 ± 0.03 a |
| 2-phenylethyl acetate | 0.11 ± 0.00 a | 0.05 ± 0.00 c | 0.10 ± 0.00 b | 0.05 ± 0.00 c |
| Fatty acids | ||||
| Butyric acid | 0.64 ± 0.01 a | 0.58 ± 0.02 b | 0.56 ± 0.01 bc | 0.54 ± 0.00 c |
| Hexanoic acid | 3.12 ± 0.21 a | 4.43 ± 0.00 a | 3.34 ± 0.12 a | 3.26 ± 0.09 a |
| Octanoic acid | 7.70 ± 1.64 b | 13.14 ± 0.02 a | 10.44 ± 0.55 ab | 9.68 ± 3.65 ab |
| Decanoic acid | 2.74 ± 0.13 b | 8.83 ± 1.24 a | 4.10 ± 0.05 b | 4.16 ± 2.67 b |
| Recipes | Q1 | Q2 | Qt |
|---|---|---|---|
| A | 3.39 ± 0.00 a | 9.41 ± 0.13 ab | 12.81 ± 0.13 b |
| B | 3.58 ± 0.16 a | 9.75 ± 0.09 a | 13.34 ± 0.07 a |
| C | 3.36 ± 0.09 a | 9.15 ± 0.21 b | 12.51 ± 0.30 bc |
| D | 3.53 ± 0.06 a | 8.73 ± 0.18 c | 12.26 ± 0.12 c |
| Sensory Attributes | Response (n = 110) | |||
|---|---|---|---|---|
| Appearance | A | B | C | D |
| Visual impression | ||||
| Very hazy | 31 | 44 | 35 | 29 |
| Hazy | 17 | 14 | 35 | 29 |
| Dull | 38 | 35 | 26 | 30 |
| Clear | 15 | 13 | 9 | 15 |
| Bright | 9 | 4 | 5 | 7 |
| Aroma | ||||
| Aroma intensity | ||||
| Low | 49 | 34 | 41 | 37 |
| Low-medium | 24 | 27 | 30 | 26 |
| Medium | 32 | 35 | 29 | 38 |
| Medium-high | 4 | 11 | 10 | 8 |
| High | 1 | 3 | 0 | 1 |
| Taste | ||||
| Acidity | ||||
| Low | 35 | 40 | 48 | 46 |
| Low-medium | 43 | 49 | 40 | 44 |
| Medium | 7 | 10 | 6 | 11 |
| Medium-high | 21 | 8 | 12 | 9 |
| High | 4 | 3 | 4 | 0 |
| Bitterness | ||||
| Low | 29 | 27 | 42 | 38 |
| Low-medium | 40 | 45 | 38 | 44 |
| Medium | 26 | 26 | 20 | 16 |
| Medium-high | 12 | 7 | 8 | 10 |
| High | 3 | 5 | 2 | 2 |
| Aftertaste intensity | ||||
| Short | 54 | 49 | 42 | 45 |
| Short-medium | 9 | 9 | 19 | 24 |
| Medium | 19 | 17 | 19 | 8 |
| Medium-long | 27 | 34 | 27 | 30 |
| Long | 1 | 1 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postigo, V.; Mauro, L.; Diaz, T.; Saiz, R.; Arroyo, T.; García, M. Autochthonous Ingredients for Craft Beer Production. Fermentation 2024, 10, 225. https://doi.org/10.3390/fermentation10050225
Postigo V, Mauro L, Diaz T, Saiz R, Arroyo T, García M. Autochthonous Ingredients for Craft Beer Production. Fermentation. 2024; 10(5):225. https://doi.org/10.3390/fermentation10050225
Chicago/Turabian StylePostigo, Vanesa, Luz Mauro, Teresa Diaz, Roberto Saiz, Teresa Arroyo, and Margarita García. 2024. "Autochthonous Ingredients for Craft Beer Production" Fermentation 10, no. 5: 225. https://doi.org/10.3390/fermentation10050225