Purification of Polymer-Grade Fumaric Acid from Fermented Spent Sulfite Liquor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spent Sulfite Liquor (SSL)-Based Fermentation Broth
2.1.1. SSL Pretreatment
2.1.2. Microorganism and Culture
2.2. Precipitation of Fumaric Acid
2.2.1. Fumaric Acid Solubility Studies
2.2.2. Fumaric acid Recovery
2.3. Use of Activated Carbon
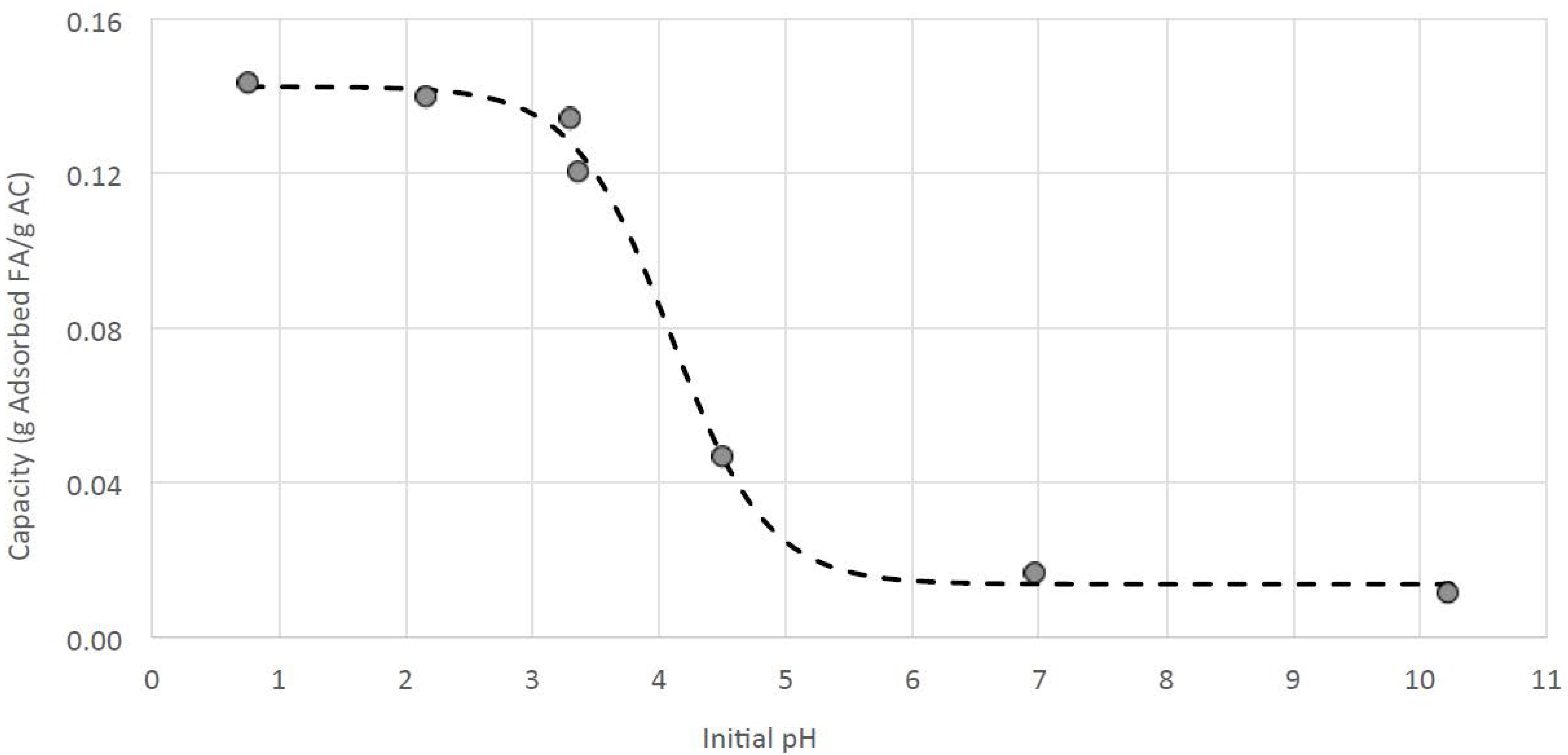
2.3.1. Determination of Fumaric Acid Adsorption Capacity
2.3.2. Removal of Contaminants of Fumaric Acid
2.3.3. Recovery of Fumaric Acid from Dilute Solutions
2.4. Analytical Methods
3. Results and Discussion
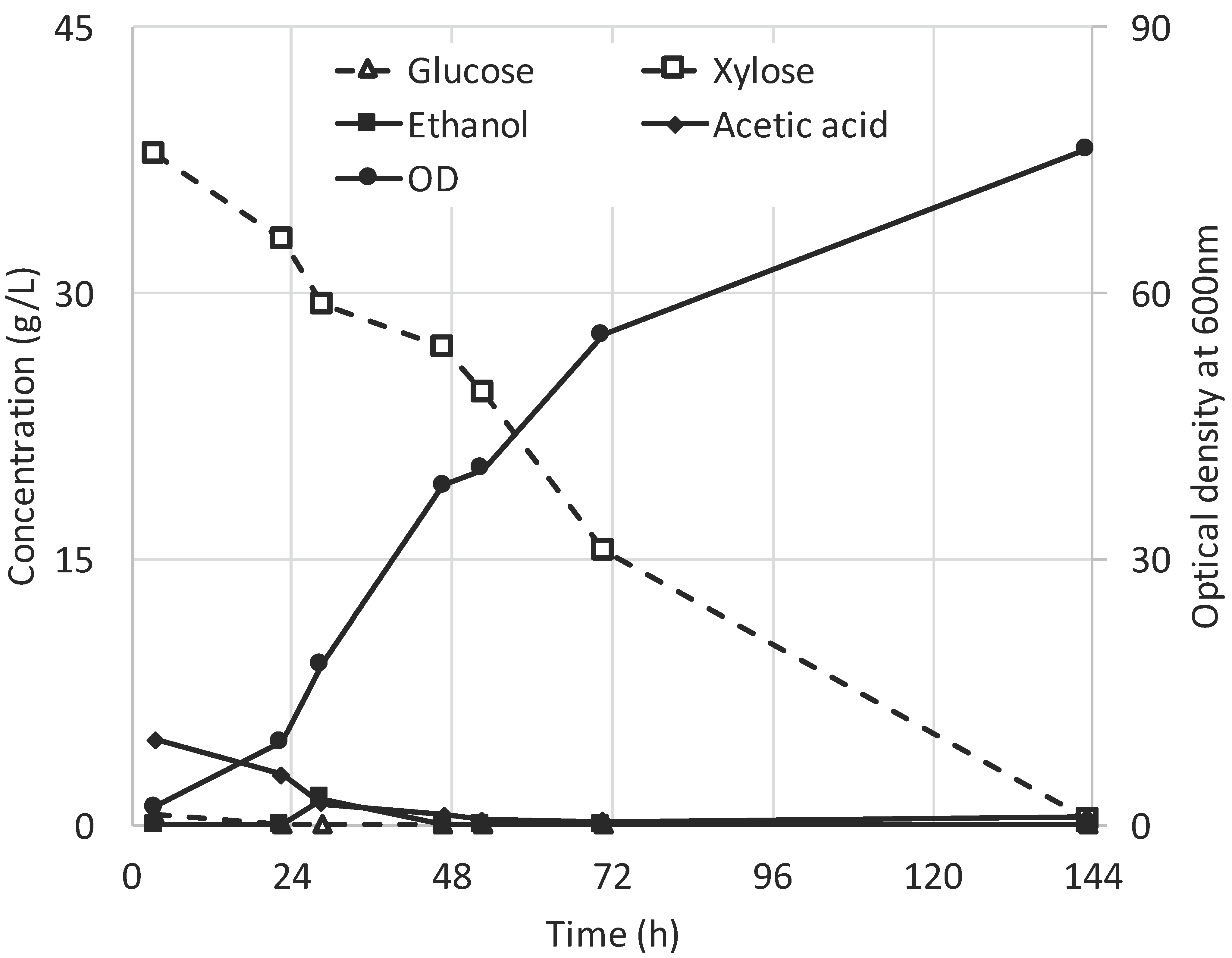
3.1. Fermentation of SSL Permeate
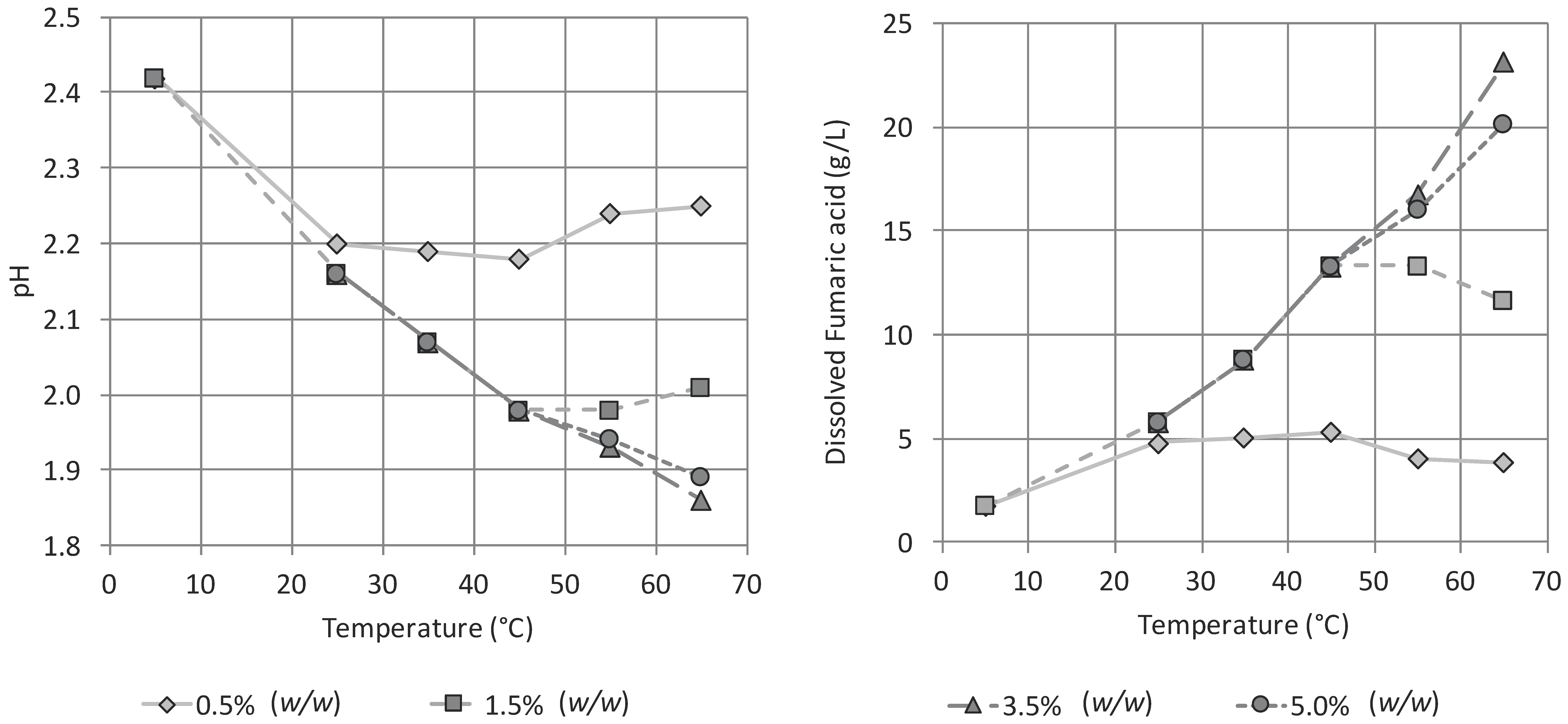
3.2. Influence of pH and Temperature on the Precipitation of Fumaric Acid
3.3. Direct Recovery of Fumaric Acid from SSL-Based Fermentation Media
3.4. Improving the Purity of Fumaric Acid with Activated Carbon
3.5. Improving the Yield of the Fumaric Acid Purification Process
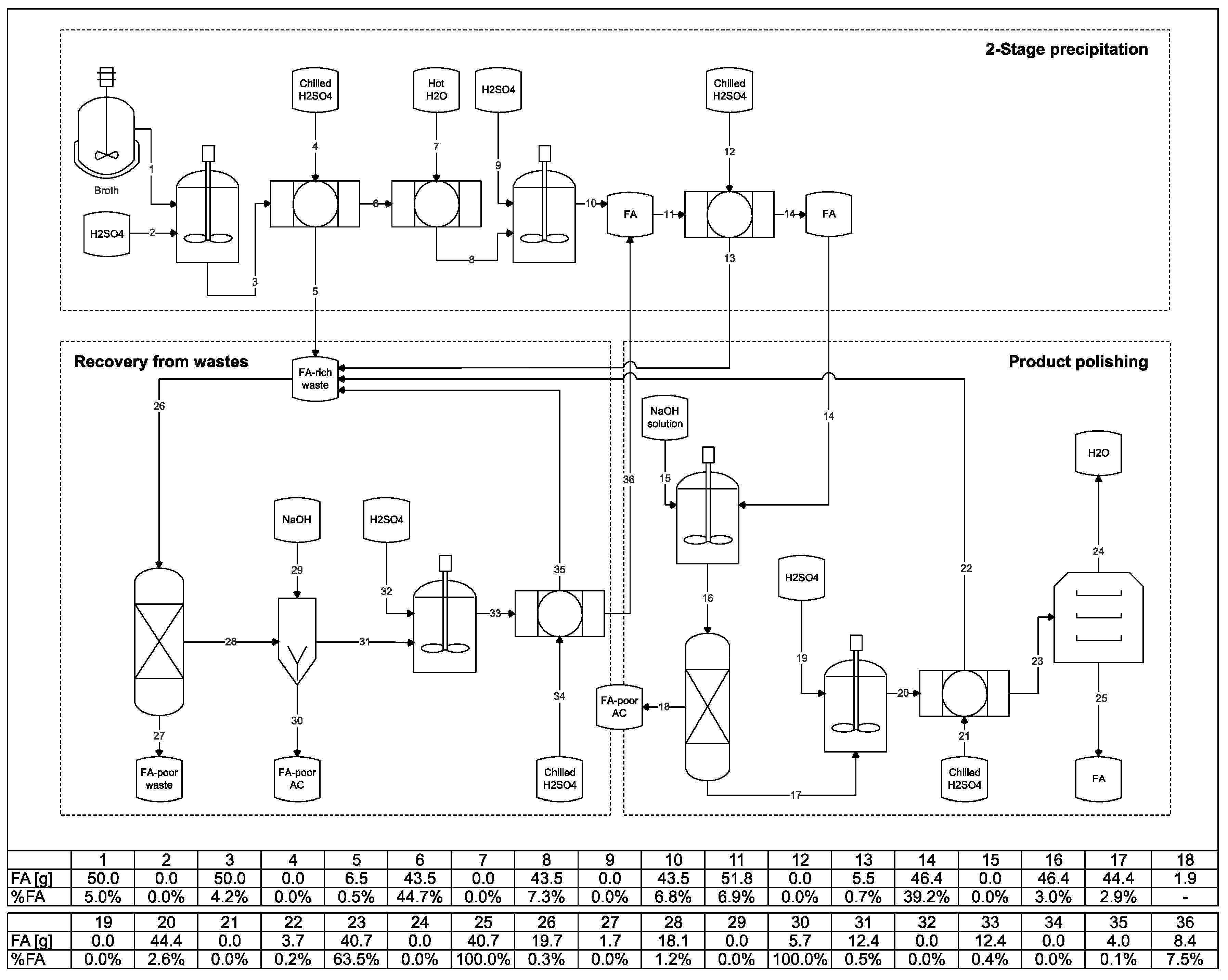
3.6. Integration of the Steps
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Towards the Circular Economy: Accelerating the Scale-Up across Global Supply Chains; World Economic Forum: Geneva, Switzerland, 2014.
- Sheridan, K. Making the bioeconomy circular: The biobased industrie’s next goal? Ind. Biotechnol. 2016, 12, 339–340. [Google Scholar] [CrossRef]
- Taha, M.; Foda, M.; Shahasavari, E.; Aburto-Medina, A.; Adetutu, E.; Ball, A. Commercial feasibility of lignocellulose biodegradation: Possibilities and challenges. Curr. Opin. Biotechnol. 2016, 38, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.B.; Jameel, H.; Chang, H.M. Integration of pulp and paper technology with bioethanol production. Biotechnol. Biofuels 2013, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Palgan, Y.V.; McCormick, K. Biorefineries in Sweden: Perspectives on the opportunities, challenges and future. Biofuels Bioprod. Biorefin. 2016, 10, 523–533. [Google Scholar] [CrossRef]
- Pereira, S.R.; Portugal-Nunes, D.J.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Advances in ethanol production from hardwood spent sulphite liquors. Proc. Biochem. 2013, 48, 272–282. [Google Scholar] [CrossRef]
- Fumaric Acid Market Size by Application (Food & Beverages, Rosin Paper Sizes, Rosin, UPR, Alkyd Resins), Competitive Analysis & Forecast, 2014–2020; Hexa Research Inc.: Felton, CA, USA, 6 September 2016; Available online: https://www.hexaresearch.com/research-report/fumaric-acid-market (accessed on 15 January 2017).
- Xu, Q.; Li, S.; Fu, Y.; Tai, C.; Huang, H. Two-stage utilization of corn straw by Rhizopus oryzae for fumaric acid production. Bioresour. Technol. 2010, 101, 6262–6264. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zou, W.; Chen, X.; Xu, N.; Liu, L.; Chen, J. Fumaric acid production in Saccharomyces cerevisiae by in silico aided metabolic engineering. PLoS ONE 2012, 7, e52086. [Google Scholar] [CrossRef] [PubMed]
- Demeke, M.M.; Dumortier, F.; Li, Y.; Broeckx, T.; Foulquié-Moreno, M.R.; Thevelein, J.M. Combining inhibitor tolerance and D-xylose fermentation in industrial Saccharomyces cerevisiae for efficient lignocellulose-based bioethanol production. Biotechnol. Biofuels 2013, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.A.; Zelle, R.M.; Pronk, J.T.; van Maris, A.J.A. Metabolic engineering of Saccharomyces cerevisiae for production of carboxylic acids: Current status and challenges. FEMS Yeast Res. 2009, 9, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Katsarava, R.; Puiggalí, J. Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: From polyesters to poly(ester amide)s. Int. J. Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 87th ed.; Taylor and Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Roa-Engel, C.A. Integration of Fermentation and Cooling Crystallisation to Produce Organic Acids. Ph.D. Thesis, Technical University of Delft, Delft, The Netherlands, 2010. [Google Scholar]
- Straathof, A.J.; van Gulik, W.M. Production of fumaric acid by fermentation. Subcell. Biochem. 2012, 64, 225–240. [Google Scholar] [PubMed]
- Fu, Y.-Q.; Li, S.; Chen, Y.; Xu, Q.; Huang, H.; Sheng, X.-Y. Enhancement of fumaric acid production by Rhizopus oryzae using a two-stage dissolved oxygen control strategy. Appl. Biochem. Biotechnol. 2010, 162, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Yang, S.-T. Fumaric acid recovery and purification from fermentation broth by activated carbon adsorption followed with desorption by acetone. Ind. Eng. Chem. Res. 2014, 53, 12802–12808. [Google Scholar] [CrossRef]


| Parameter | Non-Filtered | 5 kDa Filtrate |
|---|---|---|
| pH | 2.81 | 2.85 |
| Density (g/mL) | 1.07 | 1.04 |
| Lingnosulphonates (g/L) | 109.8 | 27.0 |
| Mg2+/Ca2+ (mM) | 144.9 | 132.1 |
| Carbohydrates (g/L) | ||
| Glucose | 1.82 | 1.29 |
| Xylose | 26.9 | 25.6 |
| Arabinose | 0.25 | 0.15 |
| Galactose | 2.61 | 2.00 |
| Mannose | 0.41 | 0.00 |
| Organic acids (g/L) | ||
| Acetic acid | 6.50 | 5.04 |
| Formic acid | 0.00 | 0.00 |
| Succinic acid | 0.00 | 0.00 |
| Malic acid | 0.03 | 0.00 |
| Fumaric acid | 0.00 | 0.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueira, D.; Cavalheiro, J.; Ferreira, B.S. Purification of Polymer-Grade Fumaric Acid from Fermented Spent Sulfite Liquor. Fermentation 2017, 3, 13. https://doi.org/10.3390/fermentation3020013
Figueira D, Cavalheiro J, Ferreira BS. Purification of Polymer-Grade Fumaric Acid from Fermented Spent Sulfite Liquor. Fermentation. 2017; 3(2):13. https://doi.org/10.3390/fermentation3020013
Chicago/Turabian StyleFigueira, Diogo, João Cavalheiro, and Bruno Sommer Ferreira. 2017. "Purification of Polymer-Grade Fumaric Acid from Fermented Spent Sulfite Liquor" Fermentation 3, no. 2: 13. https://doi.org/10.3390/fermentation3020013
APA StyleFigueira, D., Cavalheiro, J., & Ferreira, B. S. (2017). Purification of Polymer-Grade Fumaric Acid from Fermented Spent Sulfite Liquor. Fermentation, 3(2), 13. https://doi.org/10.3390/fermentation3020013






