Phenols Removal from Hemicelluloses Pre-Hydrolysate by Laccase to Improve Butanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism, Culture Maintenance and Inoculum Preparation
2.2. Fermentation Medium
2.3. Analytical Methods
2.4. Degradation with Laccase Enzymes
2.5. Preparation and Treatment of Hydrolysates
3. Results and Discussion
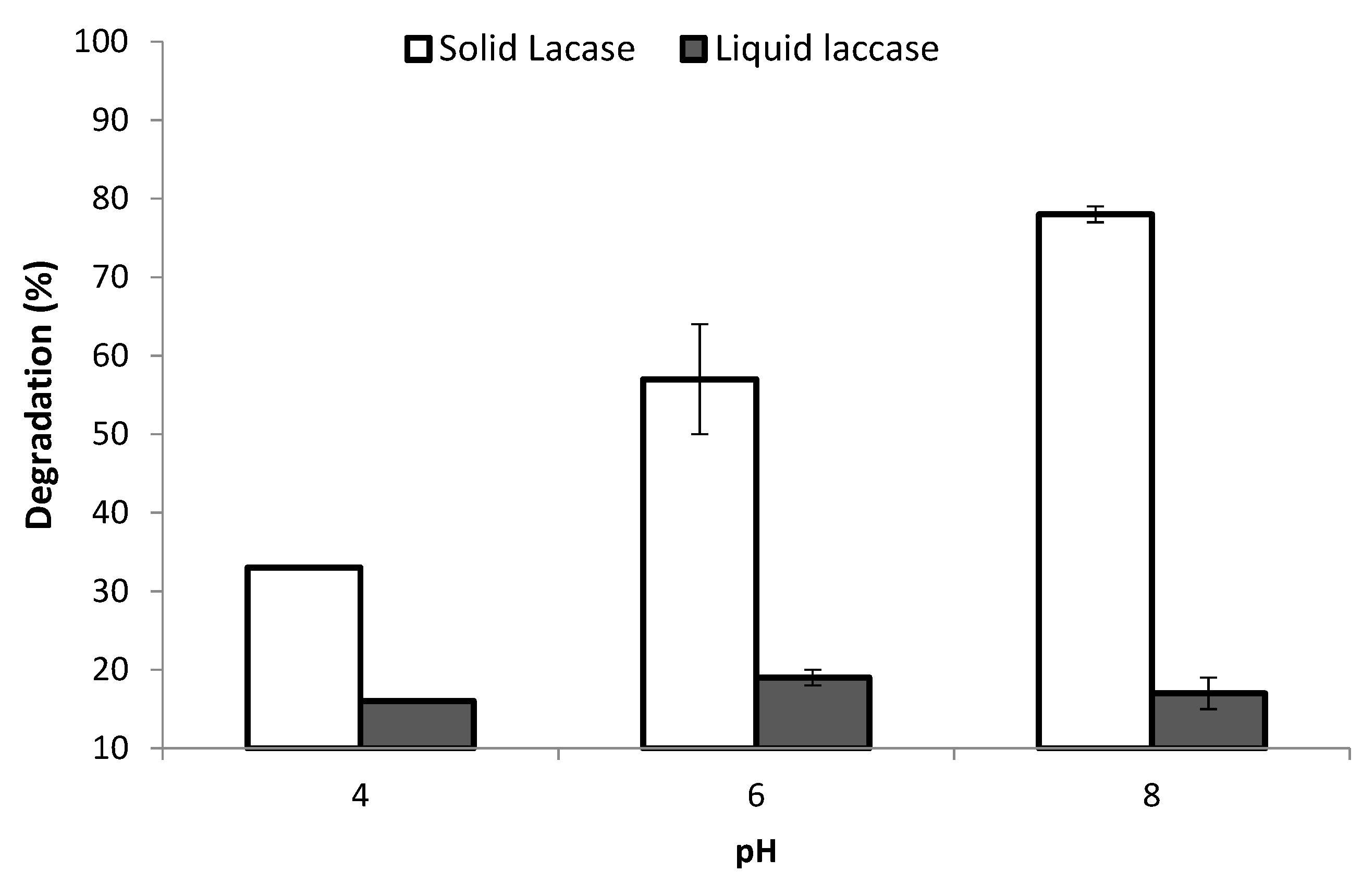
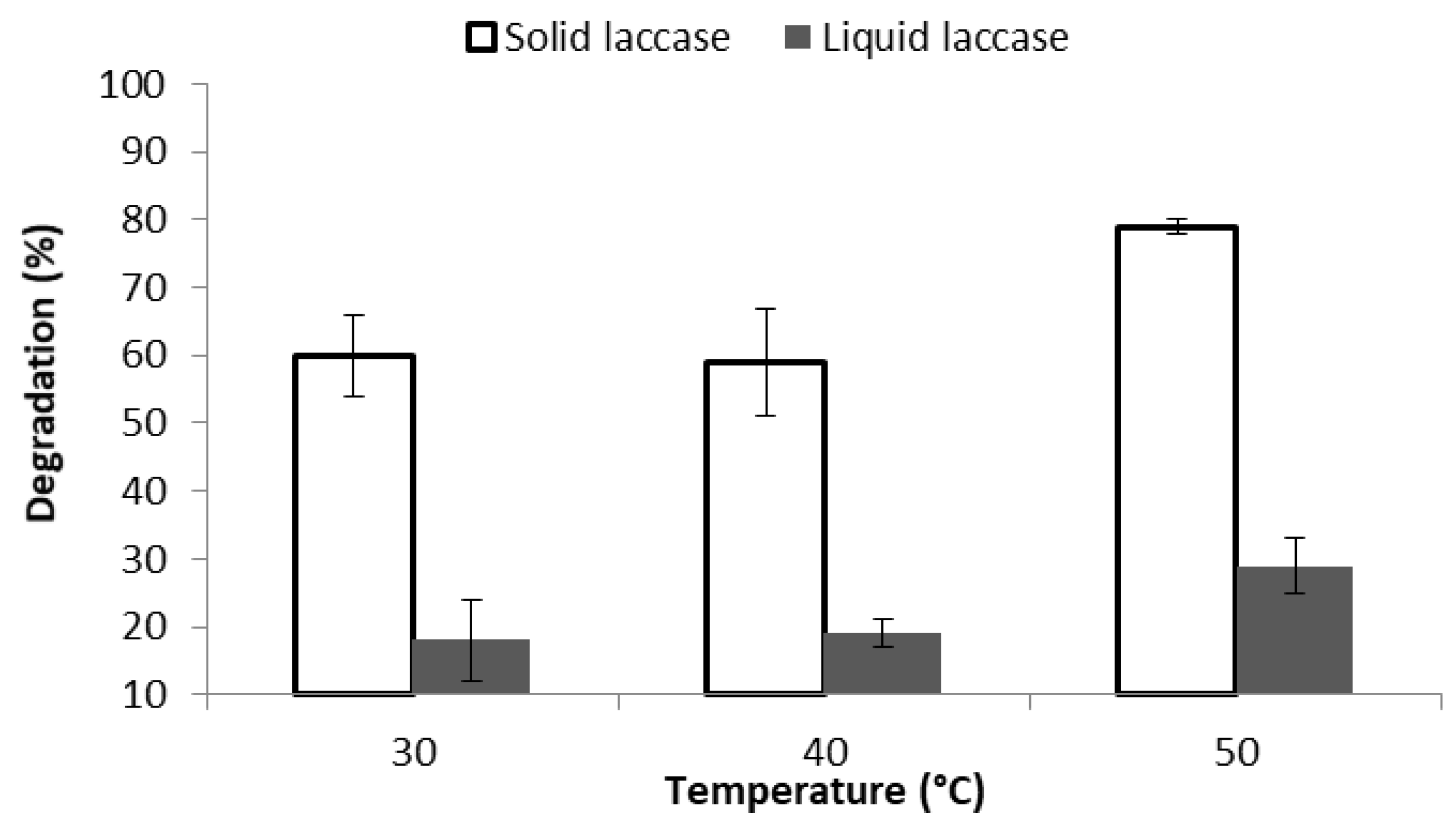
3.1. Optimization of the Degradation Conditions
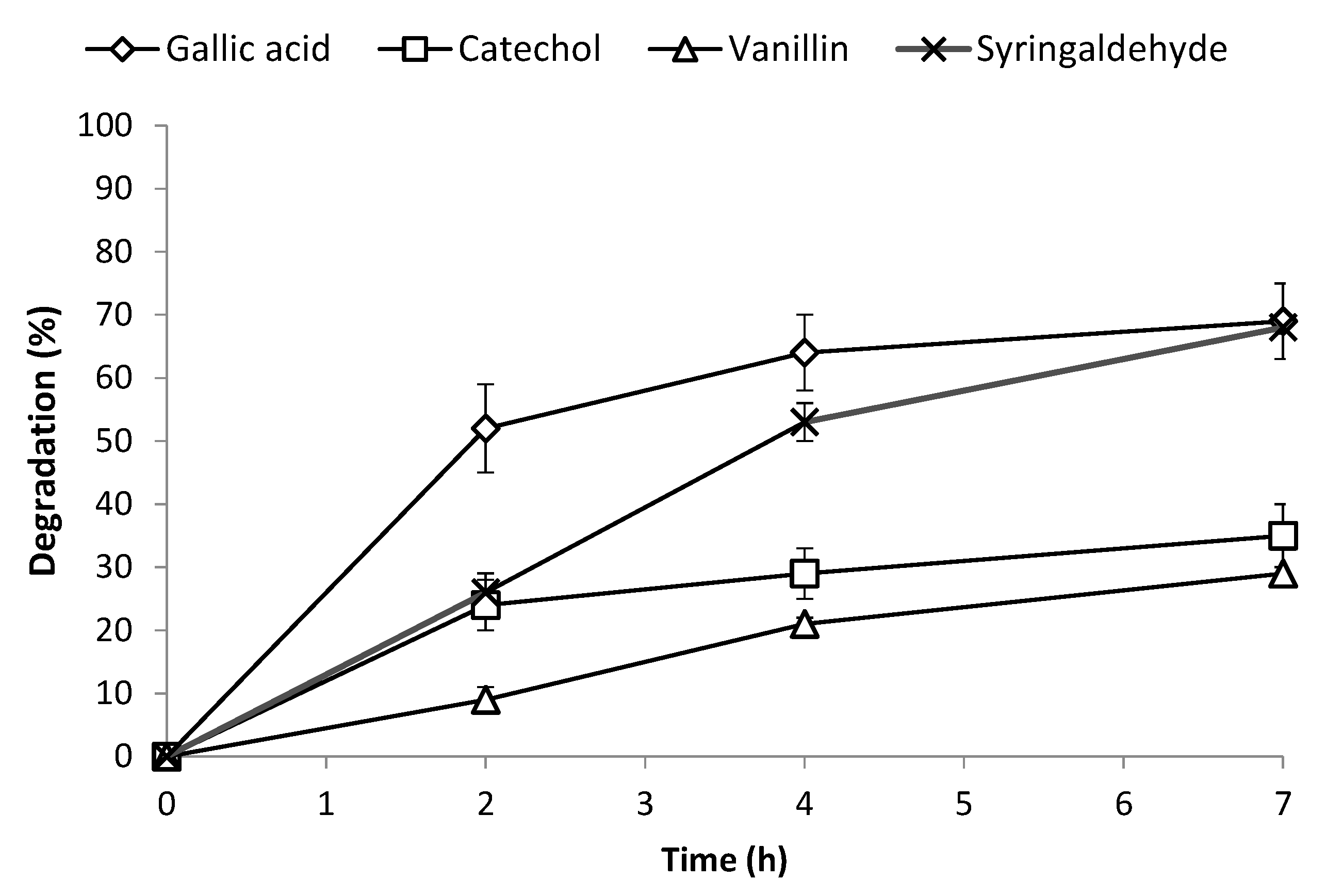
3.2. Influence of the Individual Phenolic Compounds
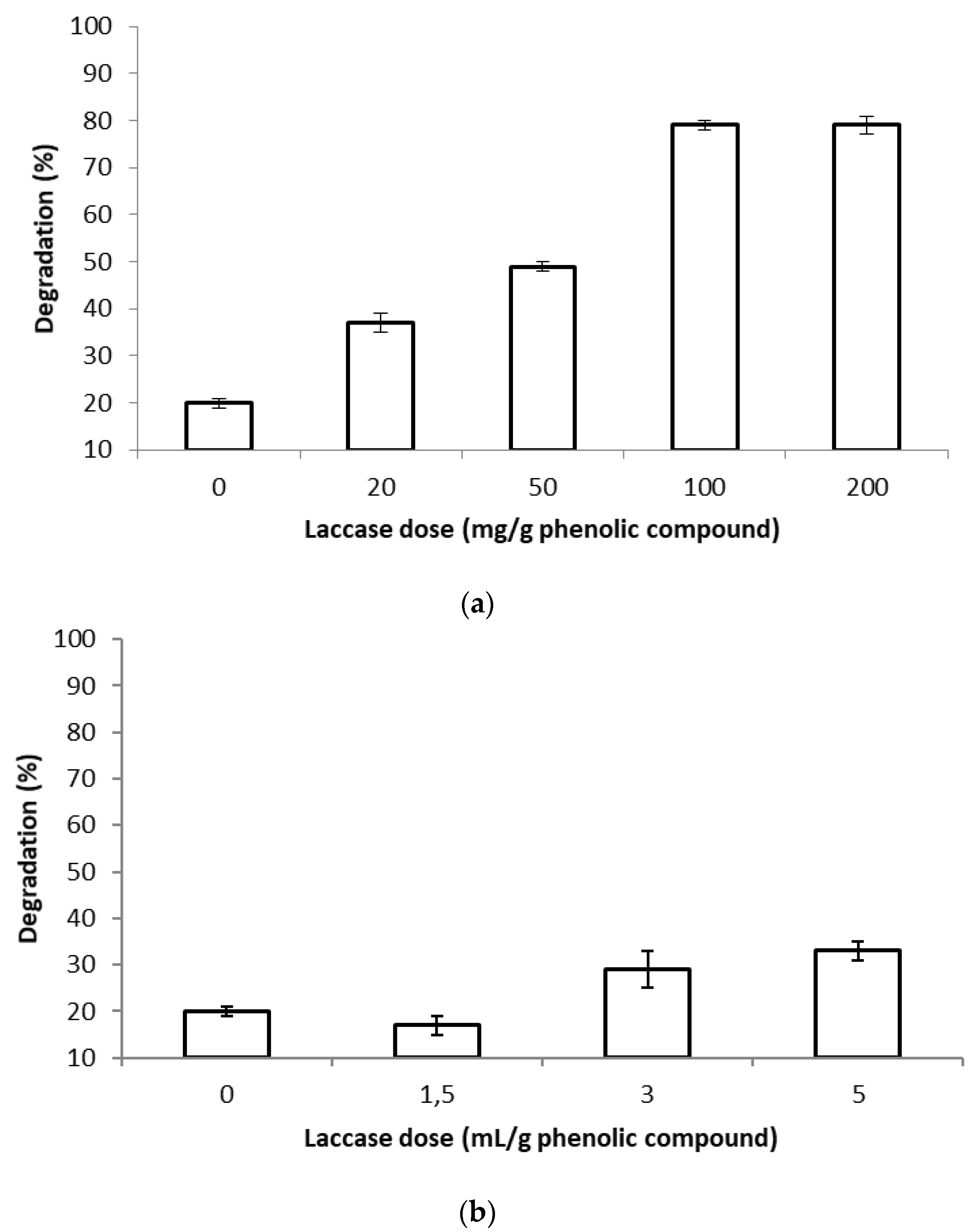
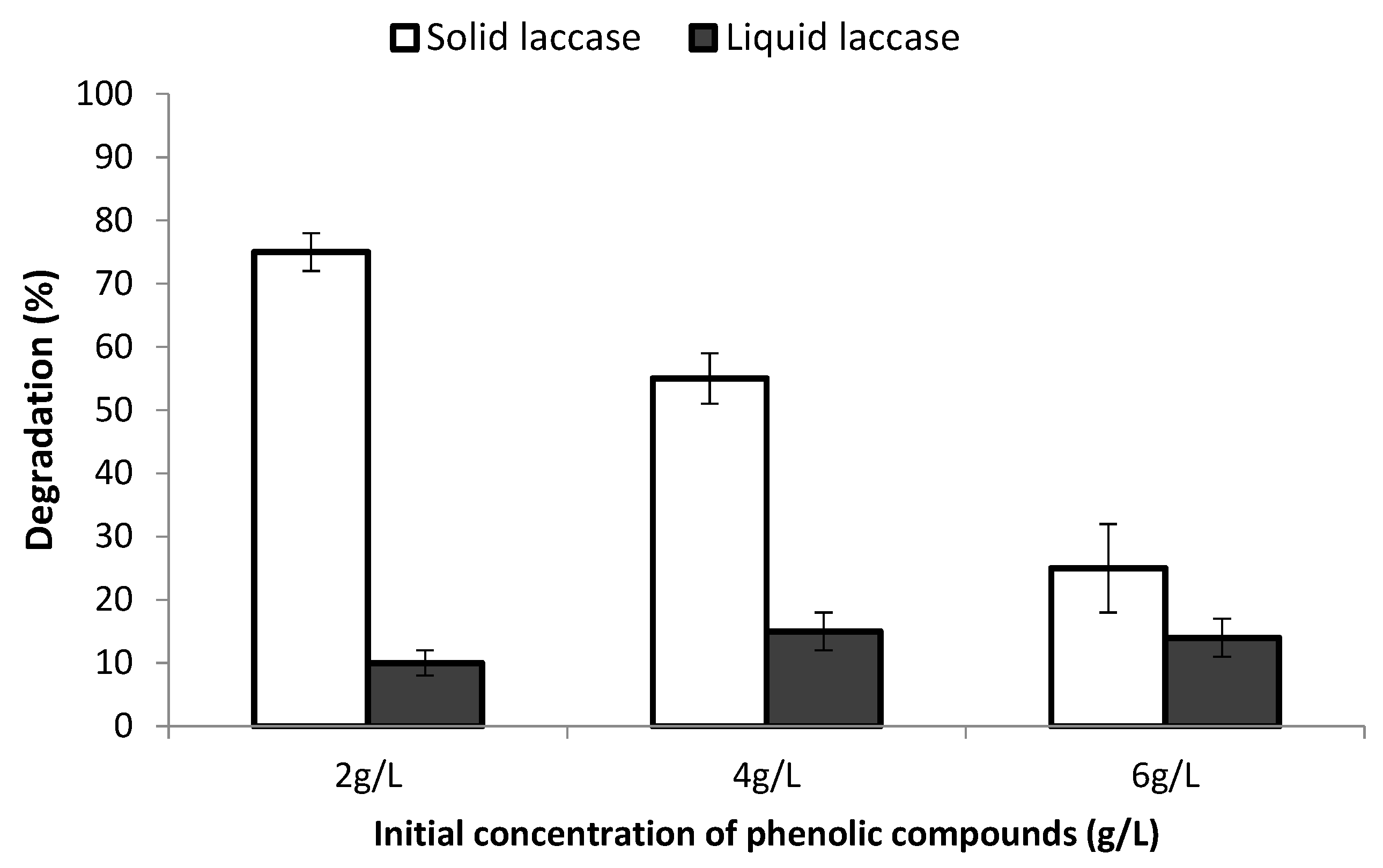
3.3. Influence of the Initial Phenolic Compounds Concentration
3.4. Hydrolysate Detoxification by Laccase
3.5. Impact of the Additional Degradation by Laccase on ABE Fermentation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, D.T.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M. n-Butanol. Chem. Mark. Report. 2006, 269, 42. [Google Scholar]
- Ajao, O.; LeHir, M.; Rahni, M.; Marinova, M.; Chadjaa, H.; Savadogo, O. Concentration and Detoxification of Kraft Prehydrolysate by Combining Nanofiltration with Flocculation. Ind. Eng. Chem. Res. 2015, 54, 1113–1122. [Google Scholar] [CrossRef]
- Ajao, O.; Rahni, M.; Marinova, M.; Chadjaa, H.; Savadogo, O. Retention and flux characteristics of nanofiltration membranes during hemicellulose prehydrolysate concentration. Chem. Eng. J. 2015, 260, 605–615. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Singh, O. V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering Toward White Biotechnology. Bioenergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Microbial inhibitors: formation and effects on acetone-butanol-ethanol fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 9151–9172. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolyzates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Mechmech, F.; Chadjaa, H.; Rahni, M.; Marinova, M.; Ben Akacha, N.; Gargouri, M. Improvement of butanol production from a hardwood hemicelluloses hydrolysate by combined sugar concentration and phenols removal. Bioresour. Technol. 2015, 192, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Lele, S.S. Laccase: properties and applications. Bioresources 2009, 4, 1694–1717. [Google Scholar]
- Wang, Z.X.; Cai, Y.J.; Liao, X.R.; Tao, G.J.; Li, Y.Y.; Zhang, F.; Zhang, D.B. Purification and characterization of two thermostable laccases with high cold adapted characteristics from Pycnoporus sp. SYBC-L1. Process Biochem. 2010, 45, 1720–1729. [Google Scholar] [CrossRef]
- Sherif, M.; Waung, D.; Korbeci, B.; Mavisakalyan, V.; Flick, R.; Brown, G.; Abou-Zaid, M.; Yakunin, A.F.; Master, E.R. Biochemical studies of the multicopper oxidase (small laccase) from Streptomyces coelicolor using bioactive phytochemicals and site-directed mutagenesis. Microb. Biotechnol. 2013, 6, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Bayramoğlu, G.; Arica, M.Y. Enzymatic removal of phenol and p-chlorophenol in enzyme reactor: Horseradish peroxidase immobilized on magnetic beads. J. Hazard. Mater. 2008, 156, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Taylor, K.E.; Bewtra, J.K.; Biswas, N. Inactivation of enzyme laccase and role of cosubstrate oxygen in enzymatic removal of phenol from water. Water Environ. Res. 2007, 79, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, C.; Petersen, B.R.; Bjerrum, M.J. Enzymatic removal of phenols from aqueous solutions by Coprinus cinereus peroxidase and hydrogen peroxide. J. Biotechnol. 1999, 73, 71–74. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Fernandez, J.L.; Ballesteros, M. Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour. Technol. 2012, 106, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Marinova, M.; Mateos-Espejel, E.; Jemaa, N.; Paris, J. Addressing the increased energy demand of a Kraft mill biorefinery: The hemicellulose extraction case. Chem. Eng. Res. Des. 2009, 87, 1269–1275. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allard-Massicotte, R.; Chadjaa, H.; Marinova, M. Phenols Removal from Hemicelluloses Pre-Hydrolysate by Laccase to Improve Butanol Production. Fermentation 2017, 3, 31. https://doi.org/10.3390/fermentation3030031
Allard-Massicotte R, Chadjaa H, Marinova M. Phenols Removal from Hemicelluloses Pre-Hydrolysate by Laccase to Improve Butanol Production. Fermentation. 2017; 3(3):31. https://doi.org/10.3390/fermentation3030031
Chicago/Turabian StyleAllard-Massicotte, Rosalie, Hassan Chadjaa, and Mariya Marinova. 2017. "Phenols Removal from Hemicelluloses Pre-Hydrolysate by Laccase to Improve Butanol Production" Fermentation 3, no. 3: 31. https://doi.org/10.3390/fermentation3030031
APA StyleAllard-Massicotte, R., Chadjaa, H., & Marinova, M. (2017). Phenols Removal from Hemicelluloses Pre-Hydrolysate by Laccase to Improve Butanol Production. Fermentation, 3(3), 31. https://doi.org/10.3390/fermentation3030031



