A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters
Abstract
1. Introduction
2. Solid State Fermentation for PHA Production
3. Kinetics of PHA Biosynthesis
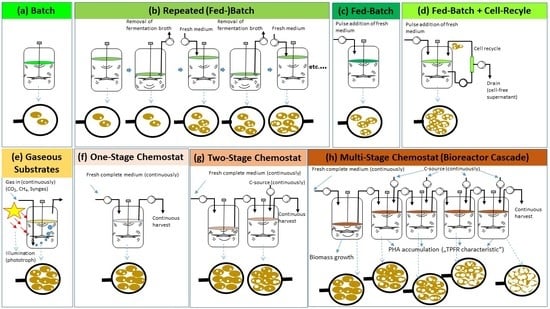
4. Discontinuous PHA Production Processes
4.1. Batch Systems
4.2. Fed-Batch Systems
4.2.1. General Aspects of Fed-Batch Processes for PHA Production
4.2.2. Fed-Batch Processes with Cell Recycling for Biomass Retention
4.2.3. Repeated Fed-Batch for PHA Production
4.3. “Continuous Fed-Batch” Systems
4.3.1. Use of Liquid Substrates
4.3.2. Use of Gaseous Substrates CH4, CO2 and Syngas
5. Continuous PHA Production Processes Operated as Chemostats
5.1. General
5.2. One-Stage Chemostats
5.2.1. One-Stage Chemostats Based on Pure Cultures
5.2.2. Dual Nutrient Limited Chemostat Cultivation to Utilize “Inefficient” Carbon Sources for PHA Biosynthesis
5.2.3. Non-Sterile Single-Stage Chemostat Processes
5.3. Two-Stage Chemostats
5.3.1. Two-Stage Chemostats under Strict Sterility Precautions
5.3.2. Non-Sterile Two-Stage Chemostat Cultivation for PHA Production
5.4. Multi-Stage Chemostats
6. Conclusions
Conflicts of Interest
References
- Pasteur, L. Mémoire sur la fermentation alcoolique. Ann. Chim. Phys. 1860, 58, 323–426. [Google Scholar]
- Choi, J.; Lee, S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 1999, 51, 13–21. [Google Scholar] [CrossRef]
- Kavitha, G.; Rengasamy, R.; Inbakandan, D. Polyhydroxybutyrate production from marine source and its application. Int. J. Biol. Macromol. 2017, 111, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.Y.U.; Castilho, N.A.S.; da Silva, M.A.C.; Miotto, M.C.; de Souza Lima, A.O. Prospecting for marine bacteria for polyhydroxyalkanoate production on low-cost substrates. Bioengineering 2017, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Dubey, S.; Singh, P.; Shrivastava, A.; Mishra, S. Biodegradable polymeric substances produced by a marine bacterium from a surplus stream of the biodiesel industry. Bioengineering 2016, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Kucera, D.; Pernicová, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I.; et al. Characterization of the promising poly(3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Bioresour. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, D.; Pfeiffer, D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ. Microbiol. 2014, 16, 2357–2373. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Koller, M.; Kucera, D.; Pernicova, I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol. Adv. 2018. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Marova, I.; Svoboda, Z.; Mikulikova, R. Use of controlled exogenous stress for improvement of poly(3-hydroxybutyrate) production in Cupriavidus necator. Folia Microbiol. 2010, 55, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Marova, I.; Stankova, M.; Mravcova, L.; Svoboda, Z. Effect of ethanol and hydrogen peroxide on poly (3-hydroxybutyrate) biosynthetic pathway in Cupriavidus necator H16. World J. Microbiol. Biotechnol. 2010, 26, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Samek, O.; Marova, I. Evaluation of 3-hydroxybutyrate as an enzyme-protective agent against heating and oxidative damage and its potential role in stress response of poly(3-hydroxybutyrate) accumulating cells. Appl. Microbiol. Biotechnol. 2016, 100, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Krzyzanek, V.; Nebesarova, J.; Samek, O.; Kucera, D.; Benesova, P.; Hrubanova, K.; Milerova, M.; et al. The presence of PHB granules in cytoplasm protects non-halophilic bacterial cells against the harmful impact of hypertonic environments. New Biotechnol. 2017, 39, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Soto, G.; Setten, L.; Lisi, C.; Maurelis, C.; Mozzicafreddo, M.; Cuccioloni, M.; Angeletti, M.; Ayub, N.D. Hydroxybutyrate prevents protein aggregation in the halotolerant bacterium Pseudomonas sp. CT13 under abiotic stress. Extremophiles 2012, 16, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Contreras, A.; Koller, M.; Braunegg, G.; Marqués-Calvo, M.S. Poly[(R)-3-hydroxybutyrate] production under different salinity conditions by a novel Bacillus megaterium strain. New Biotechnol. 2016, 33, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Krzyzanek, V.; Mravec, F.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Marova, I. Accumulation of poly(3-hydroxybutyrate) helps bacterial cells to survive freezing. PLoS ONE 2016, 11, e0157778. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, E.; Sedlacek, P.; Mravec, F.; Mullerova, L.; Samek, O.; Koller, M.; Hesko, O.; Kucera, D.; Marova, I.; Obruca, S. Light scattering on PHA granules protects bacterial cells against the harmful effects of UV radiation. Appl. Microbiol. Biotechnol. 2018, 102, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Poly(hydroxyalkanoates) for food packaging: Application and attempts towards implementation. Appl. Food Biotechnol. 2014, 1, 3–15. [Google Scholar] [CrossRef]
- De Souza, L.; Shivakumar, S. Polyhydroxyalkanoates (PHAs)-biodegradable polymers for ‘green’ food packaging materials. In Recent Advances in Biotechnology; Delhi Printer & Lamination: New Delhi, India, 2017; p. 149. [Google Scholar]
- Koller, M. Biodegradable and biocompatible polyhydroxyalkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.; Kowalczuk, M. Biomass-derived polyhydroxyalkanoates: Biomedical applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value, 1st ed.; Popa, V.I., Volf, I., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2018; pp. 271–313. ISBN 978-0-444-63774-1. [Google Scholar]
- Brigham, C.J.; Sinskey, A.J. Applications of polyhydroxyalkanoates in the medical industry. Int. J. Biotechnol. Well. Ind. 2012, 1, 52–60. [Google Scholar] [CrossRef]
- Junyu, Z.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef]
- Pierro, L.; Matturro, B.; Rossetti, S.; Sagliaschi, M.; Sucato, S.; Alesi, E.; Bartsch, E.; Arjmand, F.; Papini, M.P. Polyhydroxyalkanoate as a slow-release carbon source for in situ bioremediation of contaminated aquifers: From laboratory investigation to pilot-scale testing in the field. New Biotechnol. 2017, 37, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.; Shishatskaya, E. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int. J. Biol. Macromol. 2018, 111, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Maršálek, L.; Miranda de Sousa Dias, M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, G.; Rocher, M.; Braunegg, G. Effects of low dissolved-oxygen concentrations on poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Alcaligenes eutrophus. Appl. Environ. Microbiol. 1997, 63, 827–833. [Google Scholar] [PubMed]
- Koller, M.; Bona, R.; Chiellini, E.; Fernandes, E.G.; Horvat, P.; Kutschera, C.; Hesse, P.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour. Technol. 2008, 99, 4854–4863. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Fasl, H.; Stelzer, F.; Braunegg, G. Study on the effect of levulinic acid on whey-based biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Hydrogenophaga pseudoflava. Appl. Food Biotechnol. 2017, 4, 65–78. [Google Scholar] [CrossRef]
- Koller, M.; Miranda de Sousa Dias, M.; Rodríguez-Contreras, A.; Kunaver, M.; Žagar, E.; Kržan, A.; Braunegg, G. Liquefied wood as inexpensive precursor-feedstock for bio-mediated incorporation of (R)-3-hydroxyvalerate into polyhydroxyalkanoates. Materials 2015, 8, 6543–6557. [Google Scholar] [CrossRef] [PubMed]
- Ashby, R.D.; Solaiman, D.K.; Nuñez, A.; Strahan, G.D.; Johnston, D.B. Burkholderia sacchari DSM 17165: A source of compositionally-tunable block-copolymeric short-chain poly(hydroxyalkanoates) from xylose and levulinic acid. Bioresour. Technol. 2017, 253, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Salerno, A.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Illieva, V.; Chiellini, E.; Braunegg, E. Novel precursors for production of 3-hydroxyvalerate-containing poly[(R)-hydroxyalkanoate]s. Biocatal. Biotransform. 2014, 32, 161–167. [Google Scholar] [CrossRef]
- Johnston, B.; Jiang, G.; Hill, D.; Adamus, G.; Kwiecień, I.; Zięba, M.; Sikorska, W.; Green, M.; Kowalczuk, M.; Radecka, I. The molecular level characterization of biodegradable polymers originated from polyethylene using non-oxygenated polyethylene wax as a carbon source for polyhydroxyalkanoate production. Bioengineering 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Miranda de Sousa Dias, M.; Koller, M.; Puppi, D.; Morelli, A.; Chiellini, F.; Braunegg, G. Fed-Batch synthesis of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from sucrose and 4-hydroxybutyrate precursors by Burkholderia sacchari strain DSM 17165. Bioengineering 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.S.; de Almeida, M.C.M.; da Fonseca, M.M.R.; Cesário, M.T. Feeding strategies for tuning poly(3-hydroxybutyrate-co-4-hydroxybutyrate) monomeric composition and productivity using Burkholderia sacchari. Int. J. Biol. Macromol. 2017, 105, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Potential of various archae-and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol. Biosci. 2007, 7, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, B.B.; Bragança, J.M. Utilization of sugarcane bagasse by Halogeometricum borinquense strain E3 for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Povolo, S.; Romanelli, M.G.; Basaglia, M.; Ilieva, V.I.; Corti, A.; Morelli, A.; Chiellini, E.; Casella, S. Polyhydroxyalkanoate biosynthesis by Hydrogenophaga pseudoflava DSM 1034 from structurally unrelated carbon sources. New Biotechnol. 2013, 30, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Hokamura, A.; Yunoue, Y.; Goto, S.; Matsusaki, H. Biosynthesis of polyhydroxyalkanoate from steamed soybean wastewater by a recombinant strain of Pseudomonas sp. 61–63. Bioengineering 2017, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Hajnal, I. The ‘PHAome’. Trends Biotechnol. 2015, 33, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Ishii, N.; Sato, S.; Tsuge, T. Thermal properties and crystallization behaviors of medium-chain-length poly(3-hydroxyalkanoate)s. Polymer 2012, 53, 3026–3034. [Google Scholar] [CrossRef]
- Li, Z.J.; Qiao, K.; Che, X.M.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for the synthesis of the quadripolymer poly(glycolate-co-lactate-co-3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose. Metab. Eng. 2017, 44, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Yamada, M.; Matsumoto, K.I.; Tajima, K.; Satoh, Y.; Munekata, M.; Ohno, K.; Kohda, K.; Shimamura, T.; Kambe, H.; et al. A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 17323–17327. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Rehm, B.H. Polyester synthases: Natural catalysts for plastics. Biochem. J. 2003, 376, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Pandey, A.; Binod, P. Solid-state fermentation for the production of poly(hydroxyalkanoates). Chem. Biochem. Eng. Q. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Freire, D.M.G.; Castilho, L.R. Production of poly(3-hydroxybutyrate) by solid-state fermentation with Ralstonia eutropha. Biotechnol. Lett. 2004, 26, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.C.; Dias, M.L.; Castilho, L.R.; Freire, D.M. Characterization of poly(3-hydroxybutyrate) produced by Cupriavidus necator in solid-state fermentation. Bioresour. Technol. 2007, 98, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.R.S., Jr. Estudo das Condições de Cultivo para a Produção de PHB por Cupriavidus necator em Fermentação no Estado Sólido. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2005. (In Spanish). [Google Scholar]
- Ramadas, N.V.; Singh, S.K.; Soccol, C.R.; Pandey, A. Polyhydroxybutyrate production using agro-industrial residue as substrate by Bacillus sphaericus NCIM 5149. Braz. Arch. Biol. Technol. 2009, 52, 17–23. [Google Scholar] [CrossRef]
- Braunegg, G.; Lefebvre, G.; Renner, G.; Zeiser, A.; Haage, G.; Loidl-Lanthaler, K. Kinetics as a tool for polyhydroxyalkanoate production optimization. Can. J. Microbiol. 1995, 41, 239–248. [Google Scholar] [CrossRef]
- Braunegg, G.; Korneti, L. Pseudomonas 2 F: Kinetics of growth and accumulation of poly-d(-)-3-hydroxybutyric acid (poly-HB). Biotechnol. Lett. 1984, 6, 825–829. [Google Scholar] [CrossRef]
- Kaur, G.; Roy, I. Strategies for large-scale production of polyhydroxyalkanoates. Chem. Biochem. Eng. Q. 2015, 29, 157–172. [Google Scholar] [CrossRef]
- Rodriguez-Perez, S.; Serrano, A.; Pantión, A.A.; Alonso-Fariñas, B. Challenges of scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Koller, M.; Braunegg, M.; Horvat, P. Mathematical modelling as a tool for optimized PHA production. Chem. Biochem. Eng. Q. 2015, 29, 183–220. [Google Scholar] [CrossRef]
- Koller, M.; Vadljia, D.; Braunegg, G.; Atlić, A.; Horvat, P. Formal- and high-structured kinetic process modelling and footprint area analysis of binary imaged cells: Tools to understand and optimize multistage-continuous PHA biosynthesis. EuroBiotech J. 2017, 1, 203–211. [Google Scholar] [CrossRef]
- Cui, B.; Huang, S.; Xu, F.; Zhang, R.; Zhang, Y. Improved productivity of poly(3-hydroxybutyrate) (PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture. Appl. Microbiol. Biotechnol. 2015, 99, 6009–6019. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.O.; Kanekar, P.P.; Nilegaonkar, S.S.; Sarnaik, S.S.; Jog, J.P. Production and characterization of a biodegradable poly(hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) copolymer by moderately haloalkalitolerant Halomonas campisalis MCM B-1027 isolated from Lonar Lake, India. Bioresour. Technol. 2010, 101, 9765–9771. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.S.; Wong, Y.M.; Tsuge, T.; Sudesh, K. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymers using jatropha oil as the main carbon source. Proc. Biochem. 2011, 46, 1572–1578. [Google Scholar] [CrossRef]
- Sindhu, R.; Silviya, N.; Binod, P.; Pandey, A. Pentose-rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly-3-hydroxybutyrate. Biochem. Eng. J. 2013, 78, 67–72. [Google Scholar] [CrossRef]
- Gahlawat, G.; Srivastava, A.K. Enhancing the production of polyhydroxyalkanoate biopolymer by Azohydromonas australica using a simple empty and fill bioreactor cultivation strategy. Chem. Biochem. Eng. Q. 2018, 31, 479–485. [Google Scholar] [CrossRef]
- Marang, L.; van Loosdrecht, M.C.; Kleerebezem, R. Enrichment of PHA-producing bacteria under continuous substrate supply. New Biotechnol. 2018, 41, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. M Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, L.; Wen, Q.; Guo, Z. Efficient polyhydroxyalkanoate (PHA) accumulation by a new continuous feeding mode in three-stage mixed microbial culture (MMC) PHA production process. J. Biotechnol. 2015, 209, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.M.; Serafim, L.S.; Lemos, P.C.; Ramos, A.M.; Aguiar, F.R.; van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioproc. Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Karabegovic, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Polyhydroxyalkanoate (PHA) storage within a mixed-culture biomass with simultaneous growth as a function of accumulation substrate nitrogen and phosphorus levels. Water Res. 2015, 77, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, Z.; Wen, Q.; Zhao, L.; Lee, D.J.; Yang, L.; Wang, Y. Insights into Feast-Famine polyhydroxyalkanoate (PHA)-producer selection: Microbial community succession, relationships with system function and underlying driving forces. Water Res. 2018, 131, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Silva, C.E.; Carvalho, G.; Reis, M.A.M. Strategies for efficiently selecting PHA producing mixed microbial cultures using complex feedstocks: Feast and famine regime and uncoupled carbon and nitrogen availabilities. New Biotechnol. 2017, 37, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.; Reis, M.A.M. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Eiroa, M.; Torres, C.; Nunes, B.R.; Reis, M.A.M. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007, 130, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, D.; Carucci, G.; Petrangeli Papinia, M.; Riccardi, C.; Majone, M.; Carrasco, F. Olive oil mill effluents as a feedstock for production of biodegradable polymers. Water Res. 2005, 39, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Carucci, A.; Dionisi, D.; Majone, M.; Rolle, E.; Smurra, P. Aerobic storage by activated sludge on real wastewater. Water Res. 2001, 35, 3833–3844. [Google Scholar] [CrossRef]
- Carvalho, G.; Pedras, I.; Karst, S.M.; Oliveira, C.S.; Duque, A.F.; Nielsen, P.H.; Reis, M.A.M. Functional redundancy ensures performance robustness in 3-stage PHA-producing mixed cultures under variable feed operation. New Biotechnol. 2018, 40, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Rhu, D.H.; Lee, W.H.; Kim, J.Y.; Choi, E. Polyhydroxyalkanoate (PHA) production from waste. Water Sci. Technol. 2003, 48, 221–228. [Google Scholar] [PubMed]
- Burniol-Figols, A.; Varrone, C.; Le, S.B.; Daugaard, A.E.; Skiadas, I.V.; Gavala, H.N. Combined polyhydroxyalkanoates (PHA) and 1, 3-propanediol production from crude glycerol: Selective conversion of volatile fatty acids into PHA by mixed microbial consortia. Water Res. 2018, 136, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wusiman, A.; Liu, X.; Wan, C.; Lee, D.J.; Tay, J. Polyhydroxyalkanoates (PHA) production from phenol in an acclimated consortium: Batch study and impacts of operational conditions. J. Biotechnol. 2018, 267, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.S.; Park, S.J.; Lee, S.Y. Production of poly(3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl. Environ. Microbiol. 2000, 66, 3624–3627. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.S.; Park, S.J.; Lee, S.Y. Production of poly(3-hydroxybutyrate) from whey by cell recycle fed-batch culture of recombinant Escherichia coli. Biotechnol. Lett. 2001, 23, 235–240. [Google Scholar] [CrossRef]
- Da Cruz Pradella, J.G.; Ienczak, J.L.; Delgado, C.R.; Taciro, M.K. Carbon source pulsed feeding to attain high yield and high productivity in poly(3-hydroxybutyrate) (PHB) production from soybean oil using Cupriavidus necator. Biotechnol. Lett. 2012, 34, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co-and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [PubMed]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Schiller, M.; Kwiecień, M.; Adamus, G.; Kowalczuk, M.; Stromeier, K.; Schober, S.; et al. Biodegradable latexes from animal-derived waste: Biosynthesis and characterization of mcl-PHA accumulated by Ps. citronellolis. React. Funct. Polym. 2013, 73, 1391–1398. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Malli, K.; Strohmeier, K.; Schober, S.; Mittelbach, M.; Koller, M. Novel description of mcl-PHA biosynthesis by Pseudomonas chlororaphis from animal-derived waste. J. Biotechnol. 2013, 165, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Follonier, S.; Riesen, R.; Zinn, M. Pilot-scale production of functionalized mcl-PHA from grape pomace supplemented with fatty acids. Chem. Biochem. Eng. Q. 2015, 29, 113–121. [Google Scholar] [CrossRef]
- Ienczak, J.L.; Quines, L.K.; De Melo, A.A.; Brandellero, M.; Mendes, C.R.; Schmidell, W.; Aragão, G.M.F. High cell density strategy for poly(3-hydroxybutyrate) production by Cupriavidus necator. Braz. J. Chem. Eng. 2011, 28, 585–596. [Google Scholar] [CrossRef]
- Ienczak, J.L.; Schmidell, W.; de Aragão, G.M.F. High-cell-density culture strategies for polyhydroxyalkanoate production: A review. J. Ind. Microbiol. Biotechnol. 2013, 40, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Penloglou, G.; Vasileiadou, A.; Chatzidoukas, C.; Kiparissides, C. Model-based intensification of a fed-batch microbial process for the maximization of polyhydroxybutyrate (PHB) production rate. Bioproc. Biosyst. Eng. 2017, 40, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Benesova, P.; Marsalek, L.; Marova, I. Use of lignocellulosic materials for PHA production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Kucera, D.; Benesova, P.; Ladicky, P.; Pekar, M.; Sedlacek, P.; Obruca, S. Production of polyhydroxyalkanoates using hydrolyzates of spruce sawdust: Comparison of hydrolyzates detoxification by application of overliming, active carbon, and lignite. Bioengineering 2017, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Ienczak, J.L.; Schmidt, M.; Quines, L.K.; Zanfonato, K.; da Cruz Pradella, J.G.; Schmidell, W.; de Aragao, G.M.F. Poly(3-hydroxybutyrate) production in repeated fed-batch with cell recycle using a medium with low carbon source concentration. Appl. Biochem. Biotechnol. 2016, 178, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; El-Najjar, T.; Virgolini, N.; Smerilli, M.; Neureiter, M. High cell-density production of poly(3-hydroxybutyrate) in a membrane bioreactor. New Biotechnol. 2017, 37, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lorantfy, B.; Ruschitzka, P.; Herwig, C. Investigation of physiological limits and conditions for robust bioprocessing of an extreme halophilic archaeon using external cell retention system. Biochem. Eng. J. 2014, 90, 140–148. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Steinbüchel, A. High-cell-density cyclic fed-batch fermentation of a poly(3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10. Appl. Environ. Microbiol. 2010, 76, 7890–7895. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Yu, J. Green technology for conversion of food scraps to biodegradable thermoplastic polyhydroxyalkanoates. Environ. Sci. Technol. 2002, 36, 5511–5516. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Darani, K.; Mokhtari, Z.B.; Amai, T.; Tanaka, K. Microbial production of poly(hydroxybutyrate) from C1 carbon sources. Appl. Microbiol. Biotechnol. 2013, 97, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Rostkowski, K.H.; Criddle, C.S.; Lepech, M.D. Cradle-to-gate life cycle assessment for a cradle-to-cradle cycle: Biogas-to-bioplastic (and back). Environ. Sci. Technol. 2012, 46, 9822–9829. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Galega, W.M.; van Nostrand, J.D.; Yuan, T.; Zhou, J.; Criddle, C.S. Long-term cultivation of a stable Methylocystis-dominated methanotrophic enrichment enabling tailored production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioresour. Technol. 2015, 198, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Cal, A.J.; Sikkema, W.D.; Ponce, M.I.; Franqui-Villanueva, D.; Riiff, T.J.; Orts, W.J.; Pieja, A.J.; Lee, C.C. Methanotrophic production of polyhydroxybutyrate-co-hydroxyvalerate with high hydroxyvalerate content. Int. J. Biol. Macromol. 2016, 87, 302–307. [Google Scholar] [CrossRef] [PubMed]
- López, J.C.; Arnáiz, E.; Merchán, L.; Lebrero, R.; Muñoz, R. Biogas-based polyhydroxyalkanoates production by Methylocystis hirsuta: A step further in anaerobic digestion biorefineries. Chem. Eng. J. 2018, 333, 529–536. [Google Scholar] [CrossRef]
- García-Pérez, T.; López, J.C.; Passos, F.; Lebrero, R.; Revah, S.; Muñoz, R. Simultaneous methane abatement and PHB production by Methylocystis hirsuta in a novel gas-recycling bubble column bioreactor. Chem. Eng. J. 2018, 334, 691–697. [Google Scholar] [CrossRef]
- Drosg, B.; Fritz, I.; Gattermayr, F.; Silvestrini, L. Photo-autotrophic production of poly (hydroxyalkanoates) in cyanobacteria. Chem. Biochem. Eng. Q. 2015, 29, 145–156. [Google Scholar] [CrossRef]
- Koller, M.; Maršalek, L. Cyanobacterial polyhydroxyalkanoate production: Status quo and Quo vadis? Curr. Biotechnol. 2015, 4, 464–480. [Google Scholar] [CrossRef]
- Ghysels, S.; Mozumder, M.S.I.; De Wever, H.; Volcke, E.I.; Garcia-Gonzalez, L. Targeted poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic production from carbon dioxide. Bioresour. Technol. 2018, 249, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M. Screening and Selection of a Cyanobacteria for Production of poly-β-hydroxybutyrate in a Closed Photobioreactor. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2009. [Google Scholar]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Kovalcik, A.; Meixner, K.; Mihalic, M.; Zeilinger, W.; Fritz, I.; Fuchs, W.; Kucharczyk, P.; Stelzer, F.; Drosg, B. Characterization of polyhydroxyalkanoates produced by Synechocystis salina from digestate supernatant. Int. J. Biol. Macromol. 2017, 102, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Meixner, K.; Kovalcik, A.; Sykacek, E.; Gruber-Brunhumer, M.; Zeilinger, W.; Markl, K.; Haas, C.; Fritz, I.; Mundigler, N.; Stelzer, F.; et al. Cyanobacteria biorefinery—Production of poly (3-hydroxybutyrate) with Synechocystis salina and utilisation of residual biomass. J. Biotechnol. 2018, 265, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.K.; Costa, J.A.V.; de Morais, M.G. Polyhydroxybutyrate (PHB) synthesis by Spirulina sp. LEB 18 using biopolymer extraction waste. Appl. Biochem. Biotechnol. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Recycling of waste streams of the biotechnological poly(hydroxyalkanoate) production by Haloferax mediterranei on whey. Int. J. Polym. Sci. 2015, 2015, 370164. [Google Scholar] [CrossRef]
- Chaudhari, S.T.; Dalai, A.K.; Bakhshi, N.N. Production of hydrogen and/or syngas (H2 + CO) via steam gasification of biomass-derived chars. Energy Fuel. 2003, 17, 1062–1067. [Google Scholar] [CrossRef]
- Revelles, O.; Calvillo, I.; Prieto, A.; Prieto, M.A. Syngas Fermentation for Polyhydroxyalkanoate Production in Rhodospirillum rubrum. In Hydrocarbon and Lipid Microbiology Protocols, 1st ed.; McGenity, T., Timmis, K., Nogales, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–119. ISBN 978-3-662-491317. [Google Scholar]
- Do, Y.S.; Smeenk, J.; Broer, K.M.; Kisting, C.J.; Brown, R.; Heindel, T.J.; Bobik, T.A.; DiSpririto, A.A. Growth of Rhodospirillum rubrum on synthesis gas: Conversion of CO to H2 and poly-β-hydroxyalkanoate. Biotechnol. Bioeng. 2007, 97, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; West, T.P.; Gibbons, W.R. Rhodospirillum rubrum: Utilization of condensed corn solubles for poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) production. J. Appl. Microbiol. 2008, 104, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Revelles, O.; Beneroso, D.; Menendez, J.A.; Arenillas, A.; García, J.L.; Prieto, M.A. Syngas obtained by microwave pyrolysis of household wastes as feedstock for polyhydroxyalkanoate production in Rhodospirillum rubrum. Microb. Biotechnol. 2017, 10, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Chipman, D.C.; Bents, S.C.; Brown, R.C. A techno-economic analysis of polyhydroxyalkanoate and hydrogen production from syngas fermentation of gasified biomass. Appl. Biochem. Biotechnol. 2014, 160, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Karmann, S.; Follonier, S.; Egger, D.; Hebel, D.; Panke, S.; Zinn, M. Tailor-made PAT platform for safe syngas fermentations in batch, fed-batch and chemostat mode with Rhodospirillum rubrum. Microb. Biotechnol. 2017, 10, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Klask, C.; Raberg, M.; Heinrich, D.; Steinbüchel, A. Heterologous expression of various PHA synthase genes in Rhodospirillum rubrum. Chem. Biochem. Eng. Q. 2015, 29, 75–85. [Google Scholar] [CrossRef]
- Heinrich, D.; Raberg, M.; Fricke, P.; Kenny, S.T.; Morales-Gamez, L.; Babu, R.P.; O’Connor, K.E.; Steinbüchel, A. Synthesis gas (Syngas)-derived medium-chain-length polyhydroxyalkanoate synthesis in engineered Rhodospirillum rubrum. Appl. Environ. Microbiol. 2016, 82, 6132–6140. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Muhr, A. Continuous production mode as a viable process-engineering tool for efficient poly(hydroxyalkanoate) (PHA) bio-production. Chem. Biochem. Eng. Q. 2014, 28, 65–77. [Google Scholar]
- Koller, M.; Braunegg, G. Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2015, 2, 94–121. [Google Scholar] [CrossRef] [PubMed]
- Senior, P.J.; Beech, G.A.; Ritchie, G.A.F.; Dawes, E.A. The role of oxygen limitation in the formation of poly-β-hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem. J. 1972, 128, 1193. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, B.A.; Lomaliza, K.; Chavarie, C.; Dube, B.; Bataille, P.; Ramsay, J.A. Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acids. Appl. Environ. Microbiol. 1990, 56, 2093–2098. [Google Scholar] [PubMed]
- Zinn, M.; Witholt, B.; Egli, T. Dual nutrient limited growth: Models, experimental observations, and applications. J. Biotechnol. 2004, 113, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Hazenberg, W.; Prieto, M.; Witholt, B. Two-stage continuous process development for the production of medium-chain-length poly(3-hydroxyalkanoates). Biotechnol. Bioeng. 2001, 72, 19–24. [Google Scholar] [CrossRef]
- Durner, R.; Witholt, B.; Egli, T. Accumulation of poly[(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth with octanoate in continuous culture at different dilution rates. Appl. Environ. Mmicrobiol. 2000, 66, 3408–3414. [Google Scholar] [CrossRef]
- Durner, R.; Zinn, M.; Witholt, B.; Egli, T. Accumulation of poly[(R)-3-hydroxyalkanoates] in Pseudomonas oleovorans during growth in batch and chemostat culture with different carbon sources. Biotechnol. Bioeng. 2001, 72, 278–288. [Google Scholar] [CrossRef]
- Zinn, M.; Weilenmann, H.U.; Hany, R.; Schmid, M.; Egli, T.H. Tailored synthesis of poly([R]-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/HV) in Ralstonia eutropha DSM 428. Eng. Life Sci. 2003, 23, 309–316. [Google Scholar] [CrossRef]
- Hartmann, R.; Hany, R.; Geiger, T.; Egli, T.; Witholt, B.; Zinn, M. Tailored biosynthesis of olefinic medium-chain-length Poly[(R)-3-hydroxyalkanoates] in Pseudomonas putida GPo1 with improved thermal properties. Macromolecules 2004, 37, 6780–6785. [Google Scholar] [CrossRef]
- Hany, R.; Brinkmann, M.; Ferri, D.; Hartmann, R.; Pletscher, E.; Rentsch, D.; Zinn, M. Crystallization of an aromatic biopolyester. Macromolecules 2009, 42, 6322–6326. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.C.; Wu, Q.; Chen, G.Q. Halophiles, coming stars for industrial biotechnology. Biotechnol. Adv. 2015, 33, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Lillo, J.G.; Rodriguez-Valera, F. Effects of culture conditions on poly(β-hydroxybutyric acid) production by Haloferax mediterranei. Appl. Environ. Microb. 1990, 56, 2517–2521. [Google Scholar]
- Yue, H.; Ling, C.; Yang, T.; Chen, X.; Chen, Y.; Deng, H.; Wu, Q.; Chen, J.; Chen, G.Q. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates. Biotechnol. Biofuels 2014, 7, 108–119. [Google Scholar] [CrossRef]
- Du, G.; Chen, J.; Yu, J.; Lun, S. Continuous production of poly-3-hydroxybutyrate by Ralstonia eutropha in a two-stage culture system. J. Biotechnol. 2001, 88, 59–65. [Google Scholar] [CrossRef]
- Mothes, G.; Ackermann, J.U. Synthesis of poly(3-hydroxybutyrate-co-4-hydrobutyrate) with a target mole fraction of 4-hydroxybutyric acid units by two-stage continuous cultivation of Delftia acidovorans P4a. Eng. Life Sci. 2005, 5, 58–62. [Google Scholar] [CrossRef]
- Hartmann, R.; Hany, R.; Witholt, B.; Zinn, M. Simultaneous biosynthesis of two copolymers in Pseudomonas putida GPo1 using a two-stage continuous culture system. Biomacromolecules 2010, 11, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Pederson, E.N.; McChalicher, C.W.; Srienc, F. Bacterial synthesis of PHA block copolymers. Biomacromolecules 2006, 7, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Xue, Y.S.; Aibaidula, G.; Chen, G.Q. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01. Bioresour. Technol. 2011, 102, 8130–8136. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wu, Q.; Chen, J.C.; Chen, G.Q. Engineering Halomonas TD01 for the low-cost production of polyhydroxyalkanoates. Metab. Eng. 2014, 26, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Atlić, A.; Koller, M.; Scherzer, D.; Kutschera, C.; Grillo-Fernandes, E.; Horvat, P.; Chiellini, E.; Braunegg, G. Continuous production of poly([R]-3-hydroxybutyrate) by Cupriavidus necator in a multistage bioreactor cascade. Appl. Microbiol. Biotechnol. 2011, 91, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Horvat, P.; Vrana Špoljarić, I.; Lopar, M.; Atlić, A.; Koller, M.; Braunegg, G. Mathematical modelling and process optimization of a continuous 5-stage bioreactor cascade for production of poly[-(R)-3-hydroxybutyrate] by Cupriavidus necator. Bioproc. Biosyst. Eng. 2013, 36, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Lopar, M.; Vrana Špoljarić, I.; Atlić, A.; Koller, M.; Braunegg, G.; Horvat, P. Five-step continuous production of PHB analyzed by elementary flux, modes, yield space analysis and high structured metabolic model. Biochem. Eng. J. 2013, 79, 57–70. [Google Scholar] [CrossRef]
- Vadlja, D.; Koller, M.; Novak, M.; Braunegg, G.; Horvat, P. Footprint area analysis of binary imaged Cupriavidus necator cells to study PHB production at balanced, transient, and limited growth conditions in a cascade process. Appl. Microbiol. Biotechnol. 2016, 100, 10065–10080. [Google Scholar] [CrossRef] [PubMed]


| Production Strain | Raw Material | PHA Produced | Production Achieved | Reference |
|---|---|---|---|---|
| C. necator | Soya cake supplemented with molasses | PHB | 4.9 g PHB per kg solid | [49] |
| C. necator | Solid biodiesel waste supplemented with molasses | PHB | 2.1 g PHB per kg solid | [50] |
| B. sphaericus NII 0838 | Jack fruit seed hydrolysate on PU foam supports | PHB | 170 g PHB per kg PU support | [51] |
| Production Strain | Process Regime | Substrate | PHA Produced | Production Achieved | Reference |
|---|---|---|---|---|---|
| Chelatococcus daeguensis TAD1 | Batch | Glycerol | PHB | 0.81 g/g PHA in CDM, 0.01 g/(L h) | [59] |
| Halomonas campisalis | Batch | Maltose | PHB | 0.81 g/g PHA in CDM, 0.03 g/(L h) | [60] |
| Cupriavidus necator H16 | Batch | Jatropha oil | PHB | 0.9 g/g PHA in CDM, 0.17 g/(L h) | [61] |
| Bacillus firmus NI 0830 | Batch | Rice straw hydrolyzate | PHB | 0.89 g/g PHA in CDM, 0.02 g/(L h) | [62] |
| Azohydromonas australica DSM 1124 | Repeated batch | Sucrose | PHB | 0.82 g/g PHA in CDM, 0.31 g/(L h) | [63] |
| Chelatococcus sp. MW10 | Repeated batch (“cyclic batch”) | Glucose | PHB | 0.32 g/g PHA in CDM, 0.02 g/(L h) | [96] |
| C. necator DSM 545 | Fed-batch | Soybean oil | PHB | 0.81 g/g PHA in CDM, 2.5 g/(L h) | [83] |
| Burkholderia sacchari | Fed-batch | Sucrose | PHB | 0.72 g/g PHA in CDM, 1.87 g/(L h) | [34] |
| Hfx. mediterranei | Fed-batch | Crude glycerol phase | PHBHV | 0.76 g/g PHA in CDM, 0.12 g/(L h) | [84] |
| Pseudomonas citronellolis | Fed-batch | Low-quality biodiesel | mcl-PHA | 0.27 g/g PHA in CDM, 0.055 g/(L h) | [85] |
| Pseudomonas chlororaphis | Fed-batch | Low-quality biodiesel | mcl-PHA | 0.29 g/g PHA in CDM, 0.138 g/(L h) | [86] |
| Chelatococcus sp. MW10 | Fed-batch | Glucose | PHB | 0.51 g/g PHA in CDM, 0.05 g/(L h) | [96] |
| Pseudomonas putida KT2440 | Growth phase: Batch; Accumulation phase: Fed-batch | Growth phase: Grape pomace; accumulation phase: octanoic acid & 10-undecenoic acid | tailored mcl-PHA | 0.41 g/g PHA in CDM, 0.10 g/(L h) | [87] |
| Rec. Escherichia coli | Fed-batch; pH-stat | Whey powder | PHB | 0.81 g/g PHA in CDM, 2.57 g/(L h) | [81] |
| Rec. Escherichia coli | Fed-batch with cell recycle | Whey powder | PHB | 0.87 g/g PHA in CDM, 4.6 g/(L h) | [82] |
| C. necator DSM 545 | Fed-batch with cell recycle | Glucose & fructose | PHB | 0.69 g/g PHA in CDM, 1.0 g/(L h) | [93] |
| C. necator DSM 545 | Fed-batch with cell recycle | Glucose | PHB | 0.76 g/g PHA in CDM, 3.1 g/(L h) | [94] |
| Chelatococcus sp. MW10 | Repeated fed-batch (“cyclic fed-batch”) | Glucose | PHB | 0.12 g/g PHA in CDM, 0.07 g/(L h) | [96] |
| C. necator ATCC 17699 | “Continuous fed-batch” in airlift reactor | Organic acid cocktail | PHB & PHBHV | 0.60 g/g PHB in CDM; 0.73 g/g PHBHV in CDM | [97] |
| Methylocystis hirsuta | “Continuous fed-batch” in bubble column | Biogas with and without VFAs | PHB & PHBHV | 0.45 g/g PHB; 0.48-0.54 g/g PHBHV | [102] |
| Anabaena solitaria | “Continuous fed-batch” in flat panel bubble column PBR | CO2 | PHB | 0.03 g/g PHB in CDM; 0.191 g/(L d) | [107] |
| Synechocystis salina CCALA 192 | “Continuous fed-batch” in 200 L pilot plant tubular glass PBR | CO2 from industrial effluent gas | PHB | 0.09 g/g PHB in CDM | [108] |
| Rhodospirillum rubrum | “Continuous fed-batch” in bubbled and stirred laboratory reactor | Syngas from corn seed gasification | PHBHV | 0.09 g/g PHB in CDM; 0,0002 g/(L h) | [115] |
| Production Strain | Process Regime | Substrate | PHA Produced | Production Achieved | Reference |
|---|---|---|---|---|---|
| Azotobacter beijerinkii NCIB 9067 | One-stage chemostat | Glucose | PHB | 0.44 g/g PHA in CDM, g/(L h) | [124] |
| C. necator DSM 545 | One-stage chemostat | Glucose & propionic acid | PHBHV | 0.33 g/g PHA in CDM, 0.3 g/(L h) | [125] |
| P. putida GPo1 | One-stage chemostat; DNL | Octanoate | mcl-PHA | Up to 0.56 g/g PHA in CDM | [128] |
| C. necator DSM 428 | One-stage chemostat; DNL | Butyrate & valerate | PHBHV | n.r. | [130] |
| P. putida GPo1 | One-stage chemostat; DNL | 5-phenylpentanoate, octanoate, & 10-undecenoate | mcl-PHA | Up to 0.4 g/g PHA in CDM | [131] |
| Hfx. mediterranei | One stage chemostat; non-sterile | Glucose | PHB | 0.42 g/g PHA in CDM, 0.03 g/(L h) | [134] |
| Halomonas campaniensis LS21 | One stage chemostat; non-sterile | Mixed substrates | PHB | 0.7 g/g PHA in CDM, g/(L h) | [135] |
| Azohydromonas lata DSM 1124 | Two stage chemostat | Sucrose & propionic acid | PHBHV | 0.55 g/g PHA in CDM, 0. g/(L h) | [121] |
| C. necator WSH3 | Two stage chemostat | Crude glycerol phase | PHBHV | 0.72 g/g PHA in CDM, 1.24 g/(L h) | [136] |
| Delftia acidovorans | Two stage chemostat | Acetate & GBL | Poly(3HB-co-4HB) | 0.63 g/g PHA in CDM, 1.06 g/(L h) | [137] |
| Halomonas TD01 | Two stage chemostat; non-sterile | Glucose | PHB | 0.7 g/g PHA in CDM, g/(L h) | [140] |
| C. necator DSM 545 | Five stage chemostat | Glucose | PHB | 0.77 g/g PHA in CDM, 2.31 g/(L h) | [142] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30. https://doi.org/10.3390/fermentation4020030
Koller M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation. 2018; 4(2):30. https://doi.org/10.3390/fermentation4020030
Chicago/Turabian StyleKoller, Martin. 2018. "A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters" Fermentation 4, no. 2: 30. https://doi.org/10.3390/fermentation4020030
APA StyleKoller, M. (2018). A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation, 4(2), 30. https://doi.org/10.3390/fermentation4020030