Comparison of Water-Removal Efficiency of Molecular Sieves Vibrating by Rotary Shaking and Electromagnetic Stirring from Feedstock Oil for Biofuel Production
Abstract
:1. Introduction
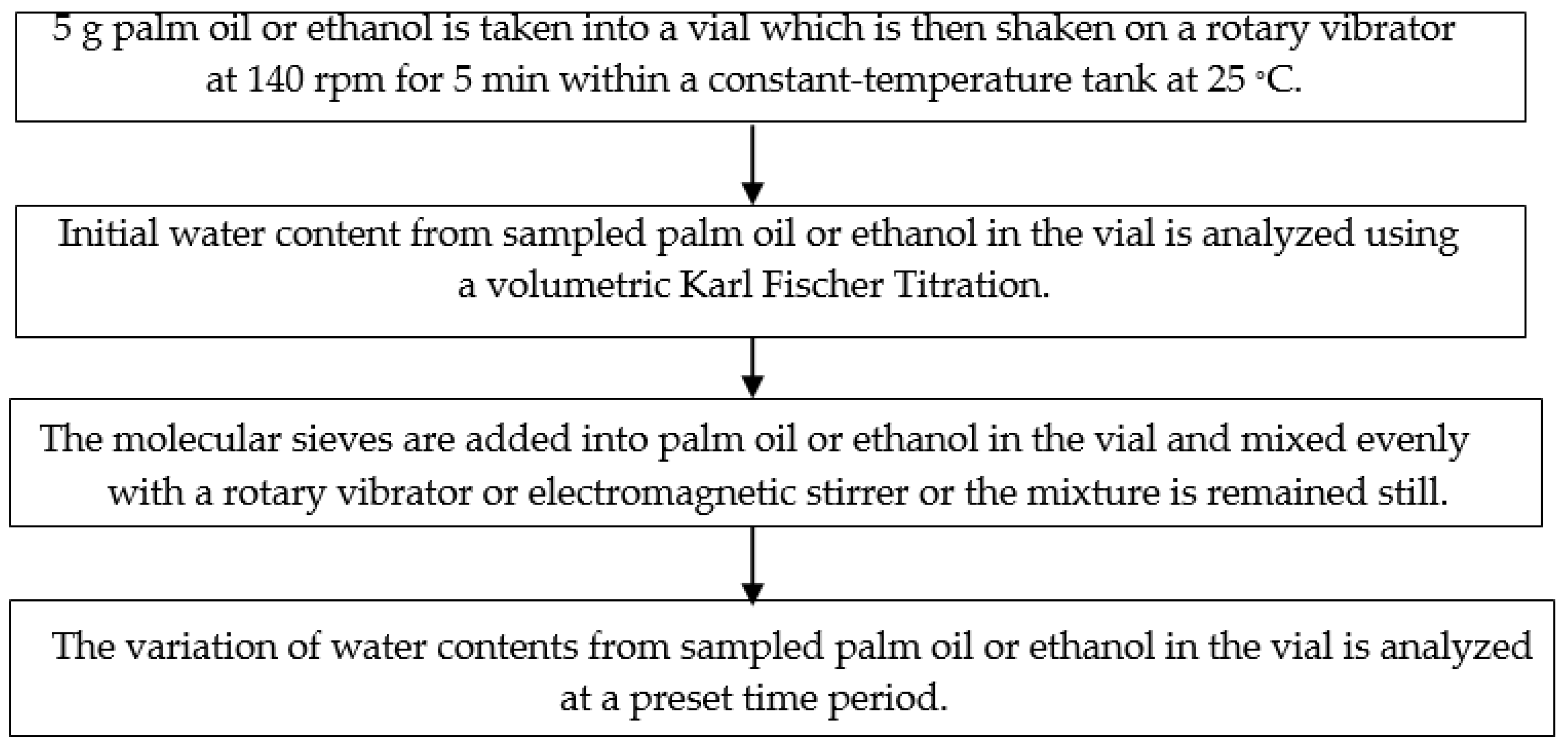
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
3. Results and Discussion
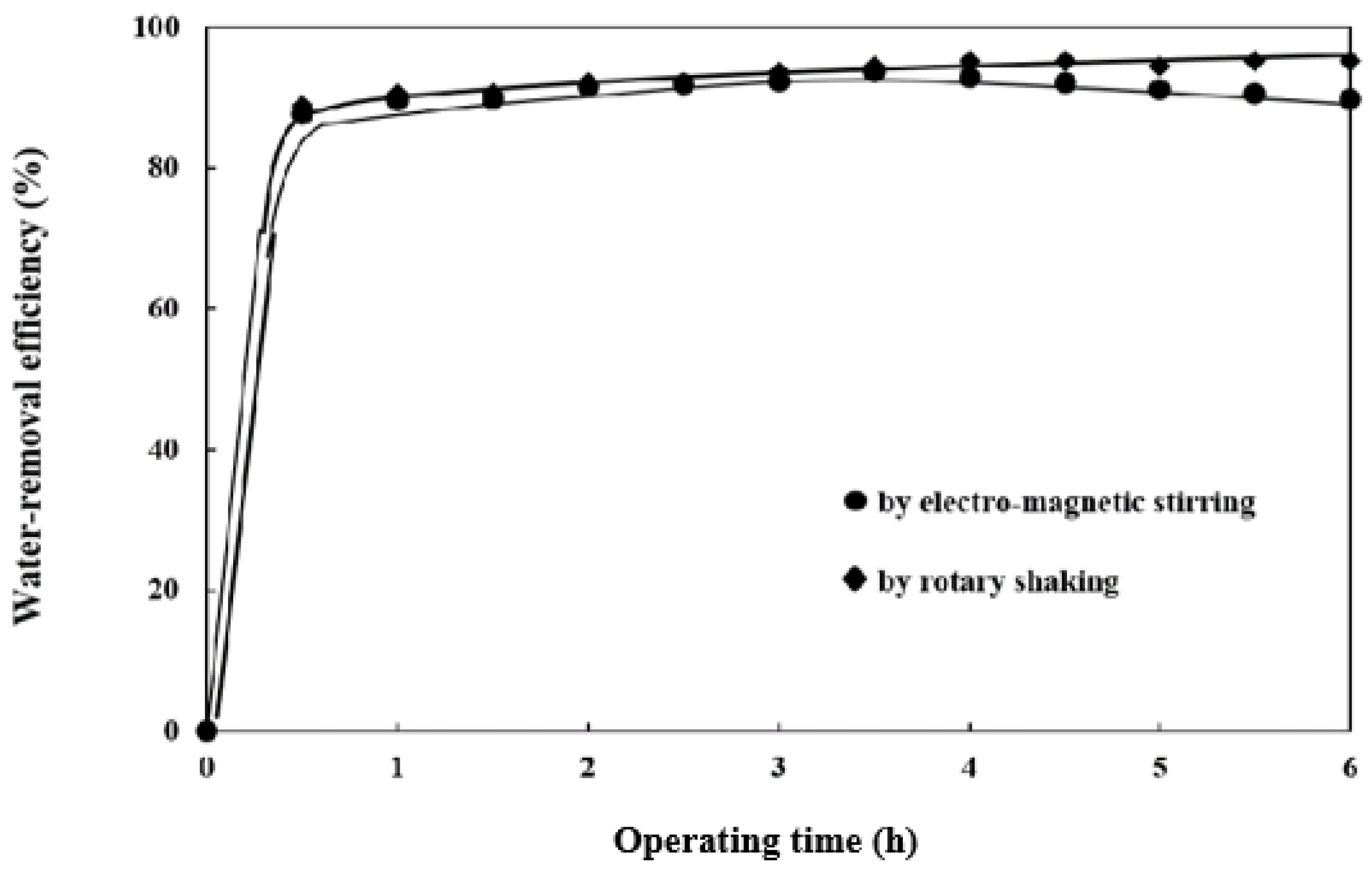
3.1. Effect of Vibration Modes on Water-Removal Efficiency
3.2. Effects of Rotary Shaking and Motionless Treatment on Water Absorbency
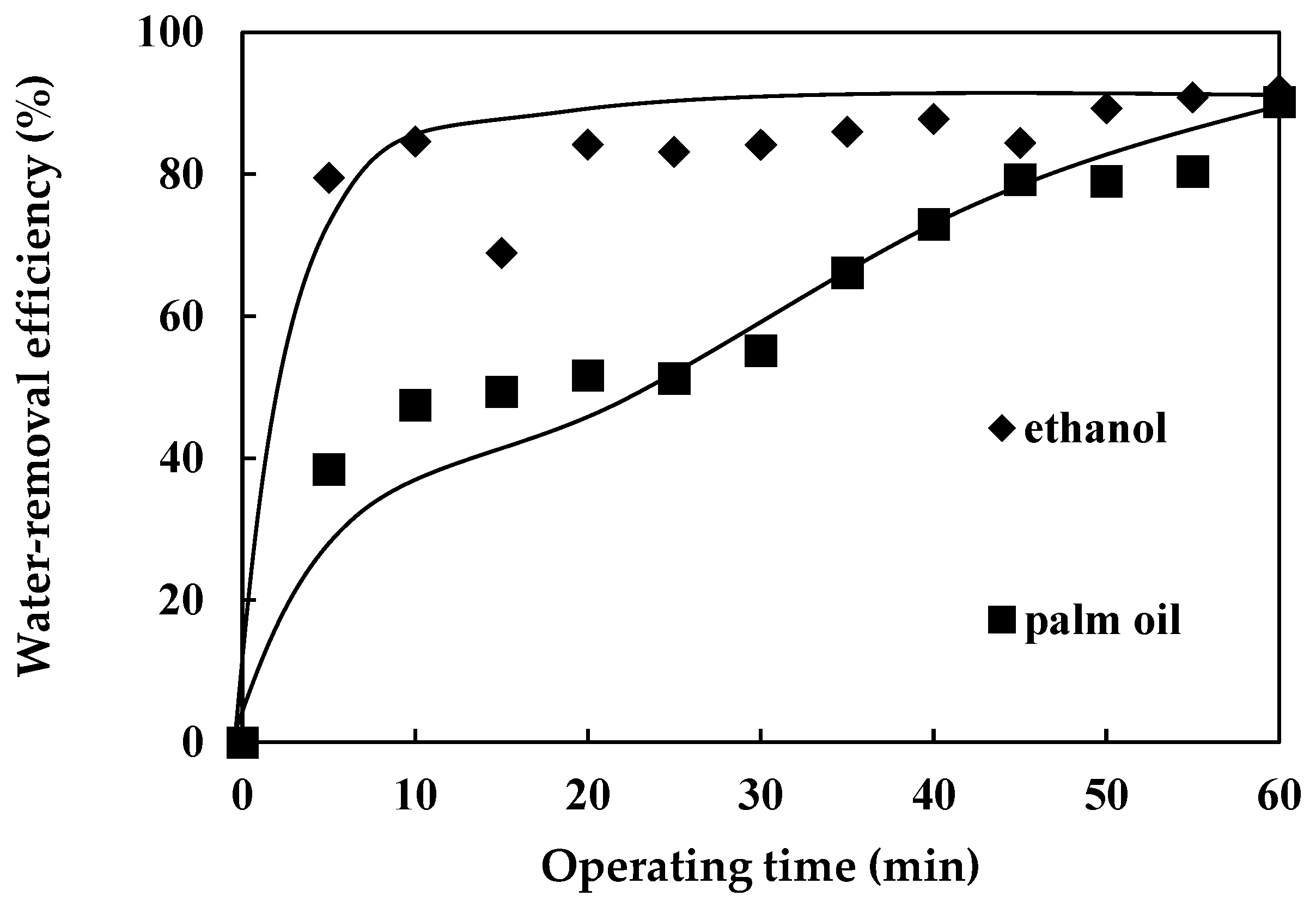
3.3. Effects of Water-Absorption Time
4. Conclusions
- The magnetic bar was prone to collide with the molecular sieves in the vial to cause structural damage of the latter during electromagnetic stirring. The shape and structure of about 66% of molecular sieves were obviously damaged due to frequent collision and friction between the molecular sieves and magnetic bar under the effect of the electromagnetic field. It resulted in fast deterioration of water-absorption capability of the molecular sieves and a slight decrease in water-removal efficiency due to the release back of absorbed water from the molecular sieves;
- The surface shapes of all the molecular sieves were nearly intact and only scaled off slightly from their surfaces after water removal from ethanol by rotary shaking motion for 6 h. The water-removing capability of the molecular sieves is almost sustained after the process accompanied by rotary shaking;
- The water-removal efficiency of the molecular sieves vibrated by a rotary shaker was higher by 6% than that by an electromagnetic stirrer after 6 h water absorption from ethanol. The electromagnetic stirring motion caused an obvious loss of water-absorption capability of the molecular sieves due to severe structural damage during the water-removal process;
- The shapes of molecular sieves were much more irregular and broken after being used for 6 h water-removal from ethanol under electromagnetic stirring than those by rotary shaking. In contrast, the molecular sieves under rotary shaking remained almost like original, ball-shaped, and much glossier. The extent of structural damage of the molecular sieves resulted in the accompanied loss of their water-adsorption capability;
- The water-absorbing process by molecular sieves vibrated by electromagnetic stirring for 6 h caused significantly larger weight loss of the molecular sieves, which accounted for 19 wt.%, nearly 10 times, than that by rotary shaking, which was less than 2 wt.%. The rotary shaking motion is considered a much more adequate agitation method to increase contact frequency and area among the reactant mixtures of feedstock oil, water, and alcohol. This results in a higher reaction rate and faster water-removal efficiency;
- The water-removal efficiency of molecular sieves vibrated by a rotary shaker is higher than that of the remaining motionless mixture of molecular sieves and ethanol by 5% after 30 min of the water-absorption process. The vibrating motion could facilitate the fluidity and mixing extents of the reactant mixture and thus accelerate the chemical reaction;
- The water-removal efficiency from ethanol was considerably higher than that from palm oil by molecular sieves vibrated by a rotary shaker. The water-absorbing capability of the molecular sieves from ethanol reached saturation and steady-state after 10 min while the water-removal efficiency from palm oil increased with time and reached that of ethanol (90%) after 60 min of operation. Ethanol is highly hydroscopic and readily absorbs or desorbs water molecules than palm oil, composed of complex fatty acids and glycerol with much higher viscosity;
- The water-removal rate of the molecular sieves from ethanol by rotary shaking motion in the first 5 min of the operation period was significantly higher and nearly twice that from palm oil in the same period of operation. The molecular structure of ethanol assures its superior miscibility with water molecules and higher water-removal rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masudi, A.; Muraza, O. Vegetable oil to biolubricants: Review on advanced porous catalysts. Energy Fuel 2018, 32, 10295–10310. [Google Scholar] [CrossRef]
- Zimmer, Y. Competitiveness of rapeseed, soybeans and palm oil. J. Oilseed Brassica 2016, 1, 84–90. [Google Scholar]
- Statista Inc. Available online: https://www.statista.com/ (accessed on 11 May 2021).
- Lin, C.Y.; Lu, C. Development perspectives of promising lignocellulose feedstocks for production of advanced generation biofuels: A review. Renew. Sust. Energy Rev. 2021, 136, 110445. [Google Scholar] [CrossRef]
- Oliverira, E.D.C.; Silva, P.R.D.; Ramos, A.P.; Aranda, D.A.G.; Freire, D.M.G. Study of soybean oil hydrolysis catalyzed by Thermomyces Ianuginosus lipase and its application to biodiesel production via hydroesterification. Enzym. Res. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porfyris, A.; Vasilakos, S.; Zotiadis, C.; Papaspyrides, C.; Moser, K.; Van Der Schueren, L.; Vouyiouka, S. Accelerated ageing and hydrolytic stabilization of poly (lactic acid) (PLA) under humidity and temperature conditioning. Polym. Test. 2018, 68, 315–332. [Google Scholar] [CrossRef]
- Carrillo, L.; Bilason, L.; Loveres, S.F.; Paurillo, J.E.; Sacobo, C.J.; Sakay, C.; Espinosa, K.; Vergara, J. Utilization of vegetable oil refinery activated carbon-bleaching earth as an additive to the production of low-density facing bricks. E&ES 2020, 463, 012092. [Google Scholar]
- Sebastian, J.; Muraleedharan, C.; Santhiagu, A. Enzyme catalyzed biodiesel production from rubber seed oil containing high free fatty acid. Int. J. Green Energy 2017, 14, 687–693. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. Intensified one-step biodiesel production from high water and free fatty acid waste cooking oils. Fuel 2018, 220, 567–574. [Google Scholar] [CrossRef]
- Gil, I.D.; Uyazán, A.M.; Agular, J.L.; Rodríguez, G.; Caicedo, L.A. Separation of ethanol and water by extractive distillation with salt and solvent as entrainer: Process simulation. Braz. J. Chem. Eng. 2008, 25, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Bui, D.T.; Nida, A.; Ng, K.C.; Chua, K.J. Water vapor permeation and dehumidification performance of poly (vinyl alcohol)/lithium chloride composite membranes. J. Membr. Sci. 2016, 498, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; He, C.; Li, Y.; Chung, K.H.; Xu, C.; Shi, Q. Fractionation and characterization of dissolved organic matter (DOM) in refinery wastewater by revised phase retention and ion-exchange adsorption solid phase extraction followed by ESI FT-ICR MS. Talanta 2017, 162, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.; Ferro, V.R.; De Riva, J.; Santiago, R.; Moya, C.; Larriba, M.; Palomar, J. Absorption refrigeration cycles based on ionic liquids: Refrigerant/absorbent selection by thermodynamic and process analysis. Appl. Energy 2018, 213, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zuo, G. Discussion on the production technology of biodiesel reaching BD100 standard. China Oil Fat 2009, 34, 59–62. [Google Scholar]
- Lawrence, M.; Jiang, Y. Porosity, pore size distribution, micro-structure. In Bio-Aggregates Based Building Materials; Springer: Dordrecht, The Netherlands, 2017; pp. 39–71. [Google Scholar]
- Besnardiere, J.; Ma, B.; Torres-Pardo, A.; Wallez, G.; Kabbour, H.; González-Calbet, J.M.; Von Bardeleben, H.J.; Fleury, F.; Buissette, V.; Sanchez, C.; et al. Structure and electrochromism of two-dimensional octahedral molecular sieve h’-WO 3. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.M.; Eljamal, O.; Amen, T.W.; Sugihara, Y.; Matsunaga, N. Optimized nano-scale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem. Eng. J. 2017, 309, 349–365. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, L.C.; Díaz Barrera, L.E.; Quintanilla-Carvajal, M.X.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Jiménez-Junca, C. Preparation of a hybrid membrane from whey protein fibrils and activated carbon to remove mercury and chromium from water. Membranes 2020, 10, 386. [Google Scholar] [CrossRef]
- Manoli, K.; Nakhla, G.; Feng, M.; Sharma, V.K.; Ray, A.K. Silica gel-enhanced oxidation of caffeine by ferrate (VI). Chem. Eng. J. 2017, 330, 987–994. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Liu, C.; Ma, W.; Yan, B.; Zhang, J. Hydrodeoxygenation of lignin-derived bio-oil using molecular sieves supported metal catalysts: A critical review. Renew. Sust. Energy Rev. 2017, 71, 296–308. [Google Scholar] [CrossRef]
- Atyaksheva, L.F.; Kasyanov, I.A.; Ivanova, I.I. Adsorptive Immobilization of proteins on mesoporous molecular sieves and zeolites. Petrol. Chem. 2019, 59, 327–337. [Google Scholar] [CrossRef]
- Tasharrofi, S.; Golchoobi, A.; Fesahat, H.; Taghdisian, H.; Hosseinnia, A. Effects of water content on so2/n2 binary adsorption capacities of 13x and 5a molecular sieve, experiment, simulation, and modeling. J. Petrol. Sci. Technol. 2019, 9, 30–45. [Google Scholar]
- Garcia, L.; Rodriguez, G.; Orjuela, A. Study of the pilot-scale pan granulation of zeolite-based molecular sieves. Braz. J. Chem. Eng. 2021, 38, 165–175. [Google Scholar] [CrossRef]
- Santos, M.G.; Correia, L.M.; de Medeiros, J.L.; Ofélia de Queiroz, F.A. Natural gas dehydration by molecular sieve in offshore plants: Impact of increasing carbon dioxide content. Energy Convers. Manag. 2017, 149, 760–773. [Google Scholar] [CrossRef]
- He, X.; Chu, Y.; Lindbråthen, A.; Hillestad, M.; Hägg, M.B. Carbon molecular sieve membranes for biogas upgrading: Techno-economic feasibility analysis. J. Clean. Prod. 2018, 194, 584–593. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R. Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite. J. Hazard. Mater. 2017, 326, 157–164. [Google Scholar] [CrossRef]
- Xia, Z.; Ying, L.; Fang, J.; Du, Y.Y.; Zhang, W.M.; Guo, X.; Yin, J. Preparation of covalently cross-linked sulfonated polybenzimidazole membranes for vanadium redox flow battery applications. J. Membr. Sci. 2017, 525, 229–239. [Google Scholar] [CrossRef]
- Lin, C.Y.; Ma, L. Influences of water content in feedstock oil on burning characteristics of fatty acid methyl esters. Processes 2020, 8, 1130. [Google Scholar] [CrossRef]
- Pahl, C.; Pasel, C.; Luckas, M.; Bathen, D. Adsorptive water removal from organic solvents in the ppm-region. Chem. Ing. Tech. 2011, 83, 177–182. [Google Scholar] [CrossRef]
- Xu, Y.M.; Tang, Y.P.; Chung, T.S.; Weber, M.; Maletzko, C. Polyarylether membranes for dehydration of ethanol and methanol via pervaporation. Sep. Purif. Technol. 2018, 193, 165–174. [Google Scholar] [CrossRef]
- Salam, M.A.; Ahmed, K.; Hossain, T.; Habib, M.S.; Uddin, M.S.; Papri, N. Prospect of molecular sieves production using rice husk in bangladesh: A review. Int. J. Chem. Math. Phys. 2019, 3, 105–134. [Google Scholar] [CrossRef]
- Gabruś, E.; Witkiewicz, K.; Nastaj, J. Modeling of regeneration stage of 3A and 4A zeolite molecular sieves in TSA process used for dewatering of aliphatic alcohols. Chem. Eng. J. 2018, 337, 416–427. [Google Scholar] [CrossRef]
- Herold, R.H.M.; Mokhatab, S. Optimal Design and Operation of Molecular Sieve Gas Dehydration Units—Part 1. Gas Processing & LNG. 2017. Available online: http://www.gasprocessingnews.com/features/201708/optimal-design-and-operation-of-molecular-sieve-gas-dehydration-units%E2%80%94part-1.aspx (accessed on 25 July 2021).
- Wang, H.; Jia, C.; Xia, X.; Karangwa, E.; Zhang, X. Enzymatic synthesis of phytosteryl lipoate and its antioxidant properties. Food Chem. 2018, 240, 736–742. [Google Scholar] [CrossRef]
- Lad, J.B.; Makkawi, Y.T. Adsorption of methyl chloride on molecular sieves, silica gels, and activated carbon. Chem. Eng. Technol. 2020, 43, 436–446. [Google Scholar] [CrossRef]
- Jemil, N.; Hmidet, N.; Ayed, H.B.; Nasri, M. Physicochemical characterization of Enterobacter cloacae C3 lipopeptides and their applications in enhancing diesel oil biodegradation. Process. Saf. Environ. 2018, 117, 399–407. [Google Scholar] [CrossRef]
- Wang, F.; Wang, E.; Zhang, L.; Jia, P.; Wang, T. Influence of electromagnetic stirring (EMS) on the microstructure and mechanical property of Incoloy825 superalloy. J. Manuf. Process. 2017, 26, 364–371. [Google Scholar] [CrossRef]
- Saremnia, B.; Esmaeili, A.; Sohrabi, M.R. Removal of total petroleum hydrocarbons from oil refinery waste using granulated NaA zeolite nanoparticles modified with hexadecyltrimethylammonium bromide. Can. J. Chem. 2016, 94, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Van Thuoc, D.; My, D.N.; Loan, T.T.; Sudesh, K. Utilization of waste fish oil and glycerol as carbon sources for polyhydroxyalkanoate production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019, 141, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Tiadi, N.; Dash, R.R.; Mohanty, C.R.; Patel, A.M. Comparative studies of adsorption of chromium (VI) ions onto different industrial wastes. J. Hazard Toxic Radioact. Waste 2020, 24, 04020021. [Google Scholar] [CrossRef]
- Dey, S.; Mehta, N.S. To optimized various parameters of Hopcalite catalysts in the synthetic processes for low temperature CO oxidation. Appl. Energy Combust. Sci 2021, 6, 100031. [Google Scholar]
- Raviadaran, R.; Ng, M.H.; Manickam, S.; Chandran, D. Ultrasound-assisted water-in-palm oil nano-emulsion: Influence of polyglycerol polyricinoleate and NaCl on its stability. Ultrason. Sonochem. 2019, 52, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.W. Addressing concerns related to the use of ethanol-blended fuels in marine vehicles. J. Sustain. Dev. Energy Water Environ. Syst. 2017, 5, 546–559. [Google Scholar] [CrossRef] [Green Version]
- Anand, V.; Juvekar, V.A.; Thaokar, R.M. Coalescence, partial coalescence, and noncoalescence of an aqueous drop at an oil–water interface under an electric field. Langmuir 2020, 36, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Purnama, K.O.; Setyaningsih, D.; Hambali, E.; Taniwiryono, D. Processing, characteristics, and potential application of red palm oil—A review. Int. J. Oil Palm 2020, 3, 40–55. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, D.; Liu, Y.; Xu, Y.; Zeng, G.; Wang, W.; Cui, F. Three-component mixed matrix organic/inorganic hybrid membranes for pervaporation separation of ethanol–water mixture. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ai, F.; Yin, X.; Hu, R.; Ma, H.; Liu, W. Research into the super-absorbent polymers on agricultural water. Agric. Water Manag. 2021, 245, 106513. [Google Scholar] [CrossRef]
- Lin, C.Y.; Ma, L. Fluid characteristics of biodiesel produced from palm oil with various initial water contents. Processes 2021, 9, 309. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Ma, L. Comparison of Water-Removal Efficiency of Molecular Sieves Vibrating by Rotary Shaking and Electromagnetic Stirring from Feedstock Oil for Biofuel Production. Fermentation 2021, 7, 132. https://doi.org/10.3390/fermentation7030132
Lin C-Y, Ma L. Comparison of Water-Removal Efficiency of Molecular Sieves Vibrating by Rotary Shaking and Electromagnetic Stirring from Feedstock Oil for Biofuel Production. Fermentation. 2021; 7(3):132. https://doi.org/10.3390/fermentation7030132
Chicago/Turabian StyleLin, Cherng-Yuan, and Lei Ma. 2021. "Comparison of Water-Removal Efficiency of Molecular Sieves Vibrating by Rotary Shaking and Electromagnetic Stirring from Feedstock Oil for Biofuel Production" Fermentation 7, no. 3: 132. https://doi.org/10.3390/fermentation7030132
APA StyleLin, C.-Y., & Ma, L. (2021). Comparison of Water-Removal Efficiency of Molecular Sieves Vibrating by Rotary Shaking and Electromagnetic Stirring from Feedstock Oil for Biofuel Production. Fermentation, 7(3), 132. https://doi.org/10.3390/fermentation7030132






