Fermentation Condition and Quality Evaluation of Pineapple Fruit Wine
Abstract
:1. Introduction
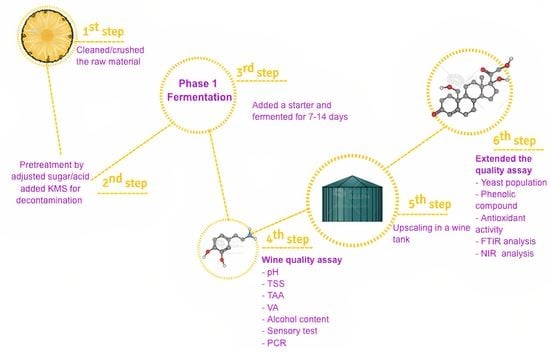
2. Materials and Methods
2.1. Preparation of Yeast Culture
2.2. Study on the Ratio of Fresh Pineapple Juice to Water
2.3. Chemical Analysis
2.4. Determination of Total Phenolics Compounds
2.5. Determination of Antioxidant Activity
2.6. Fourier Transform Infrared (FTIR) Analysis
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Study on the Ratio of Fresh Pineapple Juice to Water
3.2. Pineapple Wine Sensory Evaluation
3.3. Yeast Population
3.4. Total Phenolic Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Agriculture Extension. Situation of Pineapple Production Review. 2021. Available online: https://www.oae.go.th/view/1/%E0%B8%95%E0%B8%B2%E0%B8%A3%E0%B8%B2%E0%B8%87%E0%B9%81%E0%B8%AA%E0%B8%94%E0%B8%87%E0%B8%A3%E0%B8%B2%E0%B8%A2%E0%B8%A5%E0%B8%B0%E0%B9%80%E0%B8%AD%E0%B8%B5%E0%B8%A2%E0%B8%94%E0%B8%AA%E0%B8%B1%E0%B8%9A%E0%B8%9B%E0%B8%B0%E0%B8%A3%E0%B8%94%E0%B9%82%E0%B8%A3%E0%B8%87%E0%B8%87%E0%B8%B2%E0%B8%99/TH-TH%E0%B8%B2%E0%B8%99/TH-TH (accessed on 12 August 2021).
- Corazza, M.L.; Rodrigues, D.G. Preparation and characterization of orange wine. Quim. Nova 2001, 24, 449–452. [Google Scholar]
- Jin, Z.M.; He, J.J.; Bi, H.Q.; Cui, X.Y.; Duan, C.Q. Phenolic compound profiles in berry skins from nine red wine grape cultivars in Northwest China. Molecules 2009, 14, 4922–4935. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Food 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Moreira, J.L.; Marcos, A.M.; Barros, P. Proficiency test on FTIR wine analysis. Cienc. Tec. Vitivinic. 2002, 17, 41–51. [Google Scholar]
- IFU. Determination of Titratable Acidity. IFU Analysis No. 3. 2007. Available online: https://www.ifu-fruitjuice.com/ (accessed on 12 August 2021).
- IFU. Determination of Volatile Acids. IFU Analysis No. 5. 2005. Available online: https://www.ifu-fruitjuice.com/ (accessed on 12 August 2021).
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; The Association: Gaithersburg, MD, USA, 2006. [Google Scholar]
- CIE. Colorimetry; Publication CIE 15.2; John Wiley & Sons: Hoboken, NJ, USA, 1986. [Google Scholar]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Vidal-Gutiérrez, M.; Robles-Zepeda, R.E.; Vilegas, W.; Gonzalez-Aguilar, G.A.; Torres-Moreno, H.; López-Romero, J.C. Phenolic composition and antioxidant activity of Bursera microphylla A. Gray. Ind. Crops Prod. 2020, 152, 112412. [Google Scholar] [CrossRef]
- Augustine, S.K.; Bhavsar, S.P.; Kapadnis, B.P. A non-polyene antifungal antibiotic from Streptomyces albidoflavus PU 23. J. Biosci. 2005, 30, 201–211. [Google Scholar] [CrossRef]
- Chanprasartsuk, O.; Pheanudomkitlert, K.; Toonwai, D. Pineapple wine fermentation with yeasts isolated from fruit as single and mixed starter cultures. Asian J. Food Agro-Ind. 2012, 5, 104–111. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications; John Wiley & Sons: Chichester, UK, 2006; pp. 79–113. [Google Scholar]
- Zoecklein, B.; Fugelsang, K.; Gump, B.; Nury, F. Wine Analysis and Production; Springer Science & Business Media: New York, NY, USA, 1999. [Google Scholar]
- Pretorius, I.; Lambrechts, M. Yeast and its importance to wine aroma: A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar]
- Du Toit, W.J.; Pretorius, I.S. The occurrence, control and esoteric effect of acetic acid bacteria in winemaking. Ann. Microbiol. 2002, 52, 155–179. [Google Scholar]
- Drysdale, G.S.; Fleet, G.H. Acetic acid bacteria in winemaking: A Review. Am. J. Enol. Vitic. 1988, 39, 143–154. [Google Scholar]
- Chalermsanyakon, W.; Punyanunt, S.; Phadungath, C. Development of pineapple wine from pineapple waste. In Proceedings of the 6th Muban Chombueng Rajabhat University’s Nation Conference 2018, Chom Bueng, Ratchaburi, Thailand, 1 March 2018; Volume 6, pp. 433–438. (In Thai). [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1-1. [Google Scholar]
- Rover, M.R.; Brown, R.C. Quantification of total phenols in bio-oil using the Folin–Ciocalteu method. J. Anal. Appl. Pyrolysis 2013, 104, 366–371. [Google Scholar] [CrossRef]
- Stratil, P.; Kuban, V.; Fojtova, J. Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech J. Food Sci. 2008, 26, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Preserova, J.; Ranc, V.; Milde, C.; Kubistova, V.; Stavek, J. Study of phenolic profile and antioxidant activity in selected Moravian wines during winemaking process by FTIR spectroscopy. J. Food Sci. Technol. 2015, 52, 6405–6414. [Google Scholar] [CrossRef] [Green Version]
- Franco-Bañuelos, A.; Contreras-Martínez, C.S.; Carranza-Téllez, J.; Carranza-Concha, J. Total phenolic content and antioxidant capacity of non-native wine grapes grown in Zacatecas, Mexico. Agrociencia 2017, 51, 661–671. [Google Scholar]
- Moreira, J.L.; Santos, L. Spectroscopic interferences in Fourier transform infrared wine analysis. Anal. Chim. Acta 2004, 513, 263–268. [Google Scholar] [CrossRef]
- Versari, A.; Parpinello, G.P.; Scazzina, F.; Del Rio, D. Prediction of total antioxidant capacity of red wine by Fourier transform infrared spectroscopy. Food Control 2010, 21, 786–789. [Google Scholar] [CrossRef]
- Fragoso, S.; Acena, L.; Guasch, J.; Busto, O.; Mestres, M. Application of FT-MIR spectroscopy for fast control of red grape phenolic ripening. J. Agric. Food Chem. 2011, 59, 2175–2183. [Google Scholar] [CrossRef] [PubMed]




| Chemical Characteristics | Value ± SD |
|---|---|
| pH | 3.84 ± 0.12 |
| Total Soluble Solid (TSS, °Brix) | 14.0 ± 0.12 |
| Total Titratable acidity (TTA, as citric acid) (% w/v) | 0.378 ± 0.01 |
| Nitrogen content (% w/v) | 0.07 ± 0.01 |
| Incubation Time (Day) | Alcohol Content (%) at Ratio of Pineapple Juice and Water | Total Reducing Sugar (%) at Ratio of Pineapple Juice and Water | Total Acidity (%) at Ratio of Pineapple Juice and Water | Volatile Acidity (%) at Ratio of Pineapple Juice and Water | pH at Ratio of Pineapple Juice and Water | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2:1 | 1:1 | 1:2 | 2:1 | 1:1 | 1:2 | 2:1 | 1:1 | 1:2 | 2:1 | 1:1 | 1:2 | 2:1 | 1:1 | 1:2 | |
| 0 | 0 ± 0.01 | 0 ± 0.00 | 0 ± 0.00 | 3.10 e ± 0.04 | 3.03 f ± 0.14 | 3.33 d ± 0.14 | 0.284 a ± 0.00 | 0.286 a ± 0.01 | 0.260 b ± 0.01 | 0.0015 a ± 0.01 | 0.0012 b ± 0.00 | 0.0012 b ± 0.00 | 4 ± 0.01 | 4 ± 0.00 | 4 ± 0.00 |
| 2 | 0.35 a ± 0.08 | 0.41 a ± 0.02 | 0.14 b ± 0.01 | 5.22 a ± 0.06 | 4.96 a ± 0.08 | 4.44 b ± 0.11 | 0.314 a ± 0.01 | 0.301 a ± 0.01 | 0.277 b ± 0.01 | 0.0015 a ± 0.00 | 0.0012 b ± 0.00 | 0.0013 b ± 0.00 | 3.91 c ± 0.01 | 3.95 a ± 0.00 | 3.92 b ± 0.01 |
| 4 | 5.72 a ± 0.44 | 5.32 a ± 0.18 | 0.38 b ± 0.02 | 4.34 b ± 0.10 | 3.99 b ± 0.10 | 4.93 a ± 0.12 | 0.375 a ± 0.01 | 0.329 b ± 0.02 | 0.314 b ± 0.01 | 0.0015 a ± 0.00 | 0.0013 b ± 0.00 | 0.0013 b ± 0.00 | 3.82 ± 0.02 | 3.83 ± 0.01 | 3.9 ± 0.01 |
| 6 | 9.01 a ± 0.30 | 7.40 b ± 0.38 | 4.71 c ± 0.29 | 3.88 c ± 0.07 | 3.78 d ± 0.01 | 4.66 b ± 0.18 | 0.397 ± 0.01 | 0.383 ± 0.01 | 0.38 ± 0.01 | 0.0016 a ± 0.00 | 0.0013 b ± 0.00 | 0.0013 b ± 0.00 | 3.66 c ± 0.01 | 3.77 b ± 0.01 | 3.84 a ± 0.01 |
| 8 | 9.53 a ± 0.07 | 9.01 b ± 0.15 | 7.02 c ± 0.20 | 3.57 d ± 0.10 | 3.63 d ± 0.05 | 3.70 c ± 0.14 | 0.448 a ± 0.00 | 0.410 b ± 0.01 | 0.384 c ± 0.01 | 0.0016 a ± 0.00 | 0.0013 b ± 0.00 | 0.0013 b ± 0.00 | 3.57 c ± 0.01 | 3.74 b ± 0.02 | 3.84 ª ± 0.01 |
| 10 | 9.89 a ± 0.02 | 9.61 a ± 0.27 | 8.35 b ± 0.48 | 3.43 d ± 0.06 | 3.34 e ± 1.34 | 3.41 d ± 1.45 | 0.448 a ± 0.00 | 0.422 b ± 0.00 | 0.384 c ± 0.00 | 0.0016 a ± 0.00 | 0.0013 b ± 0.00 | 0.0013 b ± 0.00 | 3.56 c ± 0.02 | 3.73 b ± 0.03 | 3.82 a ± 0.01 |
| Ratio of Pineapple Juice to Water | Sensory Evaluation Results | ||||
|---|---|---|---|---|---|
| Appearance ns | Aroma ns | Taste ns | Sweetness | Overall Liking | |
| 2:1 | 6.93 ± 0.77 | 6.12 ± 0.98 | 6.26 ± 0.88 | 6.39 ± 0.90 a | 6.35 ± 0.96 a |
| 1:1 | 6.84 ± 0.88 | 6.03 ± 0.75 | 6.03 ± 0.95 | 5.85 ± 0.98 ab | 6.08 ± 0.81 ab |
| 1:2 | 6.53 ± 0.95 | 5.72 ± 0.99 | 5.72 ± 0.87 | 5.49 ± 1.06 b | 5.58 ± 0.81 b |
| Time (Days) | Phenolic Content (mg/L GAE) | Antioxidant Activity (mmol/L TE) | Total Cell Count (CFU/mL) |
|---|---|---|---|
| 0 | 311.57 ± 0.85 | 1.34 ± 0.18 | 8.70 × 105 |
| 1 | 228.07 ± 3.68 | 1.46 ± 0.41 | 1.10 × 107 |
| 2 | 304.69 ± 2.65 | 0.92 ± 0.05 | 1.10 × 107 |
| 3 | 307.95 ± 1.25 | 0.91 ± 0.04 | 9.50 × 106 |
| 4 | 324.25 ± 1.06 | 0.91 ± 0.03 | 6.77 × 106 |
| 5 | 322.01 ± 2.25 | 0.97 ± 0.10 | 1.96 × 106 |
| 6 | 310.57 ± 3.11 | 0.82 ± 0.05 | 9.13 × 105 |
| 7 | 337.62 ± 1.78 | 0.87 ± 0.03 | 1.55 × 105 |
| 8 | 351.53 ± 2.52 | 0.94 ± 0.04 | 1.50 × 105 |
| 9 | 358.73 ± 2.29 | 0.91 ± 0.01 | 5.75 × 104 |
| 10 | 365.80 ± 2.50 | 0.91 ± 0.02 | 9.90 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boondaeng, A.; Kasemsumran, S.; Ngowsuwan, K.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Janchai, P.; Jungtheerapanich, S.; Niyomvong, N. Fermentation Condition and Quality Evaluation of Pineapple Fruit Wine. Fermentation 2022, 8, 11. https://doi.org/10.3390/fermentation8010011
Boondaeng A, Kasemsumran S, Ngowsuwan K, Vaithanomsat P, Apiwatanapiwat W, Trakunjae C, Janchai P, Jungtheerapanich S, Niyomvong N. Fermentation Condition and Quality Evaluation of Pineapple Fruit Wine. Fermentation. 2022; 8(1):11. https://doi.org/10.3390/fermentation8010011
Chicago/Turabian StyleBoondaeng, Antika, Sumaporn Kasemsumran, Kraireuk Ngowsuwan, Pilanee Vaithanomsat, Waraporn Apiwatanapiwat, Chanaporn Trakunjae, Phornphimon Janchai, Sunee Jungtheerapanich, and Nanthavut Niyomvong. 2022. "Fermentation Condition and Quality Evaluation of Pineapple Fruit Wine" Fermentation 8, no. 1: 11. https://doi.org/10.3390/fermentation8010011
APA StyleBoondaeng, A., Kasemsumran, S., Ngowsuwan, K., Vaithanomsat, P., Apiwatanapiwat, W., Trakunjae, C., Janchai, P., Jungtheerapanich, S., & Niyomvong, N. (2022). Fermentation Condition and Quality Evaluation of Pineapple Fruit Wine. Fermentation, 8(1), 11. https://doi.org/10.3390/fermentation8010011