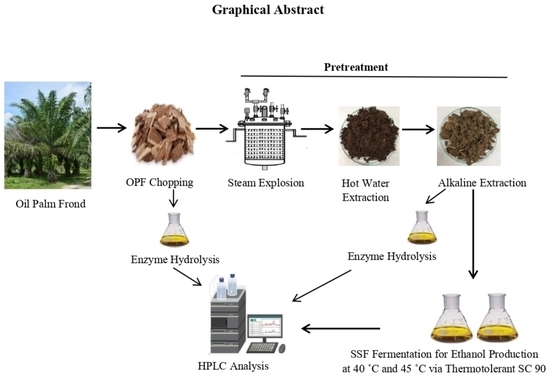
Ethanol Production through Optimized Alkaline Pretreated Elaeis guineensis Frond Waste from Krabi Province, Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Raw Materials
2.3. Pretreatment
Optimization of Alkali Extraction by the Taguchi Method
2.4. Enzyme Hydrolysis
2.5. Fermentation
2.5.1. Preparation of Yeast Growth and Preinoculums
2.5.2. Simultaneous Saccharification and Fermentation (SSF)
2.6. Analytical Methods
2.7. Analysis of Cellobiose, Glucose, and Ethanol
2.8. Statistical Analysis
3. Results
3.1. Optimization of Alkaline Extraction by the Taguchi Method
3.2. Confirmation under Optimal Conditions
3.3. Enzyme Hydrolysis
3.4. Simultaneous Saccharification and Fermentation of Alkaline Pretreated Oil Palm Frond Fibers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Ge, X.; Chang, C.; Zhang, L.; Cui, S.; Luo, X.; Hu, S.; Qin, Y.; Li, Y. Conversion of Lignocellulosic Biomass into Platform Chemicals for Biobased Polyurethane Application. In Advances in Bioenergy; Li, Y., Ge, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 161–213. [Google Scholar]
- Malinee, R.; Stratoulias, D.; Nuthammachot, N. Detection of oil palm disease in plantations in krabi province, Thailand with high spatial resolution satellite imagery. Agriculture 2021, 11, 251. [Google Scholar] [CrossRef]
- Theerapong, J. The Path to Success is the Production of Palm Oil. Oil Palm Research and Development Center, Faculty of Natural Resources, Prince of Songkla University. 2016. Available online: http://www.natres.psu.ac.th/researchcenter/Palm-Research/menu/pic-book/2559-palmbook.pdf (accessed on 13 July 2022).
- Nutongkaew, P.; Waewsak, J.; Riansut, W.; Kongruang, C.; Gagnon, Y. The potential of palm oil production as a pathway to energy security in Thailand. Sustain. Energy Technol. Assess. 2019, 1, 189–203. [Google Scholar] [CrossRef]
- Murphy, D.J. The future of oil palm as a major global crop: Opportunities and challenges. J. Oil Palm Res. 2014, 1, 1–24. [Google Scholar]
- Schoneveld, G.C.; Ekowati, D.; Andrianto, A.; Haar, S.V.D. Modeling peat and forestland conversion by oil palm smallholders in Indonesian Borneo. Environ. Res. Lett. 2019, 14, 014006. [Google Scholar] [CrossRef] [Green Version]
- Corley, R.H.V.; Tinker, P.B. The Oil Palm, 5th ed.; Wiley-Blackwell Science: Oxford, UK, 2015; pp. 1–680. [Google Scholar]
- Moraidi, A.; Sung, C.T.; Joo, C.K.; Hanif, A.H.; Ishak, C.F. Evaluation of four soil conservation practices in a nonterraced oil palm plantation. Agron. J. 2012, 104, 1727–1740. [Google Scholar] [CrossRef] [Green Version]
- Awalludin, M.F.; Sulaiman, O.; Hashim, R.; Nadhari, W.N.A.W. An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew. Sust. Energ. Rev. 2015, 50, 1469–1484. [Google Scholar] [CrossRef]
- Tareen, A.K.; Punsuvon, V.; Parakulsuksatid, P. Conversion of steam exploded hydrolyzate of oil palm trunk to furfural by using sulfuric acid, solid acid, and base catalysts in one pot. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–12. [Google Scholar] [CrossRef]
- Rizal, N.; Ibrahim, M.F.; Zakaria, M.R.; Abd-Aziz, S.; Yee, P.L.; Hassan, M.A. Pretreatment of oil palm biomass for fermentable sugars production. Molecules 2018, 23, 1381. [Google Scholar] [CrossRef] [Green Version]
- Azmi, I.S.; Azizan, A.; Salleh, M.D. Pretreatment of oil palm frond (OPF) with ionic liquid. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012071. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, S.K.; Kim, J.H.; Park, S.Y.; Kim, J.C.; Jeong, H.; Kim, H.Y.; Choi, I.G. Simultaneous production of glucose, furfural, and ethanol organosolv lignin for total utilization of high recalcitrant biomass by organosolv pretreatment. Renew. Energy 2019, 130, 952–960. [Google Scholar] [CrossRef]
- Tareen, A.K.; Punsuvon, V.; Parakulsuksatid, P. Investigation of alkaline hydrogen peroxide pretreatment to enhance enzymatic hydrolysis and phenolic compounds of oil palm trunk. 3 Biotech 2020, 10, 179. [Google Scholar] [CrossRef]
- Devi, A.; Singh, A.; Bajar, S.; Pant, D.; Din, Z.U. Ethanol from lignocellulosic biomass: An in-depth analysis of pre-treatment methods, fermentation approaches and detoxification processes. J. Environ. Chem. Eng. 2021, 9, 105798. [Google Scholar] [CrossRef]
- Kuboon, S.; Kraithong, W.; Damaurai, J.; Faungnawakij, K. Hydro-fractionation for biomass upgrading. In Renewable Resources and Biorefineries; Jacob-Lopes, E., Zepka, Q., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Matsakas, L.; Raghavendran, O.Y.; Persson, G.; Olssonc, E.; Rova, U.; Olsson, E.; Christakopoulos, P. Lignin-first biomass fractionation using a hybrid organosolv—Steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour. Technol. 2018, 273, 521–528. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotech. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Koopraserting, P.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Effect of temperature and time of steam explosion on chemical compositions of oil palm frond. In Proceedings of the 50th Kasetsart University Annual Conference (Subject Agro—Industry) Kasetsart University, Bangkok, Thailand; 2012; pp. 362–369. [Google Scholar]
- Wilaithup, A.; Sultan, I.N.; Tareen, A.K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Bioethanol production from oil palm trunk fibers using activated immobilized Saccharomyces cerevisiae SC90 under simultaneous saccharification and fermentation. Bioenerg. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Fan, Z.; Lin, J.; Wu, J.; Zhang, L.; Lyu, X.; Xiao, W.; Gong, Y.; Xu, Y.; Liu, Z. Vacuum-assisted black liquor-recycling enhances the sugar yield of sugarcane bagasse and decreases water and alkali consumption. Bioresour. Technol. 2020, 309, 123349. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, S.; Mier-Alba, E.; da Silva, S.S.; Chandel, A.K. Commercial Washing Detergents-Assisted Alkaline Pretreatment for Lignocellulosic Sugars Production: A First Report. Sugar Tech. 2021, 23, 1425–1431. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Bisht, A.; Pagolu, R.; Lai, C.; Lestari, R.; Kumar, A.; Das, D.; Kalia, V.C.; Lee, J.K. Mild Alkaline Pretreatment of Rice Straw as a Feedstock in Microbial Fuel Cells for Generation of Bioelectricity. Indian J. Microbiol. 2022, 62, 447–455. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Salini, C.N.; Sindhu, R.; Pandey, A.; Binod, P. Simultaneous saccharification and fermentation of oil palm front for the production of 2,3-butanediol. Bioresour. Technol. 2019, 278, 145–149. [Google Scholar] [CrossRef]
- Brethauer, S.; Wyman, C.E. Review: Continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour. Technol. 2010, 101, 4862–4874. [Google Scholar] [CrossRef]
- Kadar, Z.; Szengyel, Z.; Reczey, K. Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind. Crops Prod. 2004, 20, 103–110. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, X.; Li, H.; Du, X.; Liang, S.; Zhao, X. Screening and Mutation of Saccharomyces cerevisiae UV-20 with a High Yield of Second Generation Bioethanol and High Tolerance of Temperature, Glucose and Ethanol. Indian J. Microbiol. 2018, 58, 440–447. [Google Scholar] [CrossRef]
- Deepanraj, B.; Bivasubramanian, V.; Jayaraj, S. Multi-response optimization of process parameters in biogas production from food waste using taguchi-grey relational analysis. Energy Convers. Manag. 2017, 141, 429. [Google Scholar] [CrossRef]
- Taguchi, G.; Konishi, S. Taguchi Methods Orthogonal Arrays and Linear Graphs: Tools for Quality Engineering; American Supplier Institute: Dearborn, MI, USA, 1987. [Google Scholar]
- Sultan, I.N.; Khienpanya, N.; Tareen, A.K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatida, P. Kinetic study of ethanol production from different sizes of two-step pretreated oil palm trunk by fed-batch simultaneous saccharification and fermentation. Agric. Nat. Resour. 2022, 56, 287–297. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory Technical Report, NREL/TP510-42629; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Tareen, A.K.; Punsuvon, V.; Sultan, I.N.; Khan, M.W.; Parakulsuksatid, P. Cellulase addition and pre-hydrolysis effect of high solid fed-batch simultaneous saccharification and ethanol fermentation from a combined pretreated oil palm trunk. ACS Omega 2021, 6, 26119–26129. [Google Scholar] [CrossRef]
- Boateng, O.C.; Lee, K.T. Same-vessel enzymatic saccharification and fermentation of organosolv/H2O2 pretreated oil palm (Elaeis guineensis Jacq.) fronds for bioethanol production: Optimization of process parameters. Energy Convers. Manag. 2014, 78, 421–430. [Google Scholar] [CrossRef]
- Boonsawang, P.; Subkaree, Y.; Srinorakutara, T. Ethanol production from palm pressed fiber by prehydrolysis prior to simultaneous saccharification and fermentation (SSF). Biomass Bioenergy 2012, 40, 127–132. [Google Scholar] [CrossRef]
- Hong, L.S.; Ibrahim, D.; Omar, C.I. Oil palm frond for the production of bioethanol. Int. J. Biochem. Biotechnol. 2012, 1, 7–11. [Google Scholar] [CrossRef]
- Danbamrongtrakool, N.; Sultan, I.N.; Laemsak, N.; Tareen, A.K.; Sirisansaneeyakul, S.; Parakulsuksatid, P. Utilization of urea as a nitrogen source for ethanol production from oil palm trunk using simultaneous saccharification and fermentation. Agric. Nat. Resour. 2022, 56, 175–186. [Google Scholar] [CrossRef]
- Asghar, A.; Abdul Raman, A.A.; Daud, W.M.A.W.A. Comparison of Central Composite Design and Taguchi Method for Optimizing Fenton Process. Sci. World J. 2014, 2014, 869120. [Google Scholar] [CrossRef] [Green Version]
- Tareen, A.K.; Sultan, I.N.; Songprom, K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Two-step pretreatment of oil palm trunk for ethanol production by thermotolerent Saccharomyces cerevisiae SC90. Bioresour. Technol. 2021, 320, 124298. [Google Scholar] [CrossRef]
- Nlewem, K.C.; Thrash, M.E. Comparison of different pretreatment methods based on residual lignin effect on the enzymatic hydrolysis of switchgrass. Bioresour. Technol. 2010, 101, 5426–5430. [Google Scholar] [CrossRef]
- Schoenherr, S.; Ebrahimi, M.; Czermak, P. Lignin degradation processes and the purification of valuable products. In Trends and Applications; Poletto, M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Barlianti, V.; Dahnum, D.; Hendarsyah, H.; Abimanyu, H. Effect of alkaline pretreatment on properties of lignocellulosic oil palm waste. Procedia Chem. 2015, 16, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, H.; Moniruzzaman, M.; Yusup, S.; Akil, H.M. Ionic liquid pretreatment at high solids loading: A clean approach for fabrication of renewable resource based particulate composites. Polym. Compos. 2018, 39, 1994–2003. [Google Scholar] [CrossRef]
- Wang, X.; Lin, L.; Tang, Y.; Xia, H.; Zhang, X.; Yue, M.; Qiu, X.; Xu, K.; Wang, Z. Transcriptomic insights into citrus segment membrane’s cell wall components relating to fruit sensory texture. BMC Genom. 2018, 19, 280. [Google Scholar] [CrossRef]
- Pezoa, R.; Cortinez, V.; Hyvarinen, S. Use of ionic liquids in the pretreatment of forest and agricultural residues for the production of bioethanol. Cell. Chem. Technol. 2010, 44, 165–172. [Google Scholar]
- Zhang, H.; Huang, S.; Wei, W.; Zhang, J.; Xie, J. Investigation of alkaline hydrogen peroxide pretreatment and Tween 80 to enhance enzymatic hydrolysis of sugarcane bagasse. Biotechnol. Biofuels 2019, 12, 2–9. [Google Scholar] [CrossRef]
- Cara, C.; Moya, M.; Ballesteros, I.; Negro, M.J.; Gonzalez, A.; Ruiza, E. Influence of solid loading on enzymatic hydrolysis of steam exploded or liquid hot water pretreated olive tree biomass. Process Biochem. 2007, 42, 1003–1009. [Google Scholar] [CrossRef]
- Reed, G.; Peppler, H.J. Yeast Technology; The AVI Publication Company Inc.: Westport, CT, USA, 1973; Volume 19, p. 380. [Google Scholar] [CrossRef]




| Levels | A: Concentration of Sodium Hydroxide (%) | B: Temperature (°C) | C: Time (Minutes) | |||
|---|---|---|---|---|---|---|
| 1 | 15 | 70 | 30 | |||
| 2 | 20 | 80 | 60 | |||
| 3 | 25 | 90 | 90 | |||
| Experiment No. | L9 (33) | |||||
| A | B | C | NaOH (%) | Temperature (°C) | Time (min) | |
| 1 | 1 | 1 | 1 | 15 | 70 | 30 |
| 2 | 1 | 2 | 2 | 15 | 80 | 60 |
| 3 | 1 | 3 | 3 | 15 | 90 | 90 |
| 4 | 2 | 1 | 2 | 20 | 70 | 60 |
| 5 | 2 | 2 | 3 | 20 | 80 | 90 |
| 6 | 2 | 3 | 1 | 20 | 90 | 30 |
| 7 | 3 | 1 | 3 | 25 | 70 | 90 |
| 8 | 3 | 2 | 1 | 25 | 80 | 30 |
| 9 | 3 | 3 | 2 | 25 | 90 | 60 |
| Experiment No. | Factor | Chemical Composition (%) | |||
|---|---|---|---|---|---|
| NaOH (%) | Temperature (°C) | Time (Min) | Cellulose | Lignin | |
| 1 | 15 | 70 | 30 | 78.81 | 16.21 |
| 2 | 15 | 80 | 60 | 80.74 | 17.07 |
| 3 | 15 | 90 | 90 | 77.79 | 16.31 |
| 4 | 20 | 70 | 60 | 74.88 | 20.40 |
| 5 | 20 | 80 | 90 | 74.16 | 20.07 |
| 6 | 20 | 90 | 30 | 79.58 | 19.97 |
| 7 | 25 | 70 | 90 | 68.45 | 19.55 |
| 8 | 25 | 80 | 30 | 56.80 | 21.46 |
| 9 | 25 | 90 | 60 | 71.07 | 21.13 |
| Cellulose | ||||||
| Factor | DOF | SS | MS | F | S’ | Contribution (%) |
| Concentration of sodium hydroxide (%) | 2 | 5.33 | 2.67 | 3.78 | 3.92 | 79.08 |
| Temperature (°C) | 2 | 0.45 | 0.23 | 0.32 | 0 | 24.24 |
| Time (minutes) | 2 | 0.54 | 0.27 | 0.38 | 0 | 27.58 |
| Error | 2 | 1.41 | 0.71 | |||
| Total | 8 | 7.73 | 100 | |||
| Lignin | ||||||
| Factor | DOF | SS | MS | F | S’ | Contribution (%) |
| Concentration of sodium hydroxide (%) | 2 | 6.92 | 3.46 | 136.54 | 6.87 | 92.3 |
| Temperature (°C) | 2 | 0.24 | 0.12 | 4.7 | 0.19 | 2.52 |
| Time (minutes) | 2 | 0.23 | 0.12 | 4.6 | 0.18 | 2.45 |
| Error | 2 | 0.05 | 0.03 | |||
| Total | 8 | 7.47 | 100 | |||
| Factor | Factor Levels Optimized for Chemical Composition | |
|---|---|---|
| Cellulose | Lignin | |
| Concentration of sodium hydroxide (%) | 15 | 15 |
| Temperature (°C) | 90 | 70 |
| Time (min) | 60 | 90 |
| Yexpected | Predicted chemical composition based on optimal condition | |
| Cellulose | ||
| Lignin | ||
| Parameter | Cellulose | Lignin | ||
|---|---|---|---|---|
| Predicted | Experimental | Predicted | Experimental | |
| CON-1 | ||||
| Chemical composition (%) | 83.38 | 80.74 | 16.88 | 15.99 |
| Yexpected + CI | 71.08 < Yexpected < 97.80 | 14.39 < Yexpected < 19.81 | ||
| CON-2 | ||||
| Chemical composition (%) | 81.26 | 74.57 | 15.79 | 15.14 |
| Yexpected + CI | 78.84 < Yexpected < 83.76 | 15.32 < Yexpected < 16.27 | ||
| Pretreatment Steps | Yield (%) | Chemical Composition (%) | |||
|---|---|---|---|---|---|
| % Dry Weight of α-Cellulose | % Dry Weight of Lignin | % Dry Weight of Ash | % Dry Weight of Pentosan | ||
| Raw materials | 100 | 42.51 | 30.88 | 1.82 | 28.11 |
| Steam explosion | 82.55 | 36.21 | 30.32 | 1.48 | 7.41 |
| Water extraction | 49.46 | 47.78 | 32.17 | 1.44 | 6.10 |
| Alkaline extraction | 21.57 | 80.74 | 15.99 | 1.05 | 2.09 |
| Raw Material | Pretreatment Type | Fermentation Type | Fermentation Temperature (°C) | Substrate Concentration | Nitrogen Source | Ethanol Concentration | Reference |
|---|---|---|---|---|---|---|---|
| Oil palm fronds | Steam explosion and alkaline extraction | Batch SSF | 40 | 10% (w/v) | Yeast extract and peptone | C: 33.15 g/L | Current study |
| Oil palm fronds | Steam explosion and alkaline extraction | Batch SSF | 45 | 10% (w/v) | Yeast extract and peptone | C: 27.62 g/L | Current study |
| Oil palm trunk fiber | Steam explosion and alkaline extraction | Batch SSF | 40 | 10% (w/v) | Without nitrogen source | C: 29.68 g/L | [21] |
| Oil palm trunk | Steam explosion and alkaline hydrogen peroxide treatment | Fed batch fermentation | 40 | 10% (w/v) | Yeast extract and peptone | C: 31.77 g/L | [34] |
| Oil palm fronds | Organosolv/H2O2 pretreated | Batch fermentation | 30–50 | 8% (w/v) | Yeast extract and peptone | C: 20.61 g/L | [35] |
| Palm pressed | Alkali pretreated | Batch SSF | 35 | 10% (w/v) | Yeast extract, KH2PO4, (NH4)2SO4, and MgSO4 7H2O | C: 10.4 kg/m3 | [36] |
| Oil palm fronds | Biological pretreatment | Batch fermentation | 30 | 6% (w/v) | Potato dextrose and agar | C: 23.10 g/L | [37] |
| Oil palm trunk | Steam explosion and alkaline hydrogen peroxide treatment | Batch fermentation | 40 | 10% (w/v) | Urea | C: 37.41 g/L | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kooprasertying, P.; Vanichsriratana, W.; Sirisansaneeyakul, S.; Laemsak, N.; Tareen, A.K.; Ullah, Z.; Parakulsuksatid, P.; Sultan, I.N. Ethanol Production through Optimized Alkaline Pretreated Elaeis guineensis Frond Waste from Krabi Province, Thailand. Fermentation 2022, 8, 648. https://doi.org/10.3390/fermentation8110648
Kooprasertying P, Vanichsriratana W, Sirisansaneeyakul S, Laemsak N, Tareen AK, Ullah Z, Parakulsuksatid P, Sultan IN. Ethanol Production through Optimized Alkaline Pretreated Elaeis guineensis Frond Waste from Krabi Province, Thailand. Fermentation. 2022; 8(11):648. https://doi.org/10.3390/fermentation8110648
Chicago/Turabian StyleKooprasertying, Poomhatai, Wirat Vanichsriratana, Sarote Sirisansaneeyakul, Nicom Laemsak, Afrasiab Khan Tareen, Zahoor Ullah, Pramuk Parakulsuksatid, and Imrana Niaz Sultan. 2022. "Ethanol Production through Optimized Alkaline Pretreated Elaeis guineensis Frond Waste from Krabi Province, Thailand" Fermentation 8, no. 11: 648. https://doi.org/10.3390/fermentation8110648