A Review on Potential Biofuel Yields from Cover Crops
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Cover Crop for Biodiesel Production
| Methods | Description of Methods and Results | Ref. | |
|---|---|---|---|
| Plant improvements | Breeding and gene editing | Gene editing tools such as CRISPR-Cas9 to develop traits with low seed coat fiber content and low erucic acid seed oil. | [44,45,46] |
| Agronomic management | Soil fertility and health | Apply fertilizer and/amendment to improve soil fertility & health. | [47,48] |
| Irrigation and drainage | Manage irrigation and drainage to maintain soil moisture and nutrients and control groundwater levels. | [49,50] | |
| Oil extraction | Mechanical extraction | Press at a pressure of 30–40 bar and temperatures of ~95 °C. | [51] |
| Solvent oil extraction | Organic solvents i.e., n-hexane to speed up oil diffusion process. | ||
| Enzymatic oil extraction | Enzymes such as hemicellulases, cellulases to hydrolyze cell wall. | ||
| Supercritical fluid extraction | High-efficiency oil extract oil using solvents such as CO2 at a supercritical condition. | ||
| Conversion | Conventional esterification | Esterification or transesterification in the presence of alcohol. It can be acids, alkali, or enzyme-catalyzed. | [52] |
| Supercritical transesterification | Transesterification under a supercritical condition with no or reduced amount of catalyst. | ||
| Superheated conversion | Reaction at a high temperature and is rapid. | ||
3.2. Cover Crop for Ethanol Production
3.3. Cover Crop for Biogas Production
3.4. Cover Crop for Syngas and Bio-Oil Production
| Feedstock | Conditions | Non-Condensable Gas Yield, wt% of Dry Biomass | Bio-Oil Yield, wt% of Dry Biomass | Ref. |
|---|---|---|---|---|
| Alfalfa stems (early bud) | Fluidized-bed, fast pyrolysis | 16.3 | 45 | [93] |
| Alfalfa stems (full flower) | 12.8 | 53 | [93] | |
| Fodder radish seed cake | Slow pyrolysis | 16.2 | 57.8 | [99] |
| Rapeseed seed cake | Fixed bed reactor | 8–14 | 48–62 | [100] |
| Rapeseed seed cake | Flash pyrolysis | 24 | 47 | [101] |
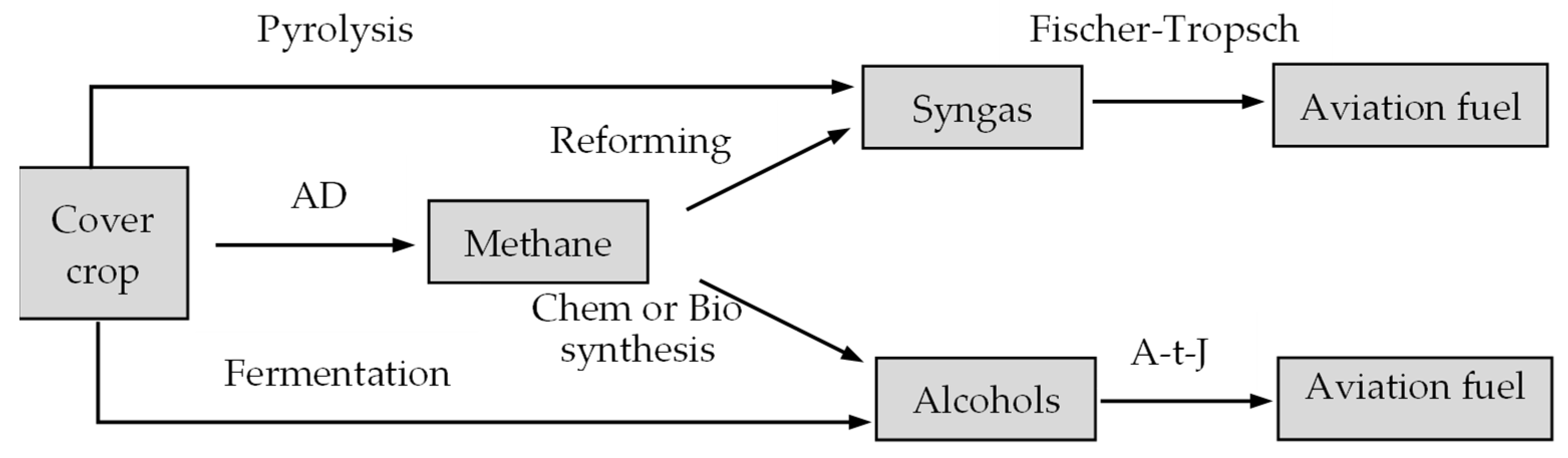
3.5. Cover Crop for SAF Production
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallander, S.; Smith, D.; Bowman, M.; Classsen, R. Cover Crop Trends, Programs, and Practices in the United States; U.S. Department of Agriculture: Washington, DC, USA, 2021.
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Change Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.; Quemada, M. Using cover crops to mitigate and adapt to climate change. A review. Agron. Sustain. Dev. 2017, 37, 4. [Google Scholar] [CrossRef]
- Chahal, I.; Vyn, R.; Mayers, D.; Eerd, L. Cumulative impact of cover crops on soil carbon sequestration and profitability in a temperate humid climate. Sci. Rep. 2020, 10, 13381. [Google Scholar] [CrossRef] [PubMed]
- USDA-ARS. Cover crop chart V4.0. Available online: https://www.ars.usda.gov/plains-area/mandan-nd/ngprl/ (accessed on 10 September 2023).
- Hamilton, A.; Mortensen, D.A.; Allen, M.K. The state of the cover crop nation and how to set realistic goals for the popular conservation practice. J. Soil Water Conserv. 2017, 72, 111A–115A. [Google Scholar] [CrossRef]
- Quinn, L. Cover Cropping Up to 7.2% in U.S. Midwest, Boosted by Government Programs. Available online: https://aces.illinois.edu/news/cover-cropping-72-us-midwest-boosted-government-programs (accessed on 10 March 2023).
- Fendrich, A.N.; Matthews, F.; Eynde, E.V.; Carozzi, M.; Li, Z.; d’Andrimont, R.; Lugato, E.; Martin, P.; Ciais, P.; Panagos, P. From regional to parcel scale: A high-resolution map of cover crops across Europe combining satellite data with statistical surveys. Sci. Total Environ. 2023, 873, 162300. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Werf, W.; Makowski, D.; Lamichhane, J.R.; Huang, W.; Li, C.; Zhang, C.; Cong, W.; Zhang, F. Cover crops promote primary crop yield in China: A meta-regression of factors affecting yield gain. Field Crops Res. 2021, 271, 108237. [Google Scholar] [CrossRef]
- Higashi, T.; Yunghui, M.; Komatsuzaki, M.; Miura, S.; Hirata, T.; Araki, H.; Kaneko, N.; Ohta, H. Tillage and cover crop species affect soil organic carbon in Andosol, Kanto, Japan. Soil Tillage Res. 2014, 138, 64–72. [Google Scholar] [CrossRef]
- Burnette, R. The Use of Green Manure/Cover Crops for Relay Cropping in Northern Thailand. Available online: https://www.echocommunity.org/en/resources/4354866e-8cf0-4a07-aaa1-a9419b076db7 (accessed on 10 September 2023).
- Smit, E.H.; Strauss, J.A.; Swanepoel, P.A. Utilisation of cover crops: Implications for conservation agriculture systems in a mediterranean climate region of South Africa. Plant Soil. 2021, 462, 207–218. [Google Scholar] [CrossRef]
- USDA. Oilseeds: World Markets and Trade. U.S. Department of Agriculture. Washington, USA. Available online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (accessed on 10 September 2023).
- Sykes, V.R.; Wilson, A.; Bates, G.; McIntosh, D.; McClure, A.T.; Raper, T.; Blair, R.; Walker, F. Cover Crop Variety Tests in Tennessee 2020. Available online: Search.utcrops.com (accessed on 10 March 2023).
- Shao, X.; DiMarco, K.; Richard, T.L.; Lynd, L.R. Winter rye as a bioenergy feedstock: Impact of crop maturity on composition, biological solubilization and potential revenue. Biotechnol. Biofuels 2015, 8, 35. [Google Scholar] [CrossRef]
- House, T.W. FACT SHEET: President Biden Signs Executive Order Catalyzing America’s Clean Energy Economy Through Federal Sustainability. Available online: https://www.whitehouse.gov/briefing-room/statements-releases/2021/12/08/fact-sheet-president-biden-signs-executive-order-catalyzing-americas-clean-energy-economy-through-federal-sustainability/ (accessed on 10 March 2023).
- Xu, H.; Ou, L.; Li, Y.; Hawkins, T.; Wang, M. Life Cycle Greenhouse Gas Emissions of Biodiesel and Renewable Diesel Production in the United States. Environ. Sci. Technol. 2022, 56, 7512–7521. [Google Scholar] [CrossRef]
- Statista. Production Volume of Biodiesel in the United States from 2001 to 2022. Available online: https://www.statista.com/statistics/509875/production-volume-of-biodiesel-in-the-us/ (accessed on 10 June 2023).
- ERS. U.S. Bioenergy Statistics. Available online: https://www.ers.usda.gov/data-products/u-s-bioenergy-statistics/ (accessed on 10 September 2023).
- Gesch, R.W.; Long, D.S.; Palmquist, D.; Allen, B.L.; Archer, D.W.; Brown, J.; Davis, J.B.; Hatfield, J.L.; Jabro, J.D.; Kiniry, J.R.; et al. Agronomic performance of Brassicaceae oilseeds in multiple environments across the Western USA. Bioenergy Res. 2019, 12, 509–523. [Google Scholar] [CrossRef]
- Blackshaw, R.; Johnson, E.; Gan, Y.; May, W.; McAndrew, D.; Barthet, V.; McDonald, T.; Wispinski, D. Alternative oilseed crops for biodiesel feedstock on the Canadian prairies. Can. J. Plant Sci. 2011, 91, 889–896. [Google Scholar] [CrossRef]
- Iboyi, J.; Mulvaney, M.; Balkcom, K.; Wright, D. Tillage system and seeding rate effects on the performance of Brassica carinata. Bioenergy 2021, 13, 600–617. [Google Scholar] [CrossRef]
- Blume, R.Y.; Rakhmetov, D.B.; Blume, Y.B. Evaluation of Ukrainian Camelina sativa germplasm productivity and analysis of its amenability for efficient biodiesel production. Ind. Crops Prod. 2022, 187, 115477. [Google Scholar] [CrossRef]
- Bronson, K.; hunsaker, D.; Thorp, K. Nitrogen Fertilizer and Irrigation Effects on Seed Yield and Oil in Camelina. Agronomy J. 2019, 111, 1712–1719. [Google Scholar] [CrossRef]
- Chaganti, V.; Ganjegunte, G.; Niu, G.; Ulery, A.; Enciso, J.; Flynn, R.; Meki, N.; Kiniry, J. Yield response of canola as a biofuel feedstock and soil quality changes under treated urban wastewater irrigation and soil amendment application. Ind. Crops Prod. 2021, 170, 113659. [Google Scholar] [CrossRef]
- Katuwal, K.B.; Angadi, S.V.; Singh, S.; Cho, Y.; Begna, S.; Umesh, M.R. Growth-stage-based irrigation management on biomass, yield, and yield attributes of spring canola in the southern Great Plains. Crop Sci. 2018, 58, 2623–2632. [Google Scholar] [CrossRef]
- Kandel, H. Tama Mustard Production. Available online: https://www.ndsu.edu/agriculture/ag-hub/publications/tame-mustard-production (accessed on 10 June 2023).
- Darapuneni, M.K.; Morgan, G.D.; Ibrahim, A.M.; Duncan, R.W. Evaluation of Flax Genotypes for Cold Tolerance and Yield in South-East Texas. J. Agron. Crop Sci. 2015, 201, 128–137. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, Z.; Niu, Z.; Coulter, J.; Niu, J.; Zhang, J.; Wang, B.; Yan, B.; Zhao, W.; Wang, L. Yield, oil content, and fatty acid profile of flax (Linum usitatissimum L.) as affected by phosphorus rate and seeding rate. Ind. Crops Prod. 2020, 145, 112087. [Google Scholar] [CrossRef]
- Gallardo, M.; Milisich, H.J.; Drago, S.R.; Gonzalez, R.J.; Kuk, Y.I. Effect of Cultivars and Planting Date on Yield, Oil Content, and Fatty Acid Profile of Flax Varieties (Linum usitatissimum L.). Intern. J. Agron. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Sedbrook, J.C.; Phippen, W.B.; Marks, M.D. New approaches to facilitate rapid domestication of a wild plant to an oilseed crop: Example pennycress (Thlaspi arvense L.). Plant Sci. 2014, 227, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.; Isbell, T.; Gesch, R.; Evangelista, R.; Alexopoulou, E.; Moser, B.; Monti, A. Turning a burden into an opportunity: Pennycress (Thlaspi arvense L.) a new oilseed crop for biofuel production. Biomass Bioenergy 2019, 130, 105354. [Google Scholar] [CrossRef]
- Lopez, M.; Vega, M.; Gracia, R.; Claver, A.; Alfonso, M. Agronomic potential of two European pennycress accessions as a winter crop under European Mediterranean conditions. Ind. Crops Prod. 2021, 159, 113107. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Shanmugam, M. Yield and oil content of peanut (var. TMV-7) and sunflower (var. Co-2) applied with bio-stimulant AQUASAP manufactured from seaweed. Afr. J. Agric. Res. 2015, 10, 1031–1042. [Google Scholar]
- Khudaykulov, J.; Umarova, Z.; Jong, Y. Effect of irrigation procedures on yield and oil concentration of peanut. IOP Conf. Ser. Earth Environ. Sci. 2023, 1142, 012045. [Google Scholar] [CrossRef]
- AgMRC. Peanuts. Available online: https://www.agmrc.org/commodities-products/nuts/peanut-profile (accessed on 10 September 2023).
- HAPA. Sunflower and Palm Oil Value Chain Analysis in Tanzania: Identifying forward and Backward Linkages, Challenges and Opportunities for Economic Growth; The Hub for Agricultural Policy Action (HAPA), AGRA: Nairobi, Kenya, 2022. [Google Scholar]
- Brazil, O.A.; Vilanova-Neta, J.L.; Silva, N.O.; Vieira, I.M.; Lima, A.S.; Ruzene, D.S.; Silva, D.P.; Figueiredo, R.T. Integral use of lignocellulosic residues from different sunflower accessions: Analysis of the production potential for biofuels. J. Clean. Prod. 2019, 221, 430–438. [Google Scholar] [CrossRef]
- Barontini, F.; Simone, M.; Triana, F.; Mancini, A.; Ragaglini, G.; Nocolella, C. Pilot-scale biofuel production from sunflower crops in centra lItaly. Renew. Energy 2015, 83, 954–962. [Google Scholar] [CrossRef]
- Naeve, S.; Miller-Garvin, J.; Christenson, J. 2022 United States Soybean Qaulity Annual Report; American Soybean Association: St. Louis, MO, USA, 2022; Available online: https://extension.umn.edu/soybean/soybean-seed-quality (accessed on 10 September 2023).
- Bayer. Investing in Climate Solutions. Available online: https://www.bayer.com/en/agriculture/cover-cress (accessed on 10 September 2023).
- Covercress. CoverCress Inc. Names New CEO as Winter Oilseed Edges Closer to Commercialization. Available online: https://covercress.com/pressrelease/pressRelease_20210513_ceo.pdf (accessed on 10 October 2023).
- Rezki, B.; Essamlali, Y.; Aadil, M.; Semlal, N.; Zahouily, M. Biodiesel production from rapeseed oil and low free fatty acid waste cooking oil using a cesium modified natural phosphate catalyst. RSC Adv. 2020, 10, 41065–41077. [Google Scholar] [CrossRef]
- Taylor, D.C.; Zhang, Y.; Kumar, A.; Francis, T.; Giblin, E.M.; Barton, D.L.; Ferrie, J.R.; Laroche, A.; Shah, S.; Zhu, W.; et al. Molecular modification of triacylglycerol accumulation by over-expression of DGAT1 to produce canola with increased seed oil content under field conditionsThis paper is one of a selection of papers published in a Special Issue from the National Research Council of Canada—Plant Biotechnology Institute. Botany 2009, 87, 533–543. [Google Scholar] [CrossRef]
- Phippen, W.B.; Rhykerd, R.; Sedbrook, J.C.; Handel, C.; Csonka, S. From Farm to Flight: CoverCress as a Low Carbon Intensity Cash Cover Crop for Sustainable Aviation Fuel Production. A Review of Progress Towards Commercialization. Front. Energy Res. 2022, 10, 939. [Google Scholar] [CrossRef]
- Dorn, K.M.; Fankhauser, J.D.; Wyse, D.L.; Marks, M.D. De novo assembly of the pennycress (Thlaspi arvense) transcriptome provides tools for the development of a winter cover crop and biodiesel feedstock. Plant J. Cell Mol. Biol. 2014, 75, 1028–1038. [Google Scholar] [CrossRef]
- Li, N.; Kumar, P.; Lai, L. Response of Soil Greenhouse Gas Fluxes and Soil Properties to Nitrogen Fertilizer Rates under Camelina and Carinata Nonfood Oilseed Crops. Bioenerg. Res. 2019, 12, 524–535. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, K.; Verma, P. Effect of nitrogen and zinc nanofertilizer with the organic farming practices on cereal and oil seed crops. Sci. Rep. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Shi, H.; Li, R.; Miao, Q.; Tian, F.; Yu, D.; Zhou, L.; Wang, B. Effects of Controlled Drainage on the Content Change and Migration of Moisture, Nutrients, and Salts in Soil and the Yield of Oilseed Sunflower in the Hetao Irrigation District. Sustainability 2021, 13, 9835. [Google Scholar] [CrossRef]
- Lohaus, R.H.; Neupane, D.; Mengistu, M.A.; Solomon, J.K.; Cushman, J.C. Five-Year Field Trial of Eight Camelina sativa Cultivars for Biomass to be Used in Biofuel under Irrigated Conditions in a Semi-Arid Climate. Agronomy 2020, 10, 562. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.; de Souza Vandenberghe, L.P.; Soccol, C.R. Oilseed enzymatic pretreatment for efficient oil recovery in biodiesel production industry: A review. BioEnergy Res. 2020, 13, 1016–1030. [Google Scholar] [CrossRef]
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Yu, H.; Liu, C.; Dixon, R. A gene-editing/complementation strategy for tissue-specific lignin reduction while preserving biomass yield. Biotechnol. Biofuels 2021, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Subedi, U.; Jayawardhane, K.; Pan, X.; Ozga, J.; Chen, G.; Foroud, N.; Singer, S. The Potential of Genome Editing for Improving Seed Oil Content and Fatty Acid Composition in Oilseed Crops. Lipids 2020, 55, 495–512. [Google Scholar] [CrossRef]
- MCCC. Cover Crops: Selector Tools. Available online: https://www.midwestcovercrops.org/selector-tools/ (accessed on 1 September 2023).
- Ahangari, H.; King, J.; Ehsani, A.; Yousefi, M. Supercritical fluid extraction of seed oils—A short review of current trends. Trend Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Farobie, O.; Leow, Z.M.; Samanmulya, T.; Matsumura, Y. New insights in biodiesel production using supercritical 1-propanol. Energy Convers. Manag. 2016, 124, 212–218. [Google Scholar] [CrossRef]
- EPA. Overview for Renewable Fuel Standard. Available online: https://www.epa.gov/renewable-fuel-standard-program/overview-renewable-fuel-standard (accessed on 10 August 2023).
- Tumbalam, P.; Thelen, K.; Adkins, A.; Dale, B.; Balan, V.; Gunawan, C.; Gao, J. Corn stover ethanol yield as affected by grain yield, Bt trait, and environment. Biomass Bioenergy 2016, 85, 119–125. [Google Scholar] [CrossRef]
- Saha, B.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Proc. Biochem. 2005, 40, 3693–3700. [Google Scholar] [CrossRef]
- Petersson, A.; Thomsen, M.H.; Hauggaard-Nielsen, H.; Thomsen, A.B. Potential bioethanol and biogas production using lignocellulosic biomass from winter rye, oilseed rape and faba bean. Biomass Bioenergy 2007, 31, 812–819. [Google Scholar] [CrossRef]
- Tan, L.; Zhong, J.; Jin, Y.L.; Sun, Z.Y.; Tang, Y.Q.; Kida, K. Production of bioethanol from unwashed-pretreated rapeseed straw at high solid loading. Bioresour. Technol. 2020, 303, 122949. [Google Scholar] [CrossRef] [PubMed]
- Kuglarz, M.; Alvarodo-Morales, M.; Dabkowska, K.; Angelidaki, I. Integrated production of cellulosic bioethanol and succinic acid from rapeseed straw after dilute-acid pretreatment. Bioresour. Technol. 2018, 265, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Passoth, V.; Schnurer, A.; Sandgren, M.; Stahlberg, J. Improved bio-energy yields via sequential ethanol fermentation and biogas digestion of steam exploded oat straw. Bioresour. Technol. 2011, 102, 4449–4455. [Google Scholar] [CrossRef]
- Springer, T.L.; Aiken, G.E. Harvest frequency effects on white clover forage biomass, quality, and theoretical ethanol yield. Biomass Bioenergy 2015, 78, 1–5. [Google Scholar] [CrossRef]
- Cifuentes, R.; Bressani, R.; Rolz, C. The potential of sweet sorghum as a source of ethanol and protein. Energy Sustain. Dev. 2014, 21, 13–19. [Google Scholar] [CrossRef]
- Castro, E.; Nieves, I.U.; Rondon, V.; Sagues, W.J.; Fernandez-Sandoval, M.T.; Yomano, L.P.; York, S.W.; Erickson, J.; Vermerris, W. Potential for ethanol production from different sorghum cultivars. Ind. Crops Prod. 2017, 109, 367–373. [Google Scholar] [CrossRef]
- Rose, D.J.; Santra, D.K. Proso millet (Panicum miliaceum L.) fermentation for fuel ethanolproduction. Ind. Crops Prod. 2013, 43, 602–605. [Google Scholar] [CrossRef]
- Lukajtis, R.; Kucharska, K.; Holowacz, I.; Rybarczyk, P.; Wychodnik, K.; Slupek, E.; Nowak, P.; Kaminski, M. Comparison and Optimization of Saccharification Conditions of Alkaline Pre-Treated Triticale Straw for Acid and Enzymatic Hydrolysis Followed by Ethanol Fermentation. Energies 2018, 11, 639. [Google Scholar] [CrossRef]
- Gohel, V.; Duan, G. No-Cook Process for Ethanol Production Using Indian Broken Rice and Pearl Millet. Intern. J. Microbiol. 2012, 2012, 680232. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Thomas, K.C.; Ingledew, W.M. Production of fuel ethanol from rye and triticale by very-high-gravity (VHG) fermentation. Appl. Biochem. Biotechnol. 1998, 69, 157–175. [Google Scholar] [CrossRef]
- Varize, C.; Bucker, A.; Lopes, L.; Christofoleti-Furlan, R.; Raposo, M.; Basso, L.; Stambuk, B. Increasing Ethanol Tolerance and Ethanol Production in an Industrial Fuel Ethanol Saccharomyces cerevisiae Strain. Fementation 2022, 8, 470. [Google Scholar] [CrossRef]
- Tutt, M.; Kikas, T.; Olt, J. Comparison of different pretreatment methods on degradation of rye straw. In Proceedings of the 11th International Scientific Conference: Engineering for Rural Development, Jelgava, Latvia, 24–25 May 2012. [Google Scholar]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Biores. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Ampese, L.; Sganzerla, W.G.; Ziero, H.D. Research progress, trends, and updates on anaerobic digestion technology: A bibliometric analysis. J. Cleaner Produc. 2022, 331, 130004. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential—Global and Irish perspective. Renew. Sustain. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Ervasti, S.; Kostensalo, J.; Tampio, E. Effects of seasonal and local co-feedstocks on the performance of continuous anaerobic digestion of cattle slurry. Bioresour. Technol. Rep. 2022, 19, 101207. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Ge, X.; Yang, L.; Sheet, J.P.; Yu, Z.; Li, Y. Biological conversion of methane to liquid fuels: Status and opportunities. Biotechnol. Adv. 2014, 32, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 44, 824–834. [Google Scholar] [CrossRef]
- Yang, L.; Kopsell, D.E.; Kottke, A.M.; Johnson, M.Q. Development of a cartridge design anaerobic digestion system for lignocellulosic biomass. Biosys. Eng. 2017, 160, 134–139. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y. Anaerobic digestion of giant reed for methane production. Bioresour. Technol. 2014, 171, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2014, 178, 178–186. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.J.; Mushtaq, M.; Borja, R. Evaluation and modelling of methane production from corn stover pretreated with various physicochemical techniques. Waste Manag. Res. 2021, 40, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Domanski, J.; marchut-Mikolajczyk, O.; Cieciura-Wloch, W.; Paterlski, P. Production of Methane, Hydrogen and Ethanol from Secale cereale L. Straw Pretreated with Sulfuric Acid. Molecules 2020, 25, 1013. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lamont, L.; Sedbrook, J.; Heller, N.; Kopsell, D. Anaerobic Digestion of Cereal Rye Cover Crop. Fementation 2022, 8, 617. [Google Scholar] [CrossRef]
- Vlierberghe, C.V.; Escudie, R.; Bernet, N.; Frederic, S.; Carrere, H. Long term alkaline storage and pretreatment process of cover crops for anaerobic digestion. Biores. Technol. 2021, 330, 124986. [Google Scholar] [CrossRef]
- Belle, A.; Lansing, S.; Mulbry, W.; Weil, R.R. Anaerobic co-digestion of forage radish and dairy manure in complete mix digesters. Bioresour. Technol. 2015, 178, 230–237. [Google Scholar] [CrossRef]
- Feng, L.; Perschke, Y.; Fontaine, D.; Ward, A.J.; Eriksen, J.; Sorensen, P.; Moller, H.B. Co-ensiling of cover crops and barley straw for biogas production. Renew. Energy 2019, 142, 677–683. [Google Scholar] [CrossRef]
- Vlierberghe, C.V.; Escudie, R.; Bernet, N.; Santa-Catalina, G.; Frederic, S.; Carrere, H. Conditions for efficient alkaline storage of cover crops for biomethane production. Bioresour. Technol. 2022, 348, 126722. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Perschke, Y.M.; Fontaine, D.; Nikolausz, M.; Ward, A.J.; Rocha, U.N.; Correa, F.B.; Eriksen, J.; Sorensen, P.; Moller, H.B. Anaerobic digestion of co-ensiled cover crop and barley straw: Effect of co-ensiling ratios, manure addition and impact on microbial community structure. Ind. Crops Prod. 2020, 144, 112025. [Google Scholar] [CrossRef]
- Igos, E.; Golkowska, K.; Koster, D.; Vervisch, B.; Benetto, E. Using rye as cover crop for bioenergy production: An environmental and economic assessment. Biomass Bioenergy 2016, 95, 116–123. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.; Hicks, K.B. Production of Bio-oil from Alfalfa Stems by Fluidized-Bed Fast Pyrolysis. Ind. Eng. Chem. Res. 2008, 47, 4115–4122. [Google Scholar] [CrossRef]
- Boateng, A.A.; Jung, H.G.; Adler, P.R. Pyrolysis of energy crops including alfalfa stems, reed canarygrass, and eastern gamagrass. Fuel 2006, 86, 2450–2457. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.P.; Zeng, Z.H.; Dai, L.L. Research progress on the role of common metal catalysts in biomass pyrolysis: A state-of-the-art review. Green. Chem. 2022, 24, 3922–3942. [Google Scholar] [CrossRef]
- Ge, S.B.; Yek, P.N.; Cheng, Y.W.; Xia, C.L. Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: A batch to continuous approach. Renew. Sustain. Energy Rev. 2021, 135, 110148. [Google Scholar] [CrossRef]
- Carpenter, D.L.; Bain, R.L.; Davis, R.E.; Dutta, A. Pilot-Scale Gasification of Corn Stover, Switchgrass, Wheat Straw, and Wood: 1. Parametric Study and Comparison with Literature. Ind. Eng. Chem. Res. 2010, 49, 1859–1871. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Galafassi, P.L.; Ferreira, S.D.; Godinho, M.; Pauletti, G.F.; Baldasso, C. Fodder radish seed cake biochar for soil amendment. Environ. Sci. Pollut. Res. 2018, 25, 25143–25154. [Google Scholar] [CrossRef] [PubMed]
- Ucar, S.; Ozkan, A.R. Characterization of products from the pyrolysis of rapeseed oil cake. Bioresour. Technol. 2008, 99, 8771–8776. [Google Scholar] [CrossRef]
- Smets, K.; Roukaerts, A.; Czech, J.; Reggers, G.; Schreurs, S.; Carleer, R.; Yperman, J. Slow catalytic pyrolysis of rapeseed cake: Product yield and characterization of the pyrolysis liquid. Biomass Bioenergy 2013, 57, 180–190. [Google Scholar] [CrossRef]
- Prussi, M.; Lee, U.; Wang, M.; Malina, R.; Valin, H.; Taheripour, F.; Velarde, C.; Staples, M.D.; Lonza, L.; Hileman, J. CORSIA: The first internationally adopted approach to calculate life-cycle GHG emissions for aviation fuels. Renew. Sustain. Energy Rev. 2021, 150, 111398. [Google Scholar] [CrossRef]
- IATA. Developing Sustainable Aviation Fuel (SAF). Available online: https://www.iata.org/en/iata-repository/pressroom/fact-sheets/fact-sheet---alternative-fuels/ (accessed on 1 August 2023).
- Mousavi-Avval, S.; Hkanal, S.; Shah, A. Assessment of Potential Pennycress Availability and Suitable Sites for Sustainable Aviation Fuel Refineries in Ohio. Sustainability 2023, 15, 10589. [Google Scholar] [CrossRef]
- Field, J.; Zhang, Y.; Marx, E.; Boote, K.; Easter, M. Modeling Yield, Biogenic Emissions, and Carbon Sequestration in Southeastern Cropping Systems With Winter Carinata. Sec. Bioenergy Biofuels 2022, 10, 837883. [Google Scholar] [CrossRef]
- USDA-ARS. Making Air Travel More Sustainable with Soy-Fuel Innovations. Available online: https://www.ars.usda.gov/news-events/news/research-news/2021/making-air-travel-more-sustainable-with-soy-fuel-innovations/ (accessed on 17 September 2023).
- Kotrba, R. EPA Finalizes Approval of Hydrotreated Canola Oil under RFS. Available online: https://biomassmagazine.com/articles/epa-finalizes-new-rfs-canola-oil-fuel-pathways-19562 (accessed on 23 August 2023).
- Why, E.; Ong, H.; Lee, H.; Gan, Y.; Chen, W.; Chong, C. Renewable aviation fuel by advanced hydroprocessing of biomass: Challenges and perspective. Energy Convers. Manag. 2019, 199, 112015. [Google Scholar] [CrossRef]
- Chu, P.L.; Vanderghem, C.; MacLean, H.L.; Saville, B.A. Process modeling of hydrodeoxygenation to produce renewable jet fuel and other hydrocarbon fuels. Fuel 2017, 196, 298–305. [Google Scholar] [CrossRef]
- Wastefuel. NetJets Invests in WasteFuel, Commits to Purchase 100 Million Gallons of Sustainable Aviation Fuel Over the Next Decade. Available online: https://www.wastefuel.com/netjets-and-wastefuel-partnership (accessed on 10 August 2023).
- Americas, S. Landfill Biogas to Sustainable Aviation Fuel (SAF). Available online: www.energy.gov (accessed on 10 August 2023).
- ENI. Kenya Airways Operating the First Fight from Africa Using Eni Sustainable Mobility’s Aviation Biofuel. Available online: https://www.eni.com/en-IT/media/press-release/2023/05/kenya-airways-operating-first-flight-from-africa-using-eni-sustainable-biofuel.html (accessed on 10 October 2023).
- Neuling, U.; Kaltschmitt, M. Chapter 18 Conversion routes from biomass to biokerosene. In Biokerosene: Status and Prospects; Kaltschmitt, M., Neuling, U., Eds.; Springer: Berlin, Germary, 2017. [Google Scholar]
- Ikegwu, T.; Ezegbe, C.; Odo, E.; Okola, C.; Mba, J.; Agu, H. Processing of Oilseeds in the Tropics: Prospects and Challenges. In Oilseed Crops; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Blanco-Canqui, H.; Ruis, S.J.; Proctor, C.A.; Creech, C.F.; Drewnoski, M.E.; Redfearn, D.D. Harvesting cover crops for biofuel and livestock production: Another ecosystem service? Agonomy J. 2020, 112, 2373–2400. [Google Scholar] [CrossRef]
- Miller, J. Cover Crop Biomass and Termination Considerations. Available online: https://sites.udel.edu/agronomy/2022/03/25/cover-crop-biomass-and-termination-considerations/ (accessed on 10 August 2023).
- Sun, H.; Cui, X.; Li, R.; Guo, J.; Dong, R. Ensiling process for efficient biogas production from lignocellulosic substrates: Methods, mechanisms, and measures. Bioresour. Technol. 2021, 342, 125928. [Google Scholar] [CrossRef]
- Villa, R.; Rodriguez, L.O.; Fenech, C.; Anika, O.C. Ensiling for anaerobic digestion: A review of key considerations to maximise methane yields. Renew. Sustain. Energy Rev. 2020, 134, 110401. [Google Scholar] [CrossRef]
| Cover Crop | Oil Content (%) | Oil Yield (kg/ha) | Location/Condition | Year | Ref. |
|---|---|---|---|---|---|
| B. Carinata | 33.2–36.0 | 388–400 | Seven western states in the US | 2013–2016 | [20] |
| B. Carinata | 38.0 | 380–920 1 | Five western states in Canada | 2008–2009 | [21] |
| B. Carinata | 45.9–47.1 46.2–46.6 | 678–903 2 601–835 2 | Alabama and Florida, US/Luverne, Dothan, Red Bay fine sandy loam | 2017–2019 | [22] |
| Camelina | 36.0 | 408 | Seven western states in the US | 2013–2016 | [20] |
| Camelina | 36.9–41.4 | 375 | Forest-Steppe, Steppe zones in Ukraine | 2001–2019 | [23] |
| Camelina | 40 (max.) | 740 (max.) | Arizona, US/Casa Grande sandy loam | 2013–2014 | [24] |
| Rapeseed (Canola) | 40.5–42.5 | 793–978 | Texas, US/arid, saneli silty clay loam | 2016–2017 | [25] |
| Rapeseed (Canola) | n.a. | 314–773 | New Mexico, US/semiarid, olton clay loam | 2015–2016 | [26] |
| Rapeseed (Canola) | 39.8–43.5 | 388–630 | Seven western states in the US | 2013–2016 | [20] |
| Brown mustard 3 | 37.7–39.3 | 447–473 | Seven western states in the US | 2013–2016 | [20] |
| Brown mustard | 35.8 | n.a. | North Dakota, US | n.a. | [27] |
| Brown mustard | 42.0 | 640–1290 1 | Five western states in Canada | 2008–2009 | [21] |
| White mustard | 24.1–24.5 | n.a. | North Dakota, US | n.a. | [27] |
| White mustard | 26.0–26.6 | 253–295 | Seven western states in the US | 2013–2016 | [20] |
| White mustard | 30.0 | 380–650 1 | Five western states in Canada | 2008–2009 | [21] |
| Field mustard 4 | 39.1 | 354 | Seven western states in the US | 2013–2016 | [20] |
| Field mustard | 45.0 | 450–1080 1 | Five western states in Canada | 2008–2009 | [21] |
| Flax 5 | 37.1–42.8 | n.a. | Texas US/Ships clay and San Saba clay | 2008–2011 | [28] |
| Flax | 36.9–40.3 | 633–827 | Northwest China/Ustorthents soil | 2012–2015 | [29] |
| Flax | n.a. | 644–845 | Argentina/Argiudol aquic soil | 2008 | [30] |
| Pennycress | 30–35 | 754 6 | Midwest US | n.a. | [31] |
| Pennycress | 32.7 34.2, 33.5 30.6 | n.a. | Allartos, Greece/sandy loam Bologna, Italy/silty clay loam Illinois, US/silty clay loam | 2013–2015 | [32] |
| Pennycress | 34.5–36.7 | n.a. | Spain/semiarid Mediterranean | 2016–2017 | [33] |
| Peanut | 53.9 | 958 7 | Manamadurai, India | 2012 | [34] |
| Peanut | 46.8–50.8 | n.a. | Uzbekistan/gray soil | 2012–2014 | [35] |
| Peanut | 45–52 | 1178 8 | Average conditions in the US | n.a. | [36] |
| Sunflower | n.a. | 304–660 9 | Average conditions in the Tanzania | 2010–2019 | [37] |
| Sunflower | 40–43 | 529–663 | Sergipe, Brazil/semiarid | 2018 | [38] |
| Sunflower | 49 (H31 hybrid) | 1504 | Tuscany, Italy/Mediterranean, clay loam | 2010 | [39] |
| Soybean | 19.5 | 568 10 | Average conditions in the US | 2022 | [40] |
| Cover Crop | Yield, g/g-Dry Matter | Ref. |
|---|---|---|
| Faba bean | 0.083 g | [61] |
| Oilseed rape | 0.099 | [61] |
| Rapeseed straw | 0.11 | [62] |
| Rapeseed straw | 0.12–0.15 | [63] |
| Winter rye | 0.14 | [61] |
| Oat straw | 0.15 | [64] |
| White clover | 0.19 | [65] |
| Sweet sorghum stem | 0.22 | [66] |
| Sweet sorghum bagasse | 0.25–0.28 1 | [67] |
| Proso millet | 0.28 | [68] |
| Triticale | 0.29 | [69] |
| Pearl millet | 0.30–0.33 2 | [70] |
| Triticale | 0.33–0.34 | [71] |
| Methods | Ethanol Yield Increase | Ref. |
|---|---|---|
| Alkaline pretreatment | Triticale: from 0.17 to 0.29 g/g DM | [69] |
| Alkaline pretreatment | Rye straw: increased to 0.081 g/g DM | [73] |
| Acid pretreatment | Wheat: from 13 ± 2 to 17 ± 0 g/L | [60] |
| Acid pretreatment | Rye straw: increased to 0.096 g/g DM | [73] |
| Wet oxidation pretreatment | Winter rye: from 0.0059 to 0.14 g/g DM Oilseed rape: from 0.0097 to 0.099 g/g DM Faba bean: from 0.010 to 0.083 g/g DM | [61] |
| Steam explosion | Oat: from 0.085 to 0.15 g/g DM | [64] |
| Cover Crop | Yield, L-CH4/kg-VS | Conditions | Ref. |
|---|---|---|---|
| Cereal rye | 99–347 | Sulfuric acid pretreated | [85] |
| Cereal rye | 175–225 | Size reduced | [86] |
| Cereal rye | 348 | Alkaline pretreated | [87] |
| Cereal rye | 360 | 42 °C, continuous stirring | [61] |
| Radish | 150–210 | Co-digestion | [88] |
| Barley straw | 283 | Co-ensiling | [89] |
| Oat | 294 | Alkaline pretreated | [87] |
| Red clover | 319 | Co-ensiling | [89] |
| Oilseed rape straw | 420 | 42 °C, continuous stirring | [61] |
| Faba bean straw | 440 | 42 °C, continuous stirring | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Lamont, L.D.; Liu, S.; Guo, C.; Stoner, S. A Review on Potential Biofuel Yields from Cover Crops. Fermentation 2023, 9, 912. https://doi.org/10.3390/fermentation9100912
Yang L, Lamont LD, Liu S, Guo C, Stoner S. A Review on Potential Biofuel Yields from Cover Crops. Fermentation. 2023; 9(10):912. https://doi.org/10.3390/fermentation9100912
Chicago/Turabian StyleYang, Liangcheng, Lucas D. Lamont, Shan Liu, Chunchun Guo, and Shelby Stoner. 2023. "A Review on Potential Biofuel Yields from Cover Crops" Fermentation 9, no. 10: 912. https://doi.org/10.3390/fermentation9100912





