Exploring the Prospects of Fermenting/Co-Fermenting Marine Biomass for Enhanced Bioethanol Production
Abstract
:1. Introduction
2. Chemical Composition of Different Feedstocks Hydrolysates
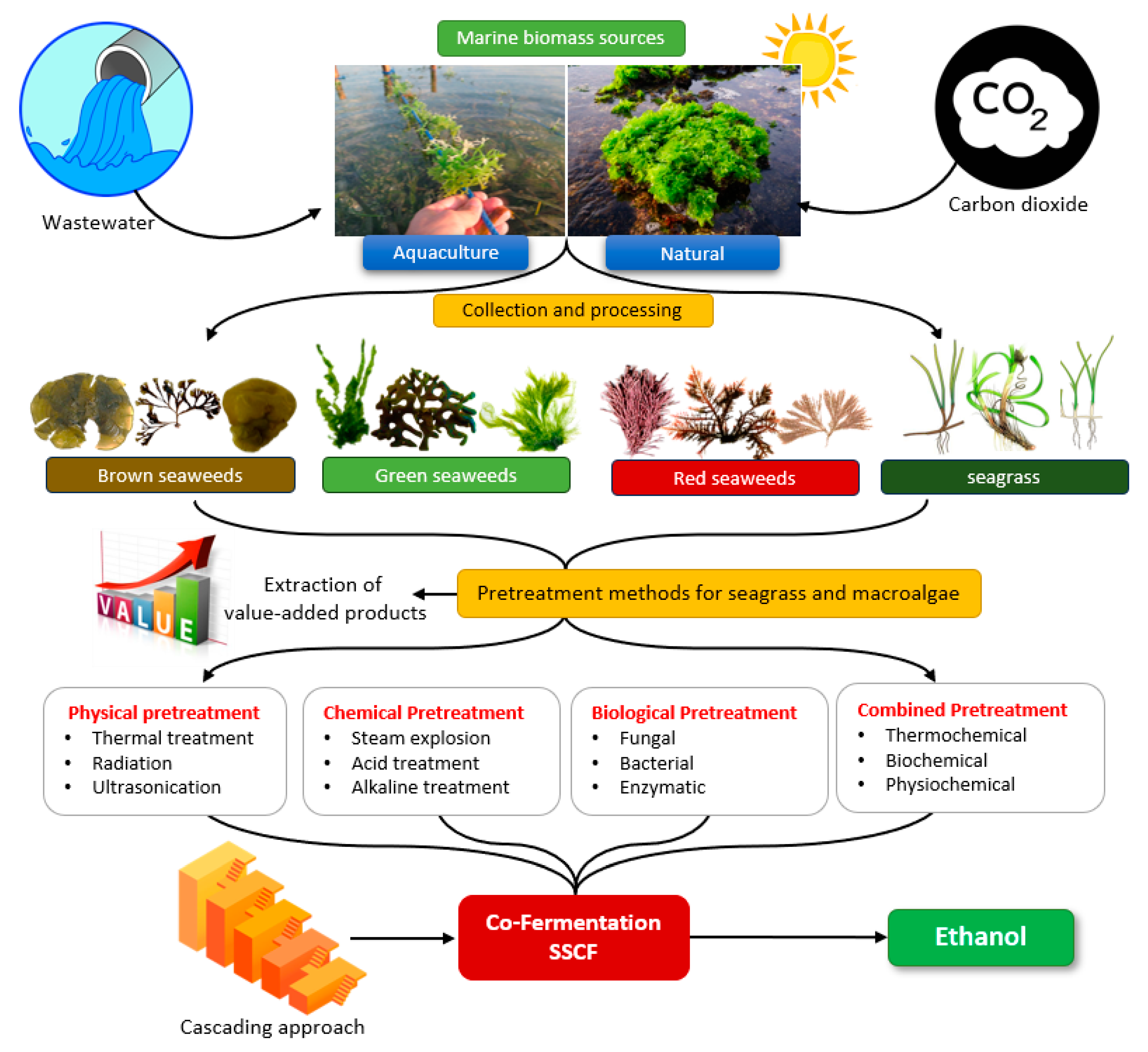
3. Macroalgae
3.1. Naturally-Growing Macroalgae
3.2. Macroalgae Farming
3.2.1. Hatchery Production
3.2.2. On-Site Seaweed Farming
4. Seagrass
4.1. Species Diversity
4.2. Cell Wall Structure
4.3. Seagrass Cultivation
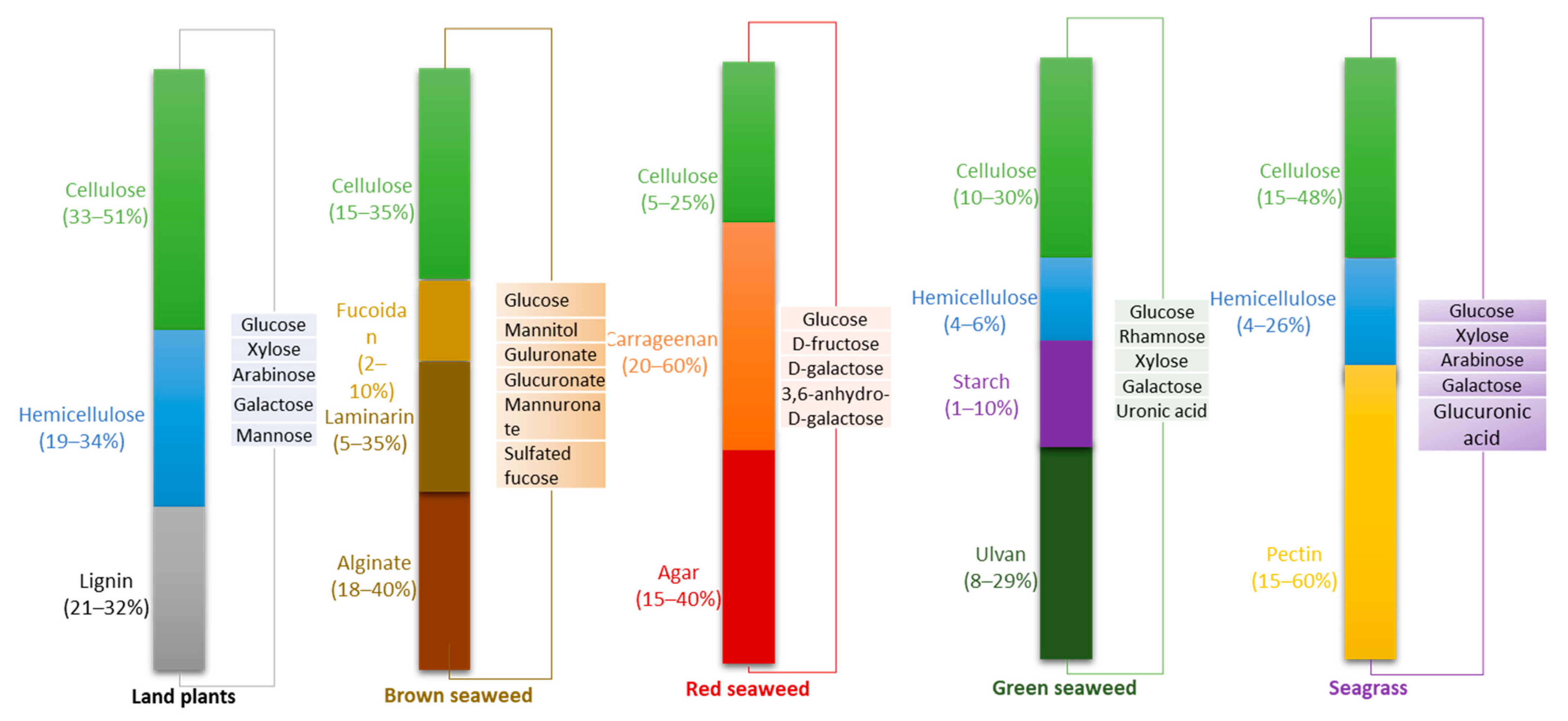
5. Biochemical Composition of Macroalgae and Seagrass
5.1. Carbohydrates in Marine Biomass
5.1.1. Green Macroalgae
| Polysaccharides | Macroalgae | Seagrass | ||
|---|---|---|---|---|
| Chlorophyta | Rhodophyta | Phaeophyta | ||
| Crystalline polysaccharides |
|
|
|
|
| Hemicellulose |
|
|
|
|
| Matrix Carboxylic polysaccharides |
| Not available |
| Not available |
| Matrix-sulfated polysaccharides |
|
|
| Not available |
| Storage carbohydrates |
|
|
|
|
5.1.2. Red Macroalgae
5.1.3. Brown Macroalgae
5.1.4. Seagrass
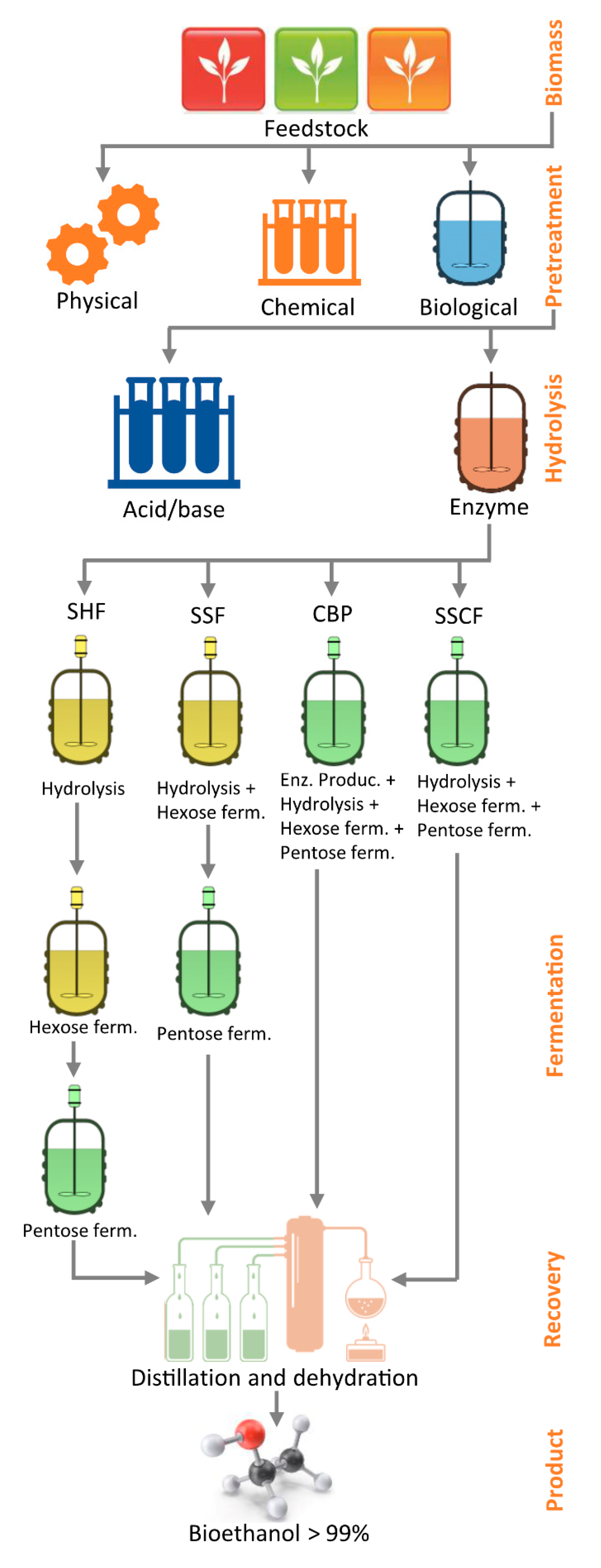
6. Marine Biomass Conversion into Bioethanol
6.1. Pretreatment
6.1.1. Physical Pretreatment
6.1.2. Chemical Pretreatments
- (a)
- Steam explosion pretreatment
- (b)
- Acid pretreatment
- (c)
- Alkaline pretreatment
6.1.3. Biological Pretreatment
6.1.4. Combined Pretreatment Method
6.2. Hydrolysis
6.2.1. Acid and Alkaline Hydrolysis
6.2.2. Enzymatic Hydrolysis
| Group | Algal Species | Substrate Concentration | Pretreatment Methods | Conditions of Process | Sugars (Yield) | Refs. |
|---|---|---|---|---|---|---|
| Red macroalgae | Gelidium elegans | 200 g L−1 | Acidolysis | 2.5% H2SO4, 120 °C, 40 min | Gal (0.238 g/g), Glu (0.243 g/g), Man (0.005 g/g), Xyl (0.010 g/g) | [175] |
| G. amansii | 120 g L−1 | Acidolysis + enzymatic hydrolysis | 144 mM H2SO4, 150 °C, 10 min, 16 U/mL Viscozyme L and Celluclast 1.5 L (1:1), 45 °C, pH 5.0, 45 min | Gal (0.238 g/g), Glu (0.187 g/g) | [176] | |
| Gracilaria salicornia | 168 g L−1 | Acidolysis Enzymatic hydrolysis | 2% H2SO4, 120 °C, 30 min 5 g/L cellulase, 40 °C, 4 h, pH 5.0 | RS (0.0043 g/g) RS (0.0138 g/g) | [133] | |
| G. lemaneiformis | 30 g L−1 5 g L−1 | Acidolysis Enzymatic hydrolysis | 0.3 M HCl, 80 °C, 2 h 10 U/mL β-agarase, 55 °C, 2 h | RS (0.200 g/g) RS (0.896 g/g) | [177] | |
| Green macroalgae | Ulva lactuca | 200 g L−1 | Acidolysis | 1% H2SO4, 125 °C, 30 min | RS (0.180 g/g), Glu (0.152 g/g) | [178] |
| 100 g L−1 | Acidolysis | 1% H2SO4, 125 °C, 30 min | Glu(0.041 g/g), Ara (0.087 g/g), Xyl (0.024 g/g) | [179] | ||
| 100 g L−1 | Acidolysis + enzymatic hydrolysis | 7.5% H2SO4, 150 °C, 10 min, 0.3 mL/g commercial cellulase cocktail, 50 °C, pH 5.0, 96 h | Glu (0.082 g/g), Rha (0.070 g/g), Xyl (0.045 g/g), Gal (0.010 g/g) | [152] | ||
| 200 g L−1 | Enzymatic hydrolysis + acidolysis | Deionized water, 150 °C, 10 min, 0.3 mL/g cellulase, 50 °C, stirring 24 h, centrifugation, 12 M H2SO4, 30 °C, 1 h, 1 M H2SO4, 100 °C, 3 h | Glu (0.113 g/g), Rha (0.090 g/g), Xyl (0.029 g/g), Gal (0.007 g/g) | [180] | ||
| U. reticulata | 50 g L−1 | Acidolysis + enzymatic hydrolysis | 0.5 M H2SO4, 120 °C, 90 min 50 IU/g Viscozyme L, 45 °C, 24 h | RS (0.609 g/g) | [181] | |
| Rhizoclonium spp. | 300 g L−1 | Acidolysis + enzymatic hydrolysis | 3% H2SO4, 95 °C, 1 h, 2.0 mL commercial enzyme cocktail (CELLIC® C TEC2), 50 °C, pH 6.3, 160 rpm, 24 h | Glu (0.558 g/g) | [182] | |
| Ulva (Enteromorpha) intestinalis | 100 g L−1 | Acidolysis + enzymatic hydrolysis | 270 mM H2SO4, 121 °C, 60 min, 16 U/mL Viscozyme L and Celluclast 1.5 L (1:1), 45 °C, pH 5.0, 150 rpm, 36 h | Glu (0.166 g/g), Xyl (0.076 g/g) | [183] | |
| Brown macroalgae | Saccharina spp. | 100 g L−1 | Grinding extraction | 65 °C, Grinding for 1 h, 20 Volume diH2O, pH 2.0 | Man (0.261 g/g), Glu (0.047 g/g) | [184] |
| Dilophus fasciola | 1 g L−1 whole biomass | Acidolysis | 5% H2SO4, 121 °C, 30 min | RS 31.98 g/L | [95] | |
| 1 g L−1 free lipid biomass | Acidolysis | 5% H2SO4, 121 °C, 30 min | RS 37.2 g/L | [95] | ||
| Padina tetrastromatica | 2 g L−1 | Acidolysis | 1% H2SO4, 100 °C, 1 h | RS (0.045 g/g) | [185] | |
| Laminaria japonica | 100 g L−1 | Acidolysis | 0.15 M H2SO4, 121 °C, 60 min | Glu (0.300 g/g) | [186] | |
| 50 g L−1 | Enzymatic hydrolysis | 10 mL/g Cellulases mixture, (NS81016; Novozymes A/S) 45 °C, 24 h | Man (0.092 g/g), Glu (0.180 g/g) | [187] | ||
| 100 g L−1 | Acid hydrolysis Acidolysis+ enzymatic hydrolysis | 0.2 M H2SO4, 121 °C, 20min Novozymes Biomass Kit, pH 5.5, 50 °C, 150 rpm, 18 h | RS (0.102 g/g) RS (0.293 g/g) | [188] | ||
| Ascophylum nodosum | 100 g L−1 | Acid hydrolysis Acidolysis + enzymatic hydrolysis | 0.2 M H2SO4, 121 °C, 20min, Novozymes Biomass Kit, pH 5.5, 50 °C, 150 rpm, 18 h | RS (0.125 g/g) RS (0.156 g/g) | [188] | |
| Sargassum fulvellum (72%), Hizikia fusiformis (18%), Undaria pinnatifida (6.2%) | 80 g L−1 | Acidolysis + enzymatic hydrolysis | 138 mM H2SO4, 160 °C, 10 min, 16 unit/mL Viscozyme L (1.2 FBG/mL), 45 °C, 48 h | Gal (0.188 g/g), Glu (0.2 g/g), Man (0.037 g/g) | [189] |
6.3. Fermentation
6.3.1. Separate Hydrolysis and Fermentation (SHF)
6.3.2. Simultaneous Saccharification and Fermentation (SSF)
6.3.3. Other Fermentation Methods
6.4. Bioethanol Recovery
7. Co-Fermentation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MMR Ethanol Market: Global Industry Analysis and Forecast (2023–2029). Available online: https://www.maximizemarketresearch.com/market-report/global-ethanol-market/25241/ (accessed on 25 September 2023).
- Panahi, H.K.S.; Dehhaghi, M.; Aghbashlo, M.; Karimi, K.; Tabatabaei, M. Shifting Fuel Feedstock from Oil Wells to Sea: Iran Outlook and Potential for Biofuel Production from Brown Macroalgae (Ochrophyta; Phaeophyceae). Renew. Sustain. Energy Rev. 2019, 112, 626–642. [Google Scholar] [CrossRef]
- Abdelsalam, I.; Elshobary, M.; Eladawy, M.M.; Nagah, M. Utilization of Multi-Tasking Non-Edible Plants for Phytoremediation and Bioenergy Source—A Review. Phyton 2019, 88, 69–90. [Google Scholar] [CrossRef]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B. An Engineered Microbial Platform for Direct Biofuel Production from Brown Macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef]
- Elsayed, M.; Abomohra, A.; Ai, P.; Wang, D.; El-Mashad, H.; Zhang, Y. Biorefining of Rice Straw by Sequential Fermentation and Anaerobic Digestion for Bioethanol and/or Biomethane Production: Comparison of Structural Properties and Energy Output. Bioresour. Technol. 2018, 268, 183–189. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Abomohra, A.; Hanelt, D. Recent Advances in Micro-/Nanoplastic (MNPs) Removal by Microalgae and Possible Integrated Routes of Energy Recovery. Microorganisms 2022, 10, 2400. [Google Scholar] [CrossRef]
- Almutairi, A.W. Full Utilization of Marine Microalgal Hydrothermal Liquefaction Liquid Products through a Closed-Loop Route: Towards Enhanced Bio-Oil Production and Zero-Waste Approach. 3 Biotech 2022, 12, 209. [Google Scholar] [CrossRef]
- Lü, J.; Sheahan, C.; Fu, P. Metabolic Engineering of Algae for Fourth Generation Biofuels Production. Energy Environ. Sci. 2011, 4, 2451–2466. [Google Scholar] [CrossRef]
- Han, S.; Jin, W.; Chen, Y.; Tu, R.; Abomohra, A. Enhancement of Lipid Production of Chlorella Pyrenoidosa Cultivated in Municipal Wastewater by Magnetic Treatment. Appl. Biochem. Biotechnol. 2016, 180, 1043–1055. [Google Scholar] [CrossRef]
- Barati, B.; Zeng, K.; Baeyens, J.; Wang, S.; Addy, M.; Gan, S.Y.; Abomohra, A. Recent Progress in Genetically Modified Microalgae for Enhanced Carbon Dioxide Sequestration. Biomass Bioenergy 2021, 145, 105927. [Google Scholar] [CrossRef]
- Lin, H.; Qin, S. Tipping Points in Seaweed Genetic Engineering: Scaling up Opportunities in the Next Decade. Mar. Drugs 2014, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic Engineering of Algae for Enhanced Biofuel Production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Elshobary, M.E.; Zabed, H.M.; Qi, X.; El-Shenody, R.A. Enhancing Biomass and Lipid Productivity of a Green Microalga Parachlorella Kessleri for Biodiesel Production Using Rapid Mutation of Atmospheric and Room Temperature Plasma. Biotechnol. Biofuels Bioprod. 2022, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Gattuso, J.P.; Hancke, K.; Gundersen, H.; Filbee-Dexter, K.; Pedersen, M.F.; Middelburg, J.J.; Burrows, M.T.; Krumhansl, K.A.; Wernberg, T.; et al. Global Estimates of the Extent and Production of Macroalgal Forests. Glob. Ecol. Biogeogr. 2022, 31, 1422–1439. [Google Scholar] [CrossRef]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Adams, J.M.; Gallagher, J.A.; Donnison, I.S. Fermentation Study on Saccharina latissima for Bioethanol Production Considering Variable Pre-Treatments. J. Appl. Phycol. 2009, 21, 569. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Iulianelli, A.; Di Paola, L.; Basile, M.; Basile, A. Ethanol from Biomass: Future and Perspectives. In Ethanol; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–59. [Google Scholar]
- Ravikumar, S. Production of Biofuel Ethanol from Pretreated Seagrass by Using Saccharomyces cerevisiae. Indian J. Sci. Technol. 2011, 4, 1087–1089. [Google Scholar] [CrossRef]
- Uchida, M.; Miyoshi, T.; Kaneniwa, M.; Ishihara, K.; Nakashimada, Y.; Urano, N. Production of 16.5% v/v Ethanol from Seagrass Seeds. J. Biosci. Bioeng. 2014, 118, 646–650. [Google Scholar] [CrossRef]
- Rajkumar, J.; Dilipan, E.; Ramachandran, M.; Panneerselvam, A.; Thajuddin, N. Bioethanol Production from Seagrass Waste, through Fermentation Process Using Cellulase Enzyme Isolated from Marine Actinobacteria. Vegetos 2021, 34, 581–591. [Google Scholar] [CrossRef]
- McMillan, J.D.; Jennings, E.W.; Mohagheghi, A.; Zuccarello, M. Comparative Performance of Precommercial Cellulases Hydrolyzing Pretreated Corn Stover. Biotechnol. Biofuels 2011, 4, 29. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Nawijn, W.; Mirończuk, A.M. Brown Seaweed Hydrolysate as a Promising Growth Substrate for Biomass and Lipid Synthesis of the Yeast Yarrowia lipolytica. Front. Bioeng. Biotechnol. 2022, 10, 944228. [Google Scholar] [CrossRef]
- Wi, S.G.; Kim, H.J.; Mahadevan, S.A.; Yang, D.-J.; Bae, H.-J. The Potential Value of the Seaweed Ceylon Moss (Gelidium amansii) as an Alternative Bioenergy Resource. Bioresour. Technol. 2009, 100, 6658–6660. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, H.; Jeong, M.; Sim, S.; Park, D.; Yang, J.; Singal, R.; Grimes, S.R.; Kim, H.; Song, H.; et al. Enzymatic Saccharification of Salix viminalis Cv. Q683 Biomass for Bioethanol Production. Biotechniques 2011, 27, 143–149. [Google Scholar]
- Cosenza, V.A.; Navarro, D.A.; Ponce, N.M.A.; Stortz, C.A. Seaweed Polysaccharides: Structure and Applications. In Industrial Applications of Renewable Biomass Products: Past, Present, Future; Springer: Cham, Switzerland, 2017; pp. 75–116. [Google Scholar]
- Ibraheem, O.; Ndimba, B.K. Molecular Adaptation Mechanisms Employed by Ethanologenic Bacteria in Response to Lignocellulose-Derived Inhibitory Compounds. Int. J. Biol. Sci. 2013, 9, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, L.; van Erven, G.; Sinclair, E.A.; Duarte, C.M.; Kabel, M.A.; Classen, B. Profiling the Cell Walls of Seagrasses from A (Amphibolis) to Z (Zostera). BMC Plant Biol. 2022, 22, 63. [Google Scholar] [CrossRef]
- Torbatinejad, N.M.; Annison, G.; Rutherfurd-Markwick, K.; Sabine, J.R. Structural Constituents of the Seagrass Posidonia australis. J. Agric. Food Chem. 2007, 55, 4021–4026. [Google Scholar] [CrossRef]
- Opsahl, S.; Benner, R. Decomposition of Senescent Blades of the Seagrass Halodule wrightii in a Subtropical Lagoon. Mar. Ecol. Prog. Ser. 1993, 94, 191. [Google Scholar] [CrossRef]
- Syed, N.F.N.; Zakaria, M.H.; Bujang, J.S. Fiber Characteristics and Papermaking of Seagrass Using Hand-Beaten and Blended Pulp. BioResources 2016, 11, 5358–5380. [Google Scholar] [CrossRef]
- Silva, J.; Dantas-Santos, N.; Gomes, D.L.; Costa, L.S.; Cordeiro, S.L.; Costa, M.S.S.P.; Silva, N.B.; Freitas, M.L.; Scortecci, K.C.; Leite, E.L. Biological Activities of the Sulfated Polysaccharide from the Vascular Plant Halodule wrightii. Rev. Bras. Farmacogn. 2012, 22, 94–101. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Fakhfakh, J.; Krichen, F.; Jribi, I.; Chiarore, A.; Patti, F.P.; Blecker, C.; Allouche, N.; Belghith, H.; Belghith, K. Structural Characterization and Functional Properties of Antihypertensive Cymodocea nodosa Sulfated Polysaccharide. Carbohydr. Polym. 2016, 151, 511–522. [Google Scholar] [CrossRef]
- Barakat, K.M.; Ismail, M.M.; Abou El Hassayeb, H.E.; El Sersy, N.A.; Elshobary, M.E. Chemical Characterization and Biological Activities of Ulvan Extracted from Ulva fasciata (Chlorophyta). Rend. Lincei. Sci. Fis. e Nat. 2022, 33, 829–841. [Google Scholar] [CrossRef]
- Gloaguen, V.; Brudieux, V.; Closs, B.; Barbat, A.; Krausz, P.; Sainte-Catherine, O.; Kraemer, M.; Maes, E.; Guerardel, Y. Structural Characterization and Cytotoxic Properties of an Apiose-Rich Pectic Polysaccharide Obtained from the Cell Wall of the Marine Phanerogam Zostera marina. J. Nat. Prod. 2010, 73, 1087–1092. [Google Scholar] [CrossRef]
- Lv, Y.; Shan, X.; Zhao, X.; Cai, C.; Zhao, X.; Lang, Y.; Zhu, H.; Yu, G. Extraction, Isolation, Structural Characterization and Anti-Tumor Properties of an Apigalacturonan-Rich Polysaccharide from the Sea Grass Zostera caespitosa Miki. Mar. Drugs 2015, 13, 3710–3731. [Google Scholar] [CrossRef]
- Cunha, J.T.; Soares, P.O.; Romaní, A.; Thevelein, J.M.; Domingues, L. Xylose Fermentation Efficiency of Industrial Saccharomyces cerevisiae Yeast with Separate or Combined Xylose Reductase/Xylitol Dehydrogenase and Xylose Isomerase Pathways. Biotechnol. Biofuels 2019, 12, 20. [Google Scholar] [CrossRef]
- Katahira, S.; Mizuike, A.; Fukuda, H.; Kondo, A. Ethanol Fermentation from Lignocellulosic Hydrolysate by a Recombinant Xylose- and Cellooligosaccharide-Assimilating Yeast Strain. Appl. Microbiol. Biotechnol. 2006, 72, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Alper, H.; Yang, Y.T.; Stephanopoulos, G. Improvement of Xylose Uptake and Ethanol Production in Recombinant Saccharomyces cerevisiae through an Inverse Metabolic Engineering Approach. Appl. Environ. Microbiol. 2005, 71, 8249–8256. [Google Scholar] [CrossRef]
- Oh, E.J.; Jin, Y.-S. Engineering of Saccharomyces cerevisiae for Efficient Fermentation of Cellulose. FEMS Yeast Res. 2020, 20, foz089. [Google Scholar] [CrossRef]
- Sze, P. A Biology of the Algae, 2nd ed.; Wm, C., Ed.; Brown Publishers: Dubuque, IA, USA, 1993. [Google Scholar]
- Lobban, C.S.; Harrison, P.J.; Duncan, M.J. The Physiological Ecology of Seaweeds; Cambridge University Press: New York, NY, USA, 1985. [Google Scholar]
- Gao, G.; Burgess, J.G.; Wu, M.; Wang, S.; Gao, K. Using Macroalgae as Biofuel: Current Opportunities and Challenges. Bot. Mar. 2020, 63, 355–370. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Essa, D.I.; Attiah, A.M.; Salem, Z.E.; Qi, X. Algal Community and Pollution Indicators for the Assessment of Water Quality of Ismailia Canal, Egypt. Stoch. Environ. Res. Risk Assess. 2020, 34, 1089–1103. [Google Scholar] [CrossRef]
- Ismail, M.M.; Ismail, G.A.; Elshobary, M.E. Morpho-Anatomical, and Chemical Characterization of Some Calcareous Mediterranean Red Algae Species. Bot. Stud. 2023, 64, 10. [Google Scholar] [CrossRef]
- Knoll, A.H. The Multiple Origins of Complex Multicellularity. Annu. Rev. Earth Planet. Sci. 2011, 39, 217–239. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and Nutritional Characteristics of Brown Seaweed Lipids: A Review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Kim, H.; Ra, C.H.; Kim, S.-K. Ethanol Production from Seaweed (Undaria pinnatifida) Using Yeast Acclimated to Specific Sugars. Biotechnol. Bioprocess Eng. 2013, 18, 533–537. [Google Scholar] [CrossRef]
- Yazdani, P.; Zamani, A.; Karimi, K.; Taherzadeh, M.J. Characterization of Nizimuddinia zanardini Macroalgae Biomass Composition and Its Potential for Biofuel Production. Bioresour. Technol. 2015, 176, 196–202. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A Review of Macroalgae Production, with Potential Applications in Biofuels and Bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Gao, K.; McKinley, K.R. Use of Macroalgae for Marine Biomass Production and CO2 Remediation: A Review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Potentials of Macroalgae as Feedstocks for Biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.G.; Bidwell, R.G.S. Mechanism of Photosynthetic Carbon Dioxide Uptake by the Red Macroalga, Chondrus crispus. Plant Physiol. 1989, 89, 93–99. [Google Scholar] [CrossRef]
- Duarte, C.M. Reviews and Syntheses: Hidden Forests, the Role of Vegetated Coastal Habitats in the Ocean Carbon Budget. Biogeosciences 2017, 14, 301–310. [Google Scholar] [CrossRef]
- Serrano, O.; Lovelock, C.E.; Atwood, T.B.; Macreadie, P.I.; Canto, R.; Phinn, S.; Arias-Ortiz, A.; Bai, L.; Baldock, J.; Bedulli, C.; et al. Australian Vegetated Coastal Ecosystems as Global Hotspots for Climate Change Mitigation. Nat. Commun. 2019, 10, 4313. [Google Scholar] [CrossRef] [PubMed]
- Weathers, K.C.; Strayer, D.L.; Likens, G.E. Fundamentals of Ecosystem Science, 2nd ed.; Academic Press: Cambridge, MA, USA, 2021; ISBN 9780128127629. [Google Scholar]
- FAO. The State of Food and Agriculture 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry. FAO Fish. Tech. Pap. 2003, 441, 105. [Google Scholar]
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Health Benefits of Algal Polysaccharides in Human Nutrition. Adv. Food Nutr. Res. 2012, 66, 75–145. [Google Scholar] [PubMed]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global Seaweed Farming and Processing in the Past 20 Years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]
- Roesijadi, G.; Jones, S.B.; Snowden-Swan, L.J.; Zhu, Y. Macroalgae as a Biomass Feedstock: A Preliminary Analysis; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2010.
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for Lignocellulosic Biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Roesijadi, G.; Copping, A.E.; Huesemann, M.H.; Forster, J.; Benemann, J.R. Techno-Economic Feasibility Analysis of Offshore Seaweed Farming for Bioenergy and Biobased Products; Battelle Pacific Northwest Div. Rep. Number PNWD-3931; Battelle Pacific Northwest Division: Richland, WA, USA, 2008; p. 115.
- Mooney-McAuley, K.M.; Edwards, M.D.; Champenois, J.; Gorman, E. Best Practice Guidelines for Seaweed Cultivation and Analysis: Public Output Report Report [WP1A5. 01] of the EnAlgae Project; EnAlgae, Swansea University, Centre for Sustainable Aquatic Research: Swansea, UK, 2016. [Google Scholar]
- de los Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; van Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, J.L.; Roca, G.; et al. Recent Trend Reversal for Declining European Seagrass Meadows. Nat. Commun. 2019, 10, 3356. [Google Scholar] [CrossRef]
- Papenbrock, J.; Teichberg, M. Editorial: Current Advances in Seagrass Research. Front. Plant Sci. 2023, 14, 1196437. [Google Scholar] [CrossRef]
- Yue, S.; Zhou, Y.; Xu, S.; Zhang, X.; Liu, M.; Qiao, Y.; Gu, R.; Xu, S.; Zhang, Y. Can the Non-Native Salt Marsh Halophyte Spartina Alterniflora Threaten Native Seagrass (Zostera japonica) Habitats? A Case Study in the Yellow River Delta, China. Front. Plant Sci. 2021, 12, 643425. [Google Scholar] [CrossRef]
- Han, Q.; Liu, D. Macroalgae Blooms and Their Effects on Seagrass Ecosystems. J. Ocean Univ. China 2014, 13, 791–798. [Google Scholar] [CrossRef]
- El-Shaffai, A.A.; Hanafy, M.H.; Gab-Alla, A.A. Distribution, Abundance and Species Composition of Seagrasses in Wadi El-Gemal National Park, Red Sea, Egypt. Indian J. Appl. Sci. 2011, 4, 1–8. [Google Scholar] [CrossRef]
- Waycott, M.; McMahon, K.; Mellors, J.; Calladine, A.; Kleine, D. A Guide to Tropical Seagrasses of the Indo-West Pacific; James Cook University: Townsville, Australia, 2004. [Google Scholar]
- Group, A.P.; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000; ISBN 0521661846. [Google Scholar]
- Reynolds, P.L.; Duffy, E.; Knowlton, N. Seagrass and Seagrass Beds. Ocean Portal. 2018. Available online: https://www.dcbd.nl/sites/default/files/documents/SeagrassAndSeagrass%20Beds%20_%20SmithsonianOceanPortal.pdf (accessed on 26 September 2023).
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global Seagrass Distribution and Diversity: A Bioregional Model. J. Exp. Mar. Bio. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Green, E.P.; Edmund, P.; Short, F.T. World Atlas of Seagrasses; University of California Press: Oakland, CA, USA, 2003; p. 298. [Google Scholar]
- Lee, H.; Golicz, A.A.; Bayer, P.E.; Jiao, Y.; Tang, H.; Paterson, A.H.; Sablok, G.; Krishnaraj, R.R.; Chan, C.-K.K.; Batley, J. The Genome of a Southern Hemisphere Seagrass Species (Zostera muelleri). Plant Physiol. 2016, 172, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.L.; Rouzé, P.; Verhelst, B.; Lin, Y.-C.; Bayer, T.; Collen, J.; Dattolo, E.; De Paoli, E.; Dittami, S.; Maumus, F. The Genome of the Seagrass Zostera marina Reveals Angiosperm Adaptation to the Sea. Nature 2016, 530, 331–335. [Google Scholar] [CrossRef]
- Aquino, R.S.; Landeira-Fernandez, A.M.; Valente, A.P.; Andrade, L.R.; Mourao, P.A.S. Occurrence of Sulfated Galactans in Marine Angiosperms: Evolutionary Implications. Glycobiology 2005, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-Proteins: Key Regulators at the Cell Surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zeng, W.; Bacic, A.; Johnson, K. AGPs through Time and Space. Annu. Plant Rev. Online 2018, 1, 767–804. [Google Scholar]
- Pfeifer, L.; Classen, B. The Cell Wall of Seagrasses: Fascinating, Peculiar and a Blank Canvas for Future Research. Front. Plant Sci. 2020, 11, 588754. [Google Scholar] [CrossRef] [PubMed]
- Klap, V.A.; Hemminga, M.A.; Boon, J.J. Retention of Lignin in Seagrasses: Angiosperms That Returned to the Sea. Mar. Ecol. Prog. Ser. 2000, 194, 1–11. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of Lignin in Seaweed Reveals Convergent Evolution of Cell-Wall Architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef]
- Kaal, J.; Serrano, O.; del Río, J.C.; Rencoret, J. Radically Different Lignin Composition in Posidonia Species May Link to Differences in Organic Carbon Sequestration Capacity. Org. Geochem. 2018, 124, 247–256. [Google Scholar] [CrossRef]
- ReMEDIES Seagrass Cultivation Lab—Save Our Seabed. Available online: https://saveourseabed.co.uk/seagrass-cultivation-lab/ (accessed on 4 September 2023).
- Yue, S.; Zhang, X.; Xu, S.; Zhang, Y.; Zhao, P.; Wang, X.; Zhou, Y. Reproductive Strategies of the Seagrass Zostera japonica under Different Geographic Conditions in Northern China. Front. Mar. Sci. 2020, 7, 574790. [Google Scholar] [CrossRef]
- Subhashini, P.; Raja, S.; Thangaradjou, T. Establishment of Cell Suspension Culture Protocol for a Seagrass (Halodule pinifolia): Growth Kinetics and Histomorphological Characterization. Aquat. Bot. 2014, 117, 33–40. [Google Scholar] [CrossRef]
- Potouroglou, M.; Pedder, K.; Wood, K.; Scalenghe, D. What to Know About Seagrass, the Ocean’s Overlooked Powerhouse 2022. Available online: https://www.wri.org/insights/understanding-seagrass?utm_campaign=wridigest&utm_source=wridigest-2022-08-09&utm_medium=email&utm_content=image (accessed on 1 October 2023).
- Barakat, K.M.; El-Sayed, H.S.; Khairy, H.M.; El-Sheikh, M.A.; Al-Rashed, S.A.; Arif, I.A.; Elshobary, M.E. Effects of Ocean Acidification on the Growth and Biochemical Composition of a Green Alga (Ulva fasciata) and Its Associated Microbiota. Saudi J. Biol. Sci. 2021, 28, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- El-Khodary, G.M.; El-Sayed, H.S.; Khairy, H.M.; El-Sheikh, M.A.; Qi, X.; Elshobary, M.E. Comparative Study on Growth, Survival and Pigmentation of Solea Aegyptiaca Larvae by Using Four Different Microalgal Species with Emphasize on Water Quality and Nutritional Value. Aquac. Nutr. 2020, 27, 615–629. [Google Scholar] [CrossRef]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive Compounds and Properties of Seaweeds—A Review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical Composition of Red, Green and Brown Seaweeds on the Swedish West Coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in Common Norwegian Seaweeds and Evaluation of Their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Elshobary, M.E.; El-Shenody, R.; Abomohra, A.E. Sequential Biofuel Production from Seaweeds Enhances the Energy Recovery: A Case Study for Biodiesel and Bioethanol Production. Int. J. Energy Res. 2020, 45, 6457–6467. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical Marine Macroalgae as Potential Sources of Nutritionally Important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the Search of New Functional Food Ingredients from Algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Dewsbury, B.M.; Bhat, M.; Fourqurean, J.W. A Review of Seagrass Economic Valuations: Gaps and Progress in Valuation Approaches. Ecosyst. Serv. 2016, 18, 68–77. [Google Scholar] [CrossRef]
- Touchette, B.W. Seagrass-Salinity Interactions: Physiological Mechanisms Used by Submersed Marine Angiosperms for a Life at Sea. J. Exp. Mar. Bio. Ecol. 2007, 350, 194–215. [Google Scholar] [CrossRef]
- Pradheeba, M.; Dilipan, E.; Nobi, E.P.; Thangaradjou, T.; Sivakumar, K. Evaluation of Seagrasses for Their Nutritional Value; NISCAIR-CSIR: New Delhi, India, 2011. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.T.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Han, S.; Xing, Z.; Jiang, H.; Li, W.; Huang, W. Biological Adaptive Mechanisms Displayed by a Freshwater Plant to Live in Aquatic and Terrestrial Environments. Environ. Exp. Bot. 2021, 191, 104623. [Google Scholar] [CrossRef]
- Kamenev, G.M. Fatty Acids as Markers of Food Sources in a Shallow-Water Hydrothermal Ecosystem (Kraternaya Bight, Yankich Island, Kurile Islands). Mar. Ecol. Prog. Ser. 1995, 120, 231–241. [Google Scholar]
- Burton, T.; Lyons, H.; Lerat, Y.; Stanley, M.; Rasmussen, M.B. A Review of the Potential of Marine Algae as a Source of Biofuel in Ireland; Sustainable Energy Authority of Ireland (SEAI): Dublin, Ireland, 2009. [Google Scholar]
- Lüning, K. Seaweeds: Their Environment, Biogeography, and Ecophysiology; John Wiley & Sons: Hoboken, NJ, USA, 1990; ISBN 0471624349. [Google Scholar]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates from Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–274. [Google Scholar]
- Karsten, U.; Barrow, K.D.; King, R.J. Floridoside, L-Isofloridoside, and D-Isofloridoside in the Red Alga Porphyra Columbina (Seasonal and Osmotic Effects). Plant Physiol. 1993, 103, 485–491. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Yu, S.; Blennow, A.; Bojko, M.; Madsen, F.; Olsen, C.E.; Engelsen, S.B. Physico-chemical Characterization of Floridean Starch of Red Algae. Starch-Stärke 2002, 54, 66–74. [Google Scholar] [CrossRef]
- Karsten, U.; West, J.A.; Zuccarello, G.C.; Nixdorf, O.; Barrow, K.D.; King, R.J. Low Molecular Weight Carbohydrate Patterns in the Bangiophyceae (Rhodophyta). J. Phycol. 1999, 35, 967–976. [Google Scholar] [CrossRef]
- Karsten, U.; Michalik, D.; Michalik, M.; West, J.A. A New Unusual Low Molecular Weight Carbohydrate in the Red Algal Genus Hypoglossum (Delesseriaceae, Ceramiales) and Its Possible Function as an Osmolyte. Planta 2005, 222, 319–326. [Google Scholar] [CrossRef]
- Lobban, C.S.; Wynne, M.J. The Biology of Seaweeds; University of California Press: Oakland, CA, USA, 1981; Volume 17, ISBN 0520045858. [Google Scholar]
- Adams, J.M.M.; Toop, T.A.; Donnison, I.S.; Gallagher, J.A. Seasonal Variation in Laminaria digitata and Its Impact on Biochemical Conversion Routes to Biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef]
- Song, M.; Duc Pham, H.; Seon, J.; Chul Woo, H. Marine Brown Algae: A Conundrum Answer for Sustainable Biofuels Production. Renew. Sustain. Energy Rev. 2015, 50, 782–792. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A Review of the Biochemistry of Heavy Metal Biosorption by Brown Algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Graiff, A.; Ruth, W.; Kragl, U.; Karsten, U. Chemical Characterization and Quantification of the Brown Algal Storage Compound Laminarin—A New Methodological Approach. J. Appl. Phycol. 2016, 28, 533–543. [Google Scholar] [CrossRef]
- Nøkling-Eide, K.; Langeng, A.-M.; Åslund, A.; Aachmann, F.L.; Sletta, H.; Arlov, Ø. An Assessment of Physical and Chemical Conditions in Alginate Extraction from Two Cultivated Brown Algal Species in Norway: Alaria esculenta and Saccharina latissima. Algal Res. 2023, 69, 102951. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Li, Y.; Xu, Z.; Fong, C.W.; Huang, D.; Han, Q.; Huang, H.; Tan, Y.; Liu, S. Main Seagrass Beds and Threats to Their Habitats in the Coastal Sea of South China. Chin. Sci. Bull. 2006, 51, 136–142. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Orth, R.J.; Duarte, C.M. Seagrasses: Biology, Ecology and Conservation. Phycologia 2006, 45, 5. [Google Scholar]
- Hasegawa, N.; Hori, M.; Mukai, H. Seasonal Shifts in Seagrass Bed Primary Producers in a Cold-Temperate Estuary: Dynamics of Eelgrass Zostera marina and Associated Epiphytic Algae. Aquat. Bot. 2007, 86, 337–345. [Google Scholar] [CrossRef]
- Touchette, B.W.; Burkholder, J.M. Overview of the Physiological Ecology of Carbon Metabolism in Seagrasses. J. Exp. Mar. Biol. Ecol. 2000, 250, 169–205. [Google Scholar] [CrossRef] [PubMed]
- Marbà, N.; Krause-Jensen, D.; Alcoverro, T.; Birk, S.; Pedersen, A.; Neto, J.M.; Orfanidis, S.; Garmendia, J.M.; Muxika, I.; Borja, A.; et al. Diversity of European Seagrass Indicators: Patterns within and across Regions. Hydrobiologia 2013, 704, 265–278. [Google Scholar] [CrossRef]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.T.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and Diversity of Plant Cell Walls: From Algae to Flowering Plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [PubMed]
- El-Hefnawy, M.E.; Alhayyani, S.; El-Sherbiny, M.M.; Abomohra, A.; Al-Harbi, M. Endogenous Bioethanol Production by Solid-State Prefermentation for Enhanced Crude Bio-Oil Recovery through Integrated Hydrothermal Liquefaction of Seaweeds. J. Clean. Prod. 2022, 355, 131811. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Abomohra, A. Screening of Seaweeds for Sustainable Biofuel Recovery through Sequential Biodiesel and Bioethanol Production. Environ. Sci. Pollut. Res. 2020, 27, 32481–32493. [Google Scholar] [CrossRef]
- Chirapart, A.; Praiboon, J.; Puangsombat, P.; Pattanapon, C.; Nunraksa, N. Chemical Composition and Ethanol Production Potential of Thai Seaweed Species. J. Appl. Phycol. 2014, 26, 979–986. [Google Scholar] [CrossRef]
- Kim, H.M.H.; Wi, S.G.S.; Jung, S.; Song, Y.; Bae, H.-J.H. Efficient Approach for Bioethanol Production from Red Seaweed Gelidium amansii. Bioresour. Technol. 2015, 175, 128–134. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Schmidt, A.; Gallagher, J.A. The Impact of Sample Preparation of the Macroalgae Laminaria digitata on the Production of the Biofuels Bioethanol and Biomethane. J. Appl. Phycol. 2015, 27, 985–991. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Sperber, V.E.; Faruk, O. Natural and Wood Fibre Reinforcement in Polymers; iSmithers Rapra Publishing: Shawbury, UK, 2002; Volume 13, ISBN 1859573592. [Google Scholar]
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed Bioethanol Production: A Process Selection Review on Hydrolysis and Fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef]
- Ge, L.; Wang, P.; Mou, H. Study on Saccharification Techniques of Seaweed Wastes for the Transformation of Ethanol. Renew. Energy 2011, 36, 84–89. [Google Scholar] [CrossRef]
- Enquist-Newman, M.; Faust, A.M.E.; Bravo, D.D.; Santos, C.N.S.; Raisner, R.M.; Hanel, A.; Sarvabhowman, P.; Le, C.; Regitsky, D.D.; Cooper, S.R. Efficient Ethanol Production from Brown Macroalgae Sugars by a Synthetic Yeast Platform. Nature 2014, 505, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, H.; Kim, S.K. Bioethanol Production from Brown Seaweed, Undaria pinnatifida, Using NaCl Acclimated Yeast. Bioprocess Biosyst. Eng. 2013, 36, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.L.; Teh, K.Y.; Lam, S.S.; Kaben, A.M.; Cha, T.S. Optimization of Cell Disruption Methods for Efficient Recovery of Bioactive Metabolites via NMR of Three Freshwater Microalgae (Chlorophyta). Bioresour. Technol. 2015, 190, 536–542. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Kim, T.; Shin, M.-K.; Kim, Y.J.; Yoon, J.-J.; Chang, I.S. Use of Red Algae, Ceylon Moss (Gelidium amansii), Hydrolyzate for Clostridial Fermentation. Biomass Bioenergy 2013, 56, 38–42. [Google Scholar] [CrossRef]
- Schultz-Jensen, N.; Thygesen, A.; Leipold, F.; Thomsen, S.T.; Roslander, C.; Lilholt, H.; Bjerre, A.B. Pretreatment of the Macroalgae Chaetomorpha Linum for the Production of Bioethanol—Comparison of Five Pretreatment Technologies. Bioresour. Technol. 2013, 140, 36–42. [Google Scholar] [CrossRef]
- Percival, E.; McDowell, R.H. Chemistry and Enzymology of Marine Algal Polysaccharides; Academic Press: Cambridge, MA, USA, 1967. [Google Scholar]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol Production from Agricultural Wastes: An Overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Torun, M. Radiation Pretreatment of Biomass. Appl. Ioniz. Radiat. Mater. Process. 2017, 2, 447–460. [Google Scholar]
- El-Mesery, H.S.; Mao, H.; Abomohra, A. Applications of Non-Destructive Technologies for Agricultural and Food Products Quality Inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, Z. Enhancing Enzymatic Digestibility of Switchgrass by Microwave-Assisted Alkali Pretreatment. Biochem. Eng. J. 2008, 38, 369–378. [Google Scholar] [CrossRef]
- Yoon, M.; Choi, J.; Lee, J.-W.; Park, D.-H. Improvement of Saccharification Process for Bioethanol Production from Undaria sp. by Gamma Irradiation. Radiat. Phys. Chem. 2012, 81, 999–1002. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D.J. Microwave Assisted Acid Hydrolysis of Brown Seaweed Ascophyllum Nodosum for Bioethanol Production and Characterization of Alga Residue. ACS Sustain. Chem. Eng. 2015, 3, 1359–1365. [Google Scholar] [CrossRef]
- Kassim, M.A.; Meng, T.K.; Kamaludin, R.; Hussain, A.H.; Bukhari, N.A. Bioprocessing of Sustainable Renewable Biomass for Bioethanol Production. In Value-Chain of Biofuels—Fundamentals, Technology, and Standardization; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–234. [Google Scholar] [CrossRef]
- Alam, S.N.; Khalid, Z.; Guldhe, A.; Singh, B.; Korstad, J. Harvesting and Pretreatment Techniques of Aquatic Macrophytes and Macroalgae for Production of Biofuels. Environ. Sustain. 2021, 4, 299–316. [Google Scholar] [CrossRef]
- Sahay, S. Impact of Pretreatment Technologies for Biomass to Biofuel Production. In Substrate Analysis for Effective Biofuels Production; Springer: Berlin, Germany, 2020; pp. 173–216. [Google Scholar] [CrossRef]
- Parab, P.; Khandeparker, R.; Amberkar, U.; Khodse, V. Enzymatic Saccharification of Seaweeds into Fermentable Sugars by Xylanase from Marine Bacillus Sp. Strain BT21. 3 Biotech 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Soliman, R.M.; Younis, S.A.; El-Gendy, N.S.; Mostafa, S.S.M.; El-Temtamy, S.A.; Hashim, A.I. Batch Bioethanol Production via the Biological and Chemical Saccharification of Some Egyptian Marine Macroalgae. J. Appl. Microbiol. 2018, 125, 422–440. [Google Scholar] [CrossRef]
- Fasahati, P.; Woo, H.C.; Liu, J.J. Industrial-Scale Bioethanol Production from Brown Algae: Effects of Pretreatment Processes on Plant Economics. Appl. Energy 2015, 139, 175–187. [Google Scholar] [CrossRef]
- van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; López-Contreras, A.M. Production of Acetone, Butanol, and Ethanol from Biomass of the Green Seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Solid Acid Catalysts Pretreatment and Enzymatic Hydrolysis of Macroalgae Cellulosic Residue for the Production of Bioethanol. Carbohydr. Polym. 2015, 124, 311–321. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Enzymatic Hydrolysis and Fermentation of Seaweed Solid Wastes for Bioethanol Production: An Optimization Study. Energy 2014, 78, 53–62. [Google Scholar] [CrossRef]
- Tan, Y.; Fang, M.; Jin, L.; Zhang, C.; Li, H.-P.; Xing, X.-H. Culture Characteristics of the Atmospheric and Room Temperature Plasma-Mutated Spirulina Platensis Mutants in CO2 Aeration Culture System for Biomass Production. J. Biosci. Bioeng. 2015, 120, 438–443. [Google Scholar] [CrossRef]
- Hamedi, J.; Mohammadipanah, F.; Panahi, H.K.S. Biotechnological Exploitation of Actinobacterial Members. In Halophiles: Biodiversity and Sustainable Exploitation; Springer: Berlin, Germany, 2015; pp. 57–143. [Google Scholar]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms’ Footprint in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef]
- Wainwright, M.; Sherbrock-Cox, V. Factors Influencing Alginate Degradation by the Marine Fungi: Dendryphiella salina and D. arenaria. Bot. Mar. 1981, 24, 489–492. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Mohamed, S.A.; Asiri, A.M.; Gomaa, A.M.; Ibrahim, I.H.; Al-Talhi, H.A. Saccharification and Hydrolytic Enzyme Production of Alkali Pre-Treated Wheat Bran by Trichoderma Virens under Solid State Fermentation. BMC Biotechnol. 2015, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass Pretreatment: Fundamentals toward Application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Bouwer, E. Biogas Production from Algae and Cyanobacteria through Anaerobic Digestion: A Review, Analysis, and Research Needs. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; pp. 873–975. [Google Scholar]
- Gomaa, M.; Hifney, A.F.; Fawzy, M.A.; Issa, A.A.; Abdel-Gawad, K.M. Biodegradation of Palisada perforata (Rhodophyceae) and Sargassum sp.(Phaeophyceae) Biomass by Crude Enzyme Preparations from Algicolous Fungi. J. Appl. Phycol. 2015, 27, 2395–2404. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Wang, Q.; He, Z.; Abomohra, A.; Cao, B. Influence of Torrefaction Pretreatment on the Pyrolysis Characteristics of Seaweed Biomass. Cellulose 2019, 26, 8475–8487. [Google Scholar] [CrossRef]
- Peng, J.; Abomohra, A.; Elsayed, M.; Zhang, X.; Fan, Q.; Ai, P. Compositional Changes of Rice Straw Fibers after Pretreatment with Diluted Acetic Acid: Towards Enhanced Biomethane Production. J. Clean. Prod. 2019, 230, 775–782. [Google Scholar] [CrossRef]
- Patil, J.H.; AntonyRaj, M.; Gavimath, C.C. Study on Effect of Pretreatment Methods on Biomethanation of Water Hyacinth. Int. J. Adv. Biotechnol. Res. 2011, 2, 143–147. [Google Scholar]
- Nikolaison, L.; Dahl, J.; Bech, K.S.; Bruhn, A.; Rasmussen, M.B.; Bjerre, A.B.; Nielsen, H.B.; Ambus, P.; Rost, K.A.; Kadar, Z. Energy Production Fom Macroalgae. In Proceedings of the 20th European Biomass Conference, Milan, Italy, 18–22 June 2012; pp. 18–22. [Google Scholar]
- Meinita, M.D.N.; Hong, Y.-K.; Jeong, G.-T. Detoxification of Acidic Catalyzed Hydrolysate of Kappaphycus alvarezii (Cottonii). Bioprocess Biosyst. Eng. 2012, 35, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Karray, R.; Hamza, M.; Sayadi, S. Evaluation of Ultrasonic, Acid, Thermo-Alkaline and Enzymatic Pre-Treatment on Anaerobic Digestion of Ulva Rigida for Biogas Production. Bioresour. Technol. 2015, 187, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Atalla, R.H.; Vanderhart, D.L. Native Cellulose: A Composite of Two Distinct Crystalline Forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef]
- Daroch, M.; Geng, S.; Wang, G. Recent Advances in Liquid Biofuel Production from Algal Feedstocks. Appl. Energy 2013, 102, 1371–1381. [Google Scholar] [CrossRef]
- Choi, D.; Sim, H.S.; Piao, Y.L.; Ying, W.; Cho, H. Sugar Production from Raw Seaweed Using the Enzyme Method. J. Ind. Eng. Chem. 2009, 15, 12–15. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Nakamura, K.; Ariga, O.; Nakasaki, K. Production of High Concentrations of Bioethanol from Seaweeds That Contain Easily Hydrolyzable Polysaccharides. Process Biochem. 2011, 46, 2111–2116. [Google Scholar] [CrossRef]
- Jang, J.-S.; Cho, Y.; Jeong, G.-T.; Kim, S.-K. Optimization of Saccharification and Ethanol Production by Simultaneous Saccharification and Fermentation (SSF) from Seaweed, Saccharina japonica. Bioprocess Biosyst. Eng. 2012, 35, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, F.; Wu, Y.R. Emerging Technologies for Conversion of Sustainable Algal Biomass into Value-Added Products: A State-of-the-Art Review. Sci. Total Environ. 2021, 784, 147024. [Google Scholar] [CrossRef]
- Hessami, M.J.; Cheng, S.F.; Ambati, R.R.; Yin, Y.H.; Phang, S.M. Bioethanol Production from Agarophyte Red Seaweed, Gelidium Elegans, Using a Novel Sample Preparation Method for Analysing Bioethanol Content by Gas Chromatography. 3 Biotech 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Ra, C.H.; Jeong, G.-T.; Kim, S.-K. Hyper-Thermal Acid Hydrolysis and Adsorption Treatment of Red Seaweed, Gelidium amansii for Butyric Acid Production with PH Control. Bioprocess Biosyst. Eng. 2017, 40, 403–411. [Google Scholar] [CrossRef]
- Xu, X.-Q.; Su, B.-M.; Xie, J.-S.; Li, R.-K.; Yang, J.; Lin, J.; Ye, X.-Y. Preparation of Bioactive Neoagaroligosaccharides through Hydrolysis of Gracilaria Lemaneiformis Agar: A Comparative Study. Food Chem. 2018, 240, 330–337. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy Potential of Ulva lactuca: Biomass Yield, Methane Production and Combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Potts, T.; Du, J.; Paul, M.; May, P.; Beitle, R.; Hestekin, J. The Production of Butanol from Jamaica Bay Macro Algae. Environ. Prog. Sustain. Energy 2012, 31, 29–36. [Google Scholar] [CrossRef]
- Bikker, P.; van Krimpen, M.M.; van Wikselaar, P.; Houweling-Tan, B.; Scaccia, N.; van Hal, J.W.; Huijgen, W.J.J.; Cone, J.W.; López-Contreras, A.M. Biorefinery of the Green Seaweed Ulva lactuca to Produce Animal Feed, Chemicals and Biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.T.L.; Kawata, Y.; Tam, L.T.; Thom, L.T.; Ha, N.C.; Hien, H.T.M.; Thu, N.T.H.; Huy, P.Q.; Hong, D.D. Production of Pyruvate from Ulva Reticulata Using the Alkaliphilic, Halophilic Bacterium Halomonas sp. BL6. J. Appl. Phycol. 2020, 32, 2283–2293. [Google Scholar] [CrossRef]
- Saleh, S.; Salk, M.; Pamukçu, O. Estimating Curie Point Depth and Heat Flow Map for Northern Red Sea Rift of Egypt and Its Surroundings, from Aeromagnetic Data. Pure Appl. Geophys. 2013, 170, 863–885. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sunwoo, I.Y.; Ra, C.H.; Jeong, G.-T.; Kim, S.-K. Acetone, Butanol, and Ethanol Production from the Green Seaweed Enteromorpha intestinalis via the Separate Hydrolysis and Fermentation. Bioprocess Biosyst. Eng. 2019, 42, 415–424. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Kuo, L.-J.; Urquhart, L.; Gill, G.A.; Roesijadi, G. Acetone-Butanol Fermentation of Marine Macroalgae. Bioresour. Technol. 2012, 108, 305–309. [Google Scholar] [CrossRef]
- Radha, M.; Murugesan, A.G. Enhanced Dark Fermentative Biohydrogen Production from Marine Macroalgae Padina tetrastromatica by Different Pretreatment Processes. Biofuel Res. J. 2017, 4, 551–558. [Google Scholar] [CrossRef]
- Ventura, J.-R.S.; Jahng, D. Improvement of Butanol Fermentation by Supplementation of Butyric Acid Produced from a Brown Alga. Biotechnol. Bioprocess Eng. 2013, 18, 1142–1150. [Google Scholar] [CrossRef]
- Hou, X.; From, N.; Angelidaki, I.; Huijgen, W.J.J.; Bjerre, A.-B. Butanol Fermentation of the Brown Seaweed Laminaria digitata by Clostridium Beijerinckii DSM-6422. Bioresour. Technol. 2017, 238, 16–21. [Google Scholar] [CrossRef]
- Obata, O.; Akunna, J.; Bockhorn, H.; Walker, G. Ethanol Production from Brown Seaweed Using Non-Conventional Yeasts. Bioethanol 2016, 2, 134–145. [Google Scholar] [CrossRef]
- Sunwoo, I.Y.; Hau, N.T.; Ra, C.H.; Jeong, G.-T.; Kim, S.-K. Acetone–Butanol–Ethanol Production from Waste Seaweed Collected from Gwangalli Beach, Busan, Korea, Based on PH-Controlled and Sequential Fermentation Using Two Strains. Appl. Biochem. Biotechnol. 2018, 185, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol Production from Gracilaria verrucosa, a Red Alga, in a Biorefinery Approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Fokum, E.; Zabed, H.M.; Ravikumar, Y.; Elshobary, M.E.; Chandankere, R.; Zhang, Y.; Yun, J.; Qi, X. Co-Fermentation of Glycerol and Sugars by Clostridium beijerinckii: Enhancing the Biosynthesis of 1,3-Propanediol. Food Biosci. 2021, 41, 101028. [Google Scholar] [CrossRef]
- Ahring, B.K.; Jensen, K.; Nielsen, P.; Bjerre, A.B.; Schmidt, A.S. Pretreatment of Wheat Straw and Conversion of Xylose and Xylan to Ethanol by Thermophilic Anaerobic Bacteria. Bioresour. Technol. 1996, 58, 107–113. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable Bio-Ethanol Production from Agro-Residues: A Review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Lynd, L.R.; Van Zyl, W.H.; McBride, J.E.; Laser, M. Consolidated Bioprocessing of Cellulosic Biomass: An Update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Das Neves, M.A.; Kimura, T.; Shimizu, N.; Nakajima, M. State of the Art and Future Trends of Bioethanol Production. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2007, 1, 1–14. [Google Scholar]
- Hargreaves, P.I.; Barcelos, C.A.; da Costa, A.C.A.; Pereira, N., Jr. Production of Ethanol 3G from Kappaphycus alvarezii: Evaluation of Different Process Strategies. Bioresour. Technol. 2013, 134, 257–263. [Google Scholar] [CrossRef]
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.; Tan, T. Challenges and Opportunities in Improving the Production of Bio-Ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life Cycle Assessment of Biofuel Production from Brown Seaweed in Nordic Conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Aitken, D.; Bulboa, C.; Godoy-Faundez, A.; Turrion-Gomez, J.L.; Antizar-Ladislao, B. Life Cycle Assessment of Macroalgae Cultivation and Processing for Biofuel Production. J. Clean. Prod. 2014, 75, 45–56. [Google Scholar] [CrossRef]
- Faisal, S.; Zaky, A.; Wang, Q.; Huang, J.; Abomohra, A. Integrated Marine Biogas: A Promising Approach towards Sustainability. Fermentation 2022, 8, 520. [Google Scholar] [CrossRef]
- Nguyen, T.; Sperou, N.; Su, P.; Zhang, W. Marine Biorefinery: An Environmentally Sustainable Solution to Turn Marine Biomass and Processing Wastes into Value-Added Products and Profits. Biochemist 2022, 44, 22–27. [Google Scholar] [CrossRef]
- Abomohra, A.; Faisal, S.; Ebaid, R.; Huang, J.; Wang, Q.; Elsayed, M. Recent Advances in Anaerobic Digestion of Lipid-Rich Waste: Challenges and Potential of Seaweeds to Mitigate the Inhibitory Effect. Chem. Eng. J. 2022, 449, 137829. [Google Scholar] [CrossRef]
- Shin, S.-R.; Lee, M.-K.; Im, S.; Kim, D.-H. Effect of Seaweed Addition on Enhanced Anaerobic Digestion of Food Waste and Sewage Sludge. Environ. Eng. Res. 2019, 24, 449–455. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.; Lee, C. Continuous Anaerobic Co-Digestion of Ulva Biomass and Cheese Whey at Varying Substrate Mixing Ratios: Different Responses in Two Reactors with Different Operating Regimes. Bioresour. Technol. 2016, 221, 366–374. [Google Scholar] [CrossRef]
- Abomohra, A.; Sheikh, H.M.A.; El-Naggar, A.H.; Wang, Q. Microwave Vacuum Co-Pyrolysis of Waste Plastic and Seaweeds for Enhanced Crude Bio-Oil Recovery: Experimental and Feasibility Study towards Industrialization. Renew. Sustain. Energy Rev. 2021, 149, 111335. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Cao, B.; Hu, Y.; Abomohra, A.; Wang, Q.; Qian, L.; Liu, L.; Liu, X.; He, Z.; et al. Optimization of Hydrothermal Co-Liquefaction of Seaweeds with Lignocellulosic Biomass: Merging 2 Nd and 3 Rd Generation Feedstocks for Enhanced Bio-Oil Production. Energy 2019, 173, 413–422. [Google Scholar] [CrossRef]
- Sharma, S.; Nair, A.; Sarma, S.J. Biorefinery Concept of Simultaneous Saccharification and Co-Fermentation: Challenges and Improvements. Chem. Eng. Process.-Process Intensif. 2021, 169, 108634. [Google Scholar] [CrossRef]
- Ha, S.J.; Galazka, J.M.; Kim, S.R.; Choi, J.H.; Yang, X.; Seo, J.H.; Glass, N.L.; Cate, J.H.D.; Jin, Y.S. Engineered Saccharomyces cerevisiae Capable of Simultaneous Cellobiose and Xylose Fermentation. Proc. Natl. Acad. Sci. USA 2011, 108, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, K.; Sánchez, E.; El-Halwagi, M.; Kafarov, V. Exergy Analysis and Process Integration of Bioethanol Production from Acid Pre-Treated Biomass: Comparison of SHF, SSF and SSCF Pathways. Chem. Eng. J. 2011, 176–177, 195–201. [Google Scholar] [CrossRef]
- Olofsson, K.; Rudolf, A.; Lidén, G. Designing Simultaneous Saccharification and Fermentation for Improved Xylose Conversion by a Recombinant Strain of Saccharomyces cerevisiae. J. Biotechnol. 2008, 134, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, I.Y.; Kwon, J.E.; Nguyen, T.H.; Ra, C.H.; Jeong, G.-T.; Kim, S.-K. Bioethanol Production Using Waste Seaweed Obtained from Gwangalli Beach, Busan, Korea by Co-Culture of Yeasts with Adaptive Evolution. Appl. Biochem. Biotechnol. 2017, 183, 966–979. [Google Scholar] [CrossRef]
- Althuri, A.; Gujjala, L.K.S.; Banerjee, R. Partially Consolidated Bioprocessing of Mixed Lignocellulosic Feedstocks for Ethanol Production. Bioresour. Technol. 2017, 245, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.J.; Smith, S.V. C:N:P Ratios of Benthic Marine Plants1. Limnol. Oceanogr. 1983, 28, 568–574. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kumar, P.; Mehariya, S.; Purohit, H.J.; Lee, J.-K.; Kalia, V.C. Enhancement in Hydrogen Production by Co-Cultures of Bacillus and Enterobacter. Int. J. Hydrogen Energy 2014, 39, 14663–14668. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Elshobary, M.; Abdullah, E.; Abdel-Basset, R.; Metwally, M. Application of a Novel Biological-Nanoparticle Pretreatment to Oscillatoria acuminata Biomass and Coculture Dark Fermentation for Improving Hydrogen Production. Microb. Cell Fact. 2023, 22, 34. [Google Scholar] [CrossRef]
- Laurinavichene, T.; Laurinavichius, K.; Shastik, E.; Tsygankov, A. Long-Term H2 Photoproduction from Starch by Co-Culture of Clostridium butyricum and Rhodobacter sphaeroides in a Repeated Batch Process. Biotechnol. Lett. 2018, 40, 309–314. [Google Scholar] [CrossRef]
- Sharma, A.K.; Swain, M.R.; Singh, A.; Mathur, A.S.; Gupta, R.P.; Tuli, D.; Puri, S.K.; Ramakumar, S.S.V. US20190276857A1—Method for Second Generation Ethanol Production from Lignocellulosic Biomass—Google Patents. U.S. Patent US20190276857A1, 12 September 2021. [Google Scholar]
- Hörhammer, H.; Dou, C.; Gustafson, R.; Suko, A.; Bura, R. Removal of Non-Structural Components from Poplar Whole-Tree Chips to Enhance Hydrolysis and Fermentation Performance. Biotechnol. Biofuels 2018, 11, 222. [Google Scholar] [CrossRef]
- Johnston, K.G.; Abomohra, A.; French, C.E.; Zaky, A.S. Recent Advances in Seaweed Biorefineries and Assessment of Their Potential for Carbon Capture and Storage. Sustainability 2023, 15, 13193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, M.E.H.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Hanelt, D.; Abomohra, A. Exploring the Prospects of Fermenting/Co-Fermenting Marine Biomass for Enhanced Bioethanol Production. Fermentation 2023, 9, 934. https://doi.org/10.3390/fermentation9110934
Osman MEH, Abo-Shady AM, Elshobary ME, Abd El-Ghafar MO, Hanelt D, Abomohra A. Exploring the Prospects of Fermenting/Co-Fermenting Marine Biomass for Enhanced Bioethanol Production. Fermentation. 2023; 9(11):934. https://doi.org/10.3390/fermentation9110934
Chicago/Turabian StyleOsman, Mohamed E. H., Atef M. Abo-Shady, Mostafa E. Elshobary, Mahasen O. Abd El-Ghafar, Dieter Hanelt, and Abdelfatah Abomohra. 2023. "Exploring the Prospects of Fermenting/Co-Fermenting Marine Biomass for Enhanced Bioethanol Production" Fermentation 9, no. 11: 934. https://doi.org/10.3390/fermentation9110934







