Nattokinase: Insights into Biological Activity, Therapeutic Applications, and the Influence of Microbial Fermentation
Abstract
:1. Introduction
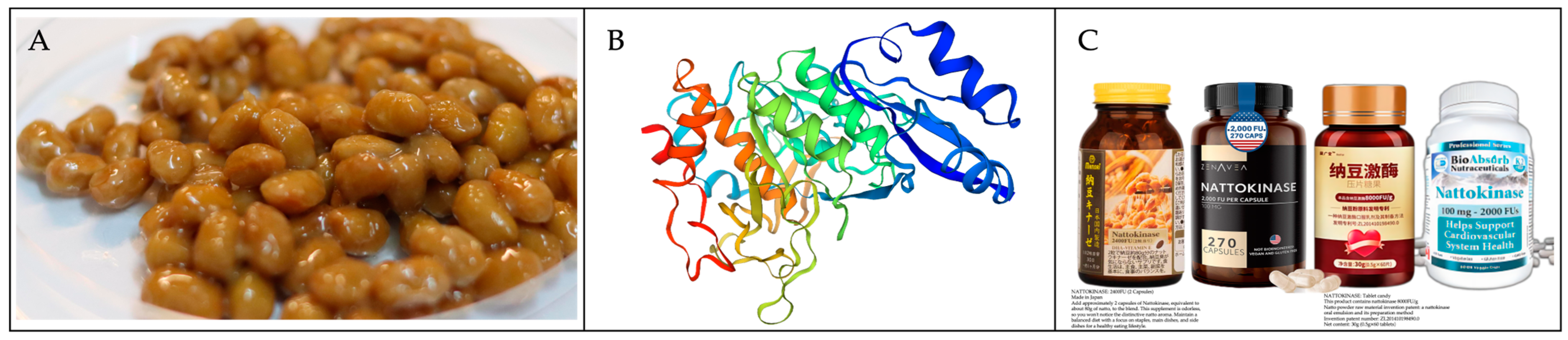
2. Physicochemical Properties and the Formation Process of Nattokinase
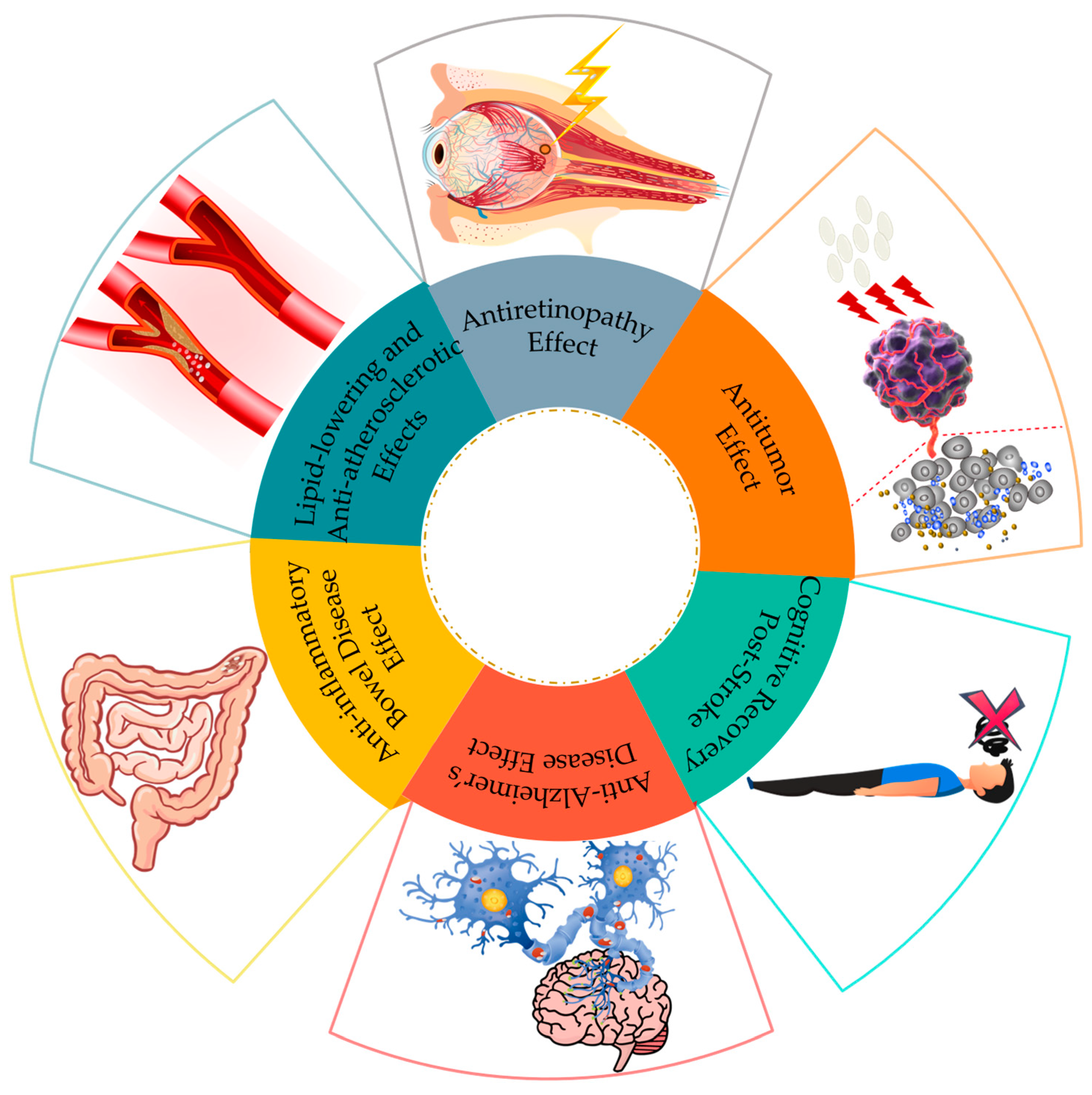
3. The Health Benefits of Nattokinase
3.1. Anti-Alzheimer’s Disease Effect of Nattokinase
3.2. Antiretinopathy Effect of Nattokinase
3.3. Antitumor Effect of Nattokinase
3.4. Anti-Hyperlipidemic and Anti-Atherosclerotic Effects of Nattokinase
3.5. Other Effects of Nattokinase
4. Enzyme Activity Assay of Nattokinase
5. Optimization Strategies for Nattokinase Production
5.1. Mutation Breeding of a Nattokinase-Producing Strain
5.2. Construction of Nattokinase Expression Systems and Molecular Modification
5.3. Optimizing Nattokinase Production through Variations in Fermentation Conditions
5.4. Purification of Nattokinase
5.5. Protection of Nattokinase
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kłosowski, G.; Mikulski, D.; Pielech-Przybylska, K. Pyrazines Biosynthesis by Bacillus Strains Isolated from Natto Fermented Soybean. Biomolecules 2021, 11, 1736. [Google Scholar] [CrossRef] [PubMed]
- Shieh, C.-J.; Phan Thi, L.-A.; Shih, I.-L. Milk-clotting Enzymes Produced by Culture of Bacillus subtilis Natto. Biochem. Eng. J. 2009, 43, 85–91. [Google Scholar] [CrossRef]
- Bus, K.; Szterk, A. Relationship between Structure and Biological Activity of Various Vitamin K Forms. Foods 2021, 10, 3136. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Wang, L.; Wu, H.; Zhao, G.; Liu, H.; Wang, P.; Zheng, Z. Coproduction of Menaquinone-7 and Nattokinase by Bacillus subtilis Using Soybean Curd Residue as a Renewable Substrate Combined with a Dissolved Oxygen Control Strategy. Ann. Microbiol. 2018, 68, 655–665. [Google Scholar] [CrossRef]
- Vieira, A.M.; Zahed, F.; Crispim, A.C.; de Souza Bento, E.; França, R.d.F.O.; Pinheiro, I.O.; Pardo, L.A.; Carvalho, B.M. Production of Levan from Bacillus subtilis var. natto and Apoptotic Effect on SH-SY5Y Neuroblastoma Cells. Carbohydr. Polym. 2021, 273, 118613. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Li, S.; Tian, Z.; Ma, X. Study on the Mechanism of Production of γ-PGA and Nattokinase in Bacillus subtilis Natto Based on RNA-seq Analysis. Microb. Cell Factories 2021, 20, 83. [Google Scholar] [CrossRef]
- Dabbagh, F.; Negahdaripour, M.; Berenjian, A.; Behfar, A.; Mohammadi, F.; Zamani, M.; Irajie, C.; Ghasemi, Y. Nattokinase: Production and Application. Appl. Microbiol. Biotechnol. 2014, 98, 9199–9206. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Xu, F.; Chen, J.; Duan, L.; Zhang, F. Acute Toxicity and Genotoxicity Evaluations of Nattokinase, a Promising Agent for Cardiovascular Diseases Prevention. Regul. Toxicol. Pharmacol. 2019, 103, 205–209. [Google Scholar] [CrossRef]
- Weng, Y.; Yao, J.; Sparks, S.; Wang, K.Y. Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease. Int. J. Mol. Sci. 2017, 18, 523. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, Y.; Wang, J.; Lyu, X.; Yang, R. Screening of a Bacillus subtilis Strain Producing both Nattokinase and Milk-clotting Enzyme and Its Application in Fermented Milk with Thrombolytic Activity. J. Dairy. Sci. 2021, 104, 9437–9449. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, Q.; Zhang, R.-h.; Zhang, Y.-z. Purification and Characterization of a Fibrinolytic Enzyme Produced by Bacillus amyloliquefaciens DC-4 Screened from douchi, a Traditional Chinese Soybean Food. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 134, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, X.; Chen, L.; Xu, X.; Li, J. Characterization of a Nattokinase from the Newly Isolated Bile Salt-Resistant Bacillus mojavensis LY-06. Foods 2022, 11, 2403. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Kim, J.-H.; Kwak, M.-S.; Sung, M.-H.; Jeong, D.-W. Functional Annotation Genome Unravels Potential Probiotic Bacillus velezensis Strain KMU01 from Traditional Korean Fermented Kimchi. Foods 2021, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wei, X.; Qiu, Y.; Chen, Y.; Chen, J.; Wen, Z.; Chen, S. High-Level Expression of Nattokinase in Bacillus licheniformis by Manipulating Signal Peptide and Signal Peptidase. J. Appl. Microbiol. 2016, 121, 704–712. [Google Scholar] [CrossRef]
- Devi, C.S.; Mohanasrinivasan, V.; Sharma, P.; Das, D.; Vaishnavi, B.; Naine, S.J. Production, Purification and Stability Studies on Nattokinase: A Therapeutic Protein Extracted from Mutant Pseudomonas aeruginosa CMSS Isolated from Bovine Milk. Int. J. Pept. Res. Ther. 2016, 22, 263–269. [Google Scholar] [CrossRef]
- Wang, C.T.; Ji, B.P.; Li, B.; Nout, R.; Li, P.L.; Ji, H.; Chen, L.F. Purification and Characterization of a Fibrinolytic Enzyme of Bacillus subtilis DC33, Isolated from Chinese Traditional Douchi. J. Ind. Microbiol. Biotechnol. 2006, 33, 750–758. [Google Scholar] [CrossRef]
- Wei, X.; Luo, M.; Xie, Y.; Yang, L.; Li, H.; Xu, L.; Liu, H. Strain Screening, Fermentation, Separation, and Encapsulation for Production of Nattokinase Functional Food. Appl. Biochem. Biotechnol. 2012, 168, 1753–1764. [Google Scholar] [CrossRef]
- Kim, W.; Choi, K.; Kim, Y.; Park, H.; Choi, J.; Lee, Y.; Oh, H.; Kwon, I.; Lee, S. Purification and Characterization of a Fibrinolytic Enzyme Produced from Bacillus sp. Strain CK 11-4 Screened from Chungkook-Jang. Appl. Environ. Microbiol. 1996, 62, 2482–2488. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, N.-S. Purification and Characterization of Subtilisin DJ-4 Secreted by Bacillus sp. Strain DJ-4 Screened from Doen-Jang. Biosci. Biotechnol. Biochem. 2000, 64, 1722–1725. [Google Scholar] [CrossRef]
- Mahajan, P.M.; Nayak, S.; Lele, S.S. Fibrinolytic Enzyme from Newly Isolated Marine Bacterium Bacillus subtilis ICTF-1: Media Optimization, Purification and Characterization. J. Biosci. Bioeng. 2012, 113, 307–314. [Google Scholar] [CrossRef]
- Vaithilingam, M.; Chandrasekaran, S.D.; Gupta, S.; Paul, D.; Sahu, P.; Selvaraj, J.N.; Babu, V. Extraction of Nattokinase Enzyme from Bacillus cereus Isolated from Rust. Natl. Acad. Sci. Lett. 2016, 39, 263–267. [Google Scholar] [CrossRef]
- Wang, S.-L.; Yeh, P.-Y. Production of a Surfactant- and Solvent-stable Alkaliphilic Protease by Bioconversion of Shrimp Shell Wastes Fermented by Bacillus subtilis TKU007. Process Biochem. 2006, 41, 1545–1552. [Google Scholar] [CrossRef]
- Kumar, D.J.M.; Rakshitha, R.; Vidhya, M.A.; Jennifer, P.S.; Prasad, S.; Kumar, M.R.; Kalaichelvan, P.T. Production, Optimization and Characterization of Fibrinolytic Enzyme by Bacillus subtilis RJAS19. Pak. J. Biol. Sci. 2014, 17, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H. A Novel Fibrinolytic Enzyme (Nattokinase) in the Vegetable Cheese Natto; a Typical and Popular Soybean Food in the Japanese Diet. Experientia 1987, 43, 1110–1111. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liangqi, C.; Xiyu, T.; Jinyao, L. Biotechnology, Bioengineering and Applications of Bacillus Nattokinase. Biomolecules 2022, 12, 980. [Google Scholar] [CrossRef]
- Yin, L.-J.; Lin, H.-H.; Jiang, S.-T. Bioproperties of Potent Nattokinase from Bacillus subtilis YJ1. J. Agric. Food Chem. 2010, 58, 5737–5742. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamagata, Y.; Ichishima, E. Nucleotide Sequence of the Subtilisin NAT Gene, aprN, of Bacillus subtilis (natto). Biosci. Biotechnol. Biochem. 1992, 56, 1869–1871. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Chatake, T.; Chiba-Kamoshida, K.; Naito, S.; Ohsugi, T.; Sumi, H.; Yasuda, I.; Morimoto, Y. Purification, Crystallization and Preliminary X-ray Diffraction Experiment of Nattokinase from Bacillus subtilis natto. Acta Cryst. 2010, 66, 1670–1673. [Google Scholar] [CrossRef]
- Bhatt, P.C.; Verma, A.; Al-Abbasi, F.A.; Anwar, F.; Kumar, V.; Panda, B.P. Development of Surface-engineered PLGA Nanoparticulate-Delivery System of Tet1-conjugated Nattokinase Enzyme for Inhibition of Aβ40 Plaques in Alzheimer’s Disease. Int. J. Nanomedicine 2017, 2017, 8749–8768. [Google Scholar] [CrossRef]
- Carter, P.; Wells, J.A. Dissecting the Catalytic Triad of a Serine Protease. Nature 1988, 332, 564–568. [Google Scholar] [CrossRef]
- Unrean, P.; Nguyen, N.H.A. Metabolic Pathway Analysis and Kinetic Studies for Production of Nattokinase in Bacillus subtilis. Bioprocess. Biosyst. Eng. 2013, 36, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Guangbo, Y.; Min, S.; Wei, S.; Lixin, M.; Chao, Z.; Yaping, W.; Zunxi, H. Heterologous Expression of Nattokinase from B. subtilis Natto Using Pichia pastoris GS115 and Assessment of Its Thrombolytic activity. BMC Biotechnol. 2021, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, H.; Li, W.; Zhang, C.; Liu, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, F. Nattokinase-Heparin Exhibits Beneficial Efficacy and Safety—An Optimal Strategy for CKD Patients on Hemodialysis. Glycoconj. J. 2019, 36, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-Y.; Kim, T.-S.; Cai, J.; Kim, J.; Kim, Y.; Shin, K.; Kim, K.S.; Park, S.K.; Lee, S.-P.; Choi, E.-K.; et al. Nattokinase Improves Blood Flow by Inhibiting Platelet Aggregation and Thrombus Formation. Lab. Anim. Res. 2013, 29, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ohnishi, K.; Takaoka, S.; Ogasawara, K.; Fukuyama, R.; Nakamuta, H. Antihypertensive Effects of Continuous Oral Administration of Nattokinase and Its Fragments in Spontaneously Hypertensive Rats. Biol. Pharm. Bull. 2011, 34, 1696–1701. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s Disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Paramanick, D.; Singh, V.D.; Singh, V.K. Neuroprotective Effect of Phytoconstituents via Nanotechnology for Treatment of Alzheimer Diseases. J. Control Release 2022, 351, 638–655. [Google Scholar] [CrossRef]
- Naik, S.; Katariya, R.; Shelke, S.; Patravale, V.; Umekar, M.; Kotagale, N.; Taksande, B. Nattokinase Prevents β-Amyloid Peptide (Aβ1-42) Induced Neuropsychiatric Complications, Neuroinflammation and BDNF Signalling Disruption in Mice. Eur. J. Pharmacol. 2023, 952, 175821. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lendel, C. Extracellular Protein Components of Amyloid Plaques and Their Roles in Alzheimer’s Disease Pathology. Mol. Neurodegener. 2021, 16, 59. [Google Scholar] [CrossRef]
- Fadl, N.N.; Ahmed, H.H.; Booles, H.F.; Sayed, A.H. Serrapeptase and Nattokinase Intervention for Relieving Alzheimer’s Disease Pathophysiology in Rat Model. Hum. Exp. Toxicol. 2013, 32, 721–735. [Google Scholar] [CrossRef]
- Sheng, M.; Sabatini, B.L.; Südhof, T.C. Synapses and Alzheimer’s Disease. Cold Spring Harb. Perspect. Biol. 2012, 4, a005777. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.-L.; Lee, K.-T.; Wang, J.-H.; Lee, L.Y.L.; Chen, R.P.Y. Amyloid-Degrading Ability of Nattokinase from Bacillus subtilis Natto. J. Agric. Food Chem. 2009, 57, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ni, A.; Li, H.; Wang, R.; Sun, R.; Zhang, Y. Degradation of Amyloid β-Peptides Catalyzed by Nattokinase in vivo and in vitro. Food Sci. Hum. Wellness 2023, 12, 1905–1916. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Elsherbiny, M.; Nussbaum, J.; Othman, A.; Megyerdi, S.; Tawfik, A. Targeting Neovascularization in Ischemic Retinopathy: Recent Advances. Expert. Rev. Ophthalmol. 2013, 8, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ng, T.K.; Chen, W.; Sun, X.; Huang, D.; Zheng, D.; Yi, J.; Xu, Y.; Zhuang, X.; Chen, S. Nattokinase Attenuates Retinal Neovascularization Via Modulation of Nrf2/HO-1 and Glial Activation. Investig. Ophthalmol. Vis. Sci. 2021, 62, 25. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Huang, Z.; Chu, W.K.; Ng, T.K.; Chen, S.; Liang, J.; Chen, C.-B.; Xu, Y.; Xie, B.; Ke, S.; Liu, Q.; et al. Protective Effects of Nattokinase Against Microvasculopathy and Neuroinflammation in Diabetic Retinopathy. J. Diabetes 2023, 15, 866–880. [Google Scholar] [CrossRef]
- Chung, E.J.; Roh, M.I.; Kwon, O.W.; Koh, H.J. Effects of Macular Ischemia on the Outcome of Intravitreal Bevacizumab Therapy for Diabetic Macular Edema. Retina 2008, 28, 957–963. [Google Scholar] [CrossRef]
- Suwa, T.; Kobayashi, M.; Nam, J.-M.; Harada, H. Tumor Microenvironment and Radioresistance. Exp. Mol. Med. 2021, 53, 1029–1035. [Google Scholar] [CrossRef]
- Emmanuel, N.K.; Antonios, K.; Athanasios, S.; Dimitrios, S.; Aikaterini, M.; Nikolaos, G.; Michail, D.; Kyveli, A.; Georgios, T.; Athanasios, P.; et al. Role of Oncogenes and Tumor-Suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009. [Google Scholar] [CrossRef]
- Kou, F.; Wu, L.; Ren, X.; Yang, L. Chromosome Abnormalities: New Insights into Their Clinical Significance in Cancer. Mol. Ther. Oncolytics 2020, 17, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.R.; Buha Djordjevic, A. Heavy Metal and Pesticide Exposure: A Mixture of Potential Toxicity and Carcinogenicity. Curr. Opin. Toxicol. 2020, 19, 72–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, P.; Zhou, H.; Xie, Y.; Yang, S.; Shen, W.; Hu, L.; Zhang, Y.; Liu, T.; Yang, K. Nattokinase-Mediated Regulation of Tumor Physical Microenvironment to Enhance Chemotherapy, Radiotherapy, and CAR-T Therapy of Solid Tumor. ACS Nano 2023, 17, 7475–7486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wei, P.; Gong, A.; Chiu, W.-T.; Lee, H.-T.; Colman, H.; Huang, H.; Xue, J.; Liu, M.; Wang, Y.; et al. FoxM1 Promotes β-Catenin Nuclear Localization and Controls Wnt Target-Gene Expression and Glioma Tumorigenesis. Cancer Cell 2011, 20, 427–442. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 01370. [Google Scholar] [CrossRef]
- Zhang, B.; Chai, J.; He, L.; Dusanbieke, M.; Gong, A. Nattokinase Produced by Natto Fermentation with Bacillus subtilis Inhibits Breast Cancer Growth. Int. J. Clin. Exp. Med. 2019, 12, 13380–13387. [Google Scholar]
- Yan, Y.; Wang, Y.; Qian, J.; Wu, S.; Ji, Y.; Liu, Y.; Zeng, J.; Gong, A. Nattokinase Crude Extract Inhibits Hepatocellular Carcinoma Growth in Mice. J. Microbiol. Biotechnol. 2019, 29, 1281–1287. [Google Scholar] [CrossRef]
- Yao, Y.S.; Li, T.D.; Zeng, Z.H. Mechanisms Underlying Direct Actions of Hyperlipidemia on Myocardium: An Updated Review. Lipids Health Dis. 2020, 19, 23. [Google Scholar] [CrossRef]
- Rauf, A.; Akram, M.; Anwar, H.; Daniyal, M.; Munir, N.; Bawazeer, S.; Bawazeer, S.; Rebezov, M.; Bouyahya, A.; Shariati, M.A.; et al. Therapeutic Potential of Herbal Medicine for the Management of Hyperlipidemia: Latest Updates. Environ. Sci. Pollut. Res. 2022, 29, 40281–40301. [Google Scholar] [CrossRef]
- Iwai, K.; Nakaya, N.; Kawasaki, Y.; Matsue, H. Antioxidative Functions of Natto, A Kind of Fermented Soybeans: Effect on LDL Oxidation and Lipid Metabolism in Cholesterol-Fed Rats. J. Agric. Food Chem. 2002, 50, 3597–3601. [Google Scholar] [CrossRef]
- Yang, N.C.; Chou, C.W.; Chen, C.Y.; Hwang, K.L.; Yang, Y.C. Combined Nattokinase with Red Yeast Rice but not Nattokinase Alone has Potent Effects on Blood Lipids in Human Subjects with Hyperlipidemia. Asia Pac. J. Clin. Nutr. 2009, 18, 310–317. [Google Scholar] [PubMed]
- Lu, Y.; Ding, H.; Jiang, X.; Zhang, H.; Ma, A.; Hu, Y.; Li, Z. The Effects of the Extract from Peanut Meal Fermented with Bacillus natto and Monascus on Lipid Metabolism and Intestinal Barrier Function of Hyperlipidemic in Mice. J. Sci. Food Agric. 2021, 101, 2561–2569. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kondo, K.; Matsumoto, Y.; Zhao, B.-Q.; Otsuguro, K.; Maeda, T.; Tsukamoto, Y.; Urano, T.; Umemura, K. Dietary Supplementation of Fermented Soybean, Natto, Suppresses Intimal Thickening and Modulates the Lysis of Mural Thrombi after Endothelial Injury in Rat Femoral Artery. Life Sci. 2003, 73, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, J.; Zhang, F.; Li, Y.; Wang, R.; Zheng, Q.; Zhang, X.; Zeng, J.; Xu, F.; Lin, Y. Effective Management of Atherosclerosis Progress and Hyperlipidemia with Nattokinase: A Clinical Study with 1,062 Participants. Front. Cardiovasc. Med. 2022, 9, 964977. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.N.; Chen, H.J.; Li, Y.; McGowan, G.W.; Lin, Y.G. A Clinical Study on the Effect of Nattokinase on Carotid Artery Atherosclerosis and Hyperlipidaemia. Natl. Med. J. China 2017, 97, 2038–2042. [Google Scholar] [CrossRef]
- Arribas, B.; Rodríguez-Cabezas, M.E.; Camuesco, D.; Comalada, M.; Bailón, E.; Utrilla, P.; Nieto, A.; Concha, A.; Zarzuelo, A.; Gálvez, J. A Probiotic Strain of Escherichia coli, Nissle 1917, Given Orally Exerts Local and Systemic Anti-Inflammatory Effects in Lipopolysaccharide-Induced Sepsis in Mice. Br. J. Pharmacol. 2009, 157, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhang, J.; Yang, Y.; Xia, Y.; Liu, L.; Liu, L.; Wang, Q.; Gao, X. Nattokinase Enhances the Preventive Effects of Escherichia coli Nissle 1917 on Dextran Sulfate Sodium-Induced Colitis in Mice. World J. Microbiol. Biotechnol. 2022, 39, 8. [Google Scholar] [CrossRef]
- Zhou, L.; Hao, N.; Li, X.; Chen, J.; Yang, R.; Song, C.; Sun, Y.; Zhang, Q. Nattokinase Mitigated Dextran Sulfate Sodium-induced Chronic Colitis by Regulating Microbiota and Suppressing Tryptophan Metabolism via Inhibiting IDO-1. J. Funct. Foods 2020, 75, 104251. [Google Scholar] [CrossRef]
- He, A.; Wang, Z.; Wu, X.; Sun, W.; Yang, K.; Feng, W.; Wang, Y.; Song, H. Incidence of Post-Stroke Cognitive Impairment in Patients with First-Ever Ischemic Stroke: A Multicenter Cross-Sectional Study in China. Lancet Reg. Health–West. Pac. 2023, 33, 100687. [Google Scholar] [CrossRef]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise Hormone Irisin is a Critical Regulator of Cognitive Function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Q.; Xu, P.; Chen, J.; Duan, L.; Xu, F.; Zhang, F. Nattokinase Promotes Post-stroke Neurogenesis and Cognition Recovery via Increasing Circulating Irisin. J. Agric. Food Chem. 2023, 71, 11418–11428. [Google Scholar] [CrossRef]
- Astrup, T.; Müllertz, S. The Fibrin Plate Method for Estimating Fibrinolytic Activity. Arch. Biochem. Biophys. 1952, 40, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; Song, J.Y.; Kim, K.M.; Kim, M.K.; Lee, I.Y.; Kim, S.B.; Kim, H.S.; Han, N.S.; Lee, B.H.; Kim, B.S. Production of Nattokinase by Batch and Fed-Batch Culture of Bacillus subtilis. N. Biotechnol. 2010, 27, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, M.; Zheng, D.; Kong, F.; Zu, G.; Feng, Y. Purification and Characterization of Nattokinase from Bacillus subtilis Natto B-12. J. Agric. Food Chem. 2009, 57, 9722–9729. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Mahanty, B.; Daverey, A.; Dutta, K. Nattokinase Production from Bacillus subtilis Using Cheese Whey: Effect of Nitrogen Supplementation and Dynamic Modelling. J. Water Process. Eng. 2020, 38, 101533. [Google Scholar] [CrossRef]
- Weng, M.; Deng, X.; Bao, W.; Zhu, L.; Wu, J.; Cai, Y.; Jia, Y.; Zheng, Z.; Zou, G. Improving the Activity of the Subtilisin Nattokinase by Site-Directed Mutagenesis and Molecular Dynamics Simulation. Biochem. Biophys. Res. Commun. 2015, 465, 580–586. [Google Scholar] [CrossRef]
- Yuki, Y.; Nakagawa, T.; Fujita, M.; Asada, A.; Nakanishi, K.; Kato, K. A Sandwich Enzyme-linked Immunosorbent Assay for Nattokinase. Biosci. Biotechnol. Biochem. 1994, 58, 366–370. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Q.; Jiang, F.; Liu, Z.; Lai, Y.; He, J.; Li, B. A New Bacillus Subtilis Strain and Its Use in Preparing Medicine for Treating Thrombosis. U.S. Patent 11/913,002, 14 August 2008. [Google Scholar]
- Yang, Y. Research on breeding of Bacillus subtilis natto by ultraviolet mutation. In Proceedings of the Biophysical Society of GuangDong Province Academic Forum: Precise Photons and Life Health (PPLH 2022), Guangzhou, China, 9–11 December 2022; SPIE: Bellingham, WA, USA, 11 December 2022; Volume 12603, pp. 21–25. [Google Scholar]
- Wang, Y.; Wang, J.; Zhang, X.; Tong, Y.; Yang, R. Genomic and Transcriptomic Analysis of Bacillus subtilis JNFE1126 with Higher Nattokinase Production Through Ultraviolet Combined 60Co-γ Ray Mutagenesis. LWT 2021, 147, 111652. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhang, C.; Yang, Y.L.; Diao, M.; Bai, M.F. Screening of a High Fibrinolytic Enzyme Producing Strain and Characterization of the Fibrinolytic Enzyme Produced from Bacillus subtilis LD-8547. World J. Microbiol. Biotechnol. 2008, 24, 475–482. [Google Scholar] [CrossRef]
- Gu, Z.; Ning, C.; Liu, Z.; Liang, Q.; Fu, X.; Tian, M.; Zhu, C.; Mou, H. High-Efficiency Heterologous Expression of Nattokinase Based on a Combinatorial Strategy. Process Biochem. 2023, 133, 65–74. [Google Scholar] [CrossRef]
- Wu, S.-M.; Feng, C.; Zhong, J.; Huan, L.-D. Enhanced Production of Recombinant Nattokinase in Bacillus subtilis by Promoter Optimization. World J. Microbiol. Biotechnol. 2011, 27, 99–106. [Google Scholar] [CrossRef]
- Li, C.; Du, Z.; Qi, S.; Zhang, X.; Wang, M.; Zhou, Y.; Lu, H.; Gu, X.; Tian, H. Food-grade expression of nattokinase in Lactobacillus delbrueckii subsp. bulgaricus and its thrombolytic activity in vitro. Biotechnol. Lett. 2020, 42, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Guo, P.-C.; Jiang, W.-L.; Fan, X.-M.; Luo, X.-Y.; Li, H.-H. Expression of Nattokinase in Escherichia coli and Renaturation of its inclusion body. J. Biotechnol. 2016, 231, 65–71. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xiong, S.; Zhang, J.; Cai, L.; Yang, Y. Expression and Purification of Recombinant Nattokinase in Spodoptera frugiperda cells. Biotechnol. Lett. 2007, 29, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, L.; Liu, J.; Li, H.; Wang, Y.; Hasi, A. Transient Expression of Optimized and Synthesized Nattokinase Gene in Melon (Cucumis melo L.) Fruit by Agroinfiltration. Plant Biotechnol. 2015, 32, 175–180. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, H.; Han, L.; Cui, W.; Zhou, L.; Zhou, Z. Improvement of the acid resistance, catalytic efficiency, and thermostability of nattokinase by multisite-directed mutagenesis. Biotechnol. Bioeng. 2019, 116, 1833–1843. [Google Scholar] [CrossRef]
- Li, T.; Zhan, C.; Guo, G.; Liu, Z.; Hao, N.; Ouyang, P. Tofu Processing Wastewater as a Low-Cost Substrate for High Activity Nattokinase Production Using Bacillus subtilis. BMC Biotechnol. 2021, 21, 57. [Google Scholar] [CrossRef]
- Ju, S.; Cao, Z.; Wong, C.; Liu, Y.; Foda, M.F.; Zhang, Z.; Li, J. Isolation and Optimal Fermentation Condition of the Bacillus subtilis Subsp. natto Strain WTC016 for Nattokinase Production. Fermentation 2019, 5, 92. [Google Scholar] [CrossRef]
- Keziah, S.M.; Devi, C.S. Fibrinolytic and ACE Inhibitory Activity of Nattokinase Extracted from Bacillus subtilis VITMS 2: A Strain Isolated from Fermented Milk of Vigna unguiculata. Protein J. 2021, 40, 876–890. [Google Scholar] [CrossRef]
- Xiao, Z.; Shen, J.; Li, Y.; Wang, Z.; Zhao, Y.; Chen, Y.; Zhao, J.-y. High and Economical Nattokinase Production with Acetoin as a Useful Byproduct from Soybean Milk and Glucose. Probiotics Antimicrob. Proteins 2022, 14, 792–803. [Google Scholar] [CrossRef]
- Mahajan, P.M.; Gokhale, S.V.; Lele, S.S. Production of Nattokinase Using Bacillus natto NRRL 3666: Media Optimization, Scale Up, and Kinetic Modeling. Food Sci. Biotechnol. 2010, 19, 1593–1603. [Google Scholar] [CrossRef]
- Pan, S.; Chen, G.; Zeng, J.; Cao, X.; Zheng, X.; Zeng, W.; Liang, Z. Fibrinolytic Enzyme Production from Low-Cost Substrates by Marine Bacillus subtilis: Process Optimization and Kinetic Modeling. Biochem. Eng. J. 2019, 141, 268–277. [Google Scholar] [CrossRef]
- Garg, R.; Thorat, B.N. Nattokinase Purification by Three Phase Partitioning and Impact of T-butanol on Freeze Drying. Sep. Purif. Technol. 2014, 131, 19–26. [Google Scholar] [CrossRef]
- Avhad, D.N.; Niphadkar, S.S.; Rathod, V.K. Ultrasound Assisted Three Phase Partitioning of a Fibrinolytic Enzyme. Ultrason. Sonochem 2014, 21, 628–633. [Google Scholar] [CrossRef]
- Hu, H.-B.; Yao, S.-J.; Mei, L.-H.; Zhu, Z.-Q.; Hur, B.-K. Partial Purification of Nattokinase from Bacillus subtilis by Expanded Bed Adsorption. Biotechnol. Lett. 2000, 22, 1383–1387. [Google Scholar] [CrossRef]
- Yang, C.; Xing, J.; Guan, Y.; Liu, H. Superparamagnetic Poly(methyl methacrylate) Beads for Nattokinase Purification from Fermentation Broth. Appl. Microbiol. Biotechnol. 2006, 72, 616–622. [Google Scholar] [CrossRef]
- Liu, C.-H.; Wu, W.-C.; Lai, H.-Y. Adsorption of Nattokinase by Amino Acid-Conjugated Magnetic Nanoadsorbents. Sep. Sci. Technol. 2013, 48, 923–930. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Zeng, X. Research Progress on the Utilisation of Embedding Technology and Suitable Delivery Systems for Improving the Bioavailability of Nattokinase: A Review. Food Struct. 2021, 30, 100219. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Kong, F.-P.; Yuan, G.-Y.; Wei, F.; Jiang, M.-L.; Li, G.-M.; Wang, Z.; Zhao, Y.-D.; Chen, H. Optimisation of Preparation Conditions and Properties of Phytosterol Liposome-Encapsulating Nattokinase. Nat. Prod. Res. 2012, 26, 548–556. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Liu, C.; Li, J.; Li, Z.; Zhao, J.; Liu, H. Synthesis of Sustained Release/Controlled Release Nanoparticles Carrying Nattokinase and Their Application in Thrombolysis. Pharmazie 2021, 76, 145–149. [Google Scholar] [CrossRef]
- Kapoor, R.; Harde, H.; Jain, S.; Panda, A.K.; Panda, B.P. Downstream Processing, Formulation Development and Antithrombotic Evaluation of Microbial Nattokinase. J. Biomed. Nanotechnol. 2015, 11, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lyu, X.; Tong, Y.; Wang, J.; Ye, J.; Yang, R. Chitosan/Casein Based Microparticles with a Bilayer Shell–core Structure for Oral Delivery of Nattokinase. Food Funct. 2020, 11, 10799–10816. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Lü, S.; Xu, X.; Ning, P.; Wang, Z.; Zhang, Z.; Gao, C.; Liu, Y.; Liu, M. A Polysaccharide-Based Micelle-Hydrogel Synergistic Therapy System for Diabetes and Vascular Diabetes Complications Treatment. Mater. Sci. Eng. C 2019, 100, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-s.; Zhou, M.-j.; Hu, L.-x.; Huang, K.; Zhao, S.-g.; Qian, S.-h.; Wang, Z.; Huang, X.-l.; Huang, J.-B.; Wang, J.; et al. Preparation and Property Studies of Enteric-Soluble Oral Nattokinase Microcapsules with Halloysite Nanotubes. Int. J. Polym. Mater. 2023, 1–13. [Google Scholar] [CrossRef]
- Wu, C.; Gao, C.; Lü, S.; Xu, X.; Wen, N.; Zhang, S.; Liu, M. Construction of Polylysine Dendrimer Nanocomposites Carrying Nattokinase and Their Application in Thrombolysis. J. Biomed. Mater. Res. Part. A 2018, 106, 440–449. [Google Scholar] [CrossRef]
- Ye, W.; Wang, N.; Hu, K.; Zhang, L.; Liu, A.; Pan, C.; Gong, T.; Liu, T.; Ding, H. Bio-inspired Microcapsule for Targeted Antithrombotic Drug Delivery. RSC Adv. 2018, 8, 27253–27259. [Google Scholar] [CrossRef]
- Deepak, V.; Ram Kumar Pandian, S.b.; Kalishwaralal, K.; Gurunathan, S. Purification, Immobilization, and Characterization of Nattokinase on PHB Nanoparticles. Bioresour. Technol. 2009, 100, 6644–6646. [Google Scholar] [CrossRef]
- Li, D.; Hu, M.; Hou, L.; Gao, Y.; Tian, Z.; Wen, W.; Fan, B.; Li, S.; Wang, F. The Structural and Functional Properties of Soybean Protein-Polyglutamic Acid Complex Effected the Stability of W/O/W Emulsion Encapsulated Nattokinase. Food Chem. 2023, 414, 135724. [Google Scholar] [CrossRef]

| Method | Principle | Pros | Cons | Ref. |
|---|---|---|---|---|
| Fibrin plate method | Nattokinase degrades the artificial thrombus to form a dissolving circle | Measures multiple samples at the same time | Detection accuracy is greatly affected by time, plate thickness, and product purity | [72] |
| Fibrin degradation method | Fibrin degradation products have an absorption peak at 275 nm | High accuracy | Poor stability and high concentration requirements for sample dilutions, as well as cumbersome operation and long determination time | [73] |
| CLT method | Timing the hydrolysis of fibrin by nattokinase for comparison with the standards | Rapid reaction, suitable for the determination of crude enzymes | Not suitable for simultaneous measurement of multiple samples and products with high sensitivity | [74] |
| Folin phenol method | Nattokinase hydrolyzes casein to produce tyrosine, which reacts with a Folin phenol reagent for color development | Multiple samples can be measured simultaneously at low cost. | Low specificity, and a determination of all protease activities | [75] |
| TAME method | Nattokinase cleaves arginine carboxy-terminal peptide and undergoes a chromogenic reaction | High reaction sensitivity and simple operation | Impurities in the crude enzyme solution has a greater impact on the results | [6] |
| Tetrapeptide substrate method | Hydrolysis of Suc-Ala-Pro-Phe-pNA with a chromogenic reaction | Simple and highly sensitive method for the rapid determination of enzyme activities | The assay does not directly reflect the fibrinolytic activity of nattokinase | [76] |
| ELISA method | Enzyme-linked reaction to determine nattokinase enzyme activity by the combination of marker enzymes and catalase | High specificity and sensitivity | Complex operation and high testing cost | [77] |
| HPLC method | Protein analytical columns for the detection of nattokinase | Fast analysis, good selectivity, and high sensitivity. | Not suitable for detecting crude enzymes, and there will be a great deal of stray peaks | [78] |
| Recombinant Expression Vectors | Hosts | Characteristics |
|---|---|---|
| pP43NMK-NKF | B. subtilis WB800 | Screening high-expression signal peptides for nattokinase and optimizing the signal peptide’s start codon from GTG to ATG. Establishing a dual promoter system and employing a combinatorial strategy to enhance nattokinase production [82]. |
| pHY10 | B. subtilis DB104 | Replacing the -10 box of the aprN promoter with the consensus sequence (TATAAT) of the σA-dependent promoter [83]. |
| pMGthyA-ppNK | Lactobacillus delbrueckii subsp. bulgaricus | Employing a chromosomal–plasmid complementation expression system, with the thyA gene serving as a complementary marker, to ensure that the product expression remains unhindered by antibiotic resistance in the system. The expression vector and host strain are both of food-grade quality [84]. |
| pHY-SP-NK | B. licheniformis△0F-3 | Constructing an expression vector within a gene-deficient host strain, whereby the aprE signal peptide is incorporated and the type I signal peptidase gene sipV is overexpressed [14]. |
| pQE30-m-NK | E. coli BL21 (DE3) | A substantial amount of inactive insoluble protein was detected in the inclusion bodies [85]. |
| pHBM905BDM-NK-Bs | Pichia pastoris GS115 | The promoter of the vector pHBM905BDM significantly enhances protein expression levels. The expression level of nattokinase reaches its maximum, when there are five copies of the aprN gene [32]. |
| Bacmid-egfp-NK | Spodoptera frugiperda cells | The Bac-to-Bac system was employed to express nattokinase that was fused with a His tag in insect cells while retaining its fibrinolytic activity [86]. |
| pPZP35SNi | Cucumis melo L. | Transient expression of the sNKi gene in melon fruit using the E8 promoter-involved expression system resulted in higher nattokinase activity [87]. |
| Strain | Nitrogen Source | Carbon Source | Mineral Salt | Loading Volume | Temperature | pH | Enzyme Activity | Ref. |
|---|---|---|---|---|---|---|---|---|
| B. subtilis 13932 | 93.669% Tofu process wastewater | 3% Glucose | 0.1% MgSO4·7H2O 0.08% CaCl2 | 70 mL/500 mL Flask | 37 °C | 7.0 | 7209 ± 195 IU/mL (fibrin plate method) | [89] |
| B. subtilis MTCC 2616 | Cheese whey 10 g/L Yeast extract | Cheese whey | - | 50 mL/250 mL Flask | 30 °C | - | 833.43 U/mL (Folin phenol method) | [75] |
| B. subtilis Subsp. Natto Strain WTC016 | 1% Peptone 0.5% Yeast extract | - | 1% NaCl | 60 mL/250 mL Flask | 30 °C | 7.0 | 3284 ± 58 IU/mL (fibrin plate method) | [90] |
| B. subtilis JNFE0126 | 3% Kidney bean flour | 2% Sucrose | 0.2% NaH2PO4 0.4% K2HPO4 0.4% NaCl 0.02% CaCl2 | - Flask | 40 °C | 7.0 | 3511 U/mL (fibrin plate method) | [10] |
| B. subtilis VITMS 2 | 2% Soya bean waste 1.5% Eggshell power | 2% Cane molasses | 10 mM CaCl2 10 mM MgSO4 10 mM Na2HPO4 10 mM K2HPO4 | - Flask | 30 °C | 7.0 | 2748.76 FU/mL (modified fibrin degradation method) | [91] |
| B. subtilis NDF | 18% Soybean milk | 10.5% Glucose | - | 4 L/6 L Batch | 30 °C | 7.0 | 10,220 IU/mL (fibrin plate method) | [92] |
| B. subtilis | 5% Peptone 0.3% NH4Cl | 1% Glucose | 0.1% K2HPO4 0.2% MgSO4·7H2O Trace elements | 2 L/5 L Batch | 37 °C | 7.0 | 3400 U/mL (modified fibrin degradation method) | [73] |
| Bacillus natto NRRL 3666 | 1% Soy peptone 0.325% Yeast extract | 2% Glucose | 0.0407% K2HPO4·3H2O 0.044% MgSO4·7H2O 0.05% CaCl2·2H2O | 2.5 L/5 L Batch | 37 °C | 6.3 | 1932 U/mL (tetrapeptide substrate method) | [93] |
| B. subtilis D21-8 | 2.32% Soybean meal | 2.41% Cassava starch | 0.16% CaCl2 | 7.5 L/10 L Batch | 34 °C | 5.0–5.2 | 3203 U/mL (fibrin plate method) | [94] |
| Embedding Material | Property | Pros | Ref. | |
|---|---|---|---|---|
| Lipids, polysaccharides, proteins | Lecithin, phytosterol, and mannite | 65.25% 1 | Biocompatibility, fast release, and easy absorption | [101] |
| Sodium alginate, carboxymethyl chitosan, and Fe3O4 | 65.24% 2 | Sustained release and magnetic targeting | [102] | |
| Chitosan, tripolyphosphate, and glutaraldehyde | >90% 1 | Inexpensive raw materials and simple manufacture | [103] | |
| Casein and chitosan | 73.8% 1 | Safer and better tolerated | [104] | |
| Chitosan/dialdehyde starch derivatives micelle-hydrogel | 89.4% 2 | Easy self-assembly | [105] | |
| Artificial synthetic polymer | Eudragit L100-55-halloysite nanotubes | 31.6% 1 | Sustained release, and a reduction in the side effects caused by excessive thrombolysis | [106] |
| Polylysine dendrimer | 117% 3 | Biocompatibility and biodegradability | [107] | |
| Polystyrene and polydopamine | 75% 4 | Targets activated platelets with high affinity | [108] | |
| Polyhydroxybutyrate | 120% 3 | Increased enzyme stability | [109] | |
| W/O/W emulsions | Soybean isolate protein and polyglutamic acid | 97.19% 1 | Compact structure and good stability | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, M.; Yuan, B.; Wang, M.; Liu, J.; Wang, Z. Nattokinase: Insights into Biological Activity, Therapeutic Applications, and the Influence of Microbial Fermentation. Fermentation 2023, 9, 950. https://doi.org/10.3390/fermentation9110950
Fang M, Yuan B, Wang M, Liu J, Wang Z. Nattokinase: Insights into Biological Activity, Therapeutic Applications, and the Influence of Microbial Fermentation. Fermentation. 2023; 9(11):950. https://doi.org/10.3390/fermentation9110950
Chicago/Turabian StyleFang, Mudannan, Beichen Yuan, Meng Wang, Junfeng Liu, and Zheng Wang. 2023. "Nattokinase: Insights into Biological Activity, Therapeutic Applications, and the Influence of Microbial Fermentation" Fermentation 9, no. 11: 950. https://doi.org/10.3390/fermentation9110950




