Research on the Effect of Simultaneous and Sequential Fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum on Antioxidant Activity and Flavor of Apple Cider
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material, Yeast and Bacterial Strains, and Culture Media
2.2. Apple Cider Making
2.3. Basic Oenological Analysis
2.4. Determination of Microbial Counts
2.5. Determination of Antioxidant Activity
2.6. Volatile Compound Analysis
2.7. Non-Volatile Compound Analysis
2.8. Sensory Analysis
2.9. Statistical Analyses
3. Results
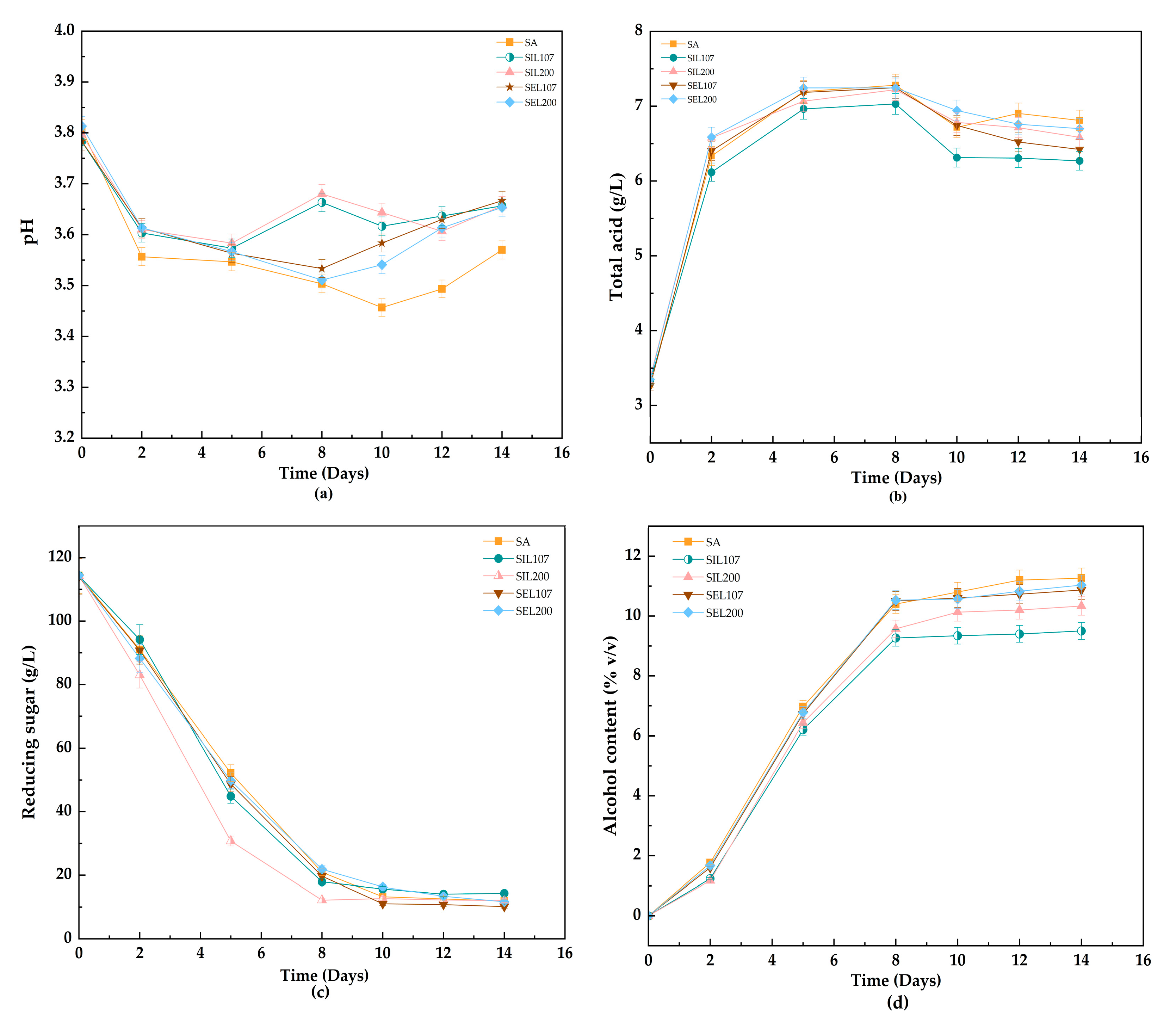
3.1. Dynamic Evolution of Basic Oenological Parameters
3.2. Evolution of Microbial Populations
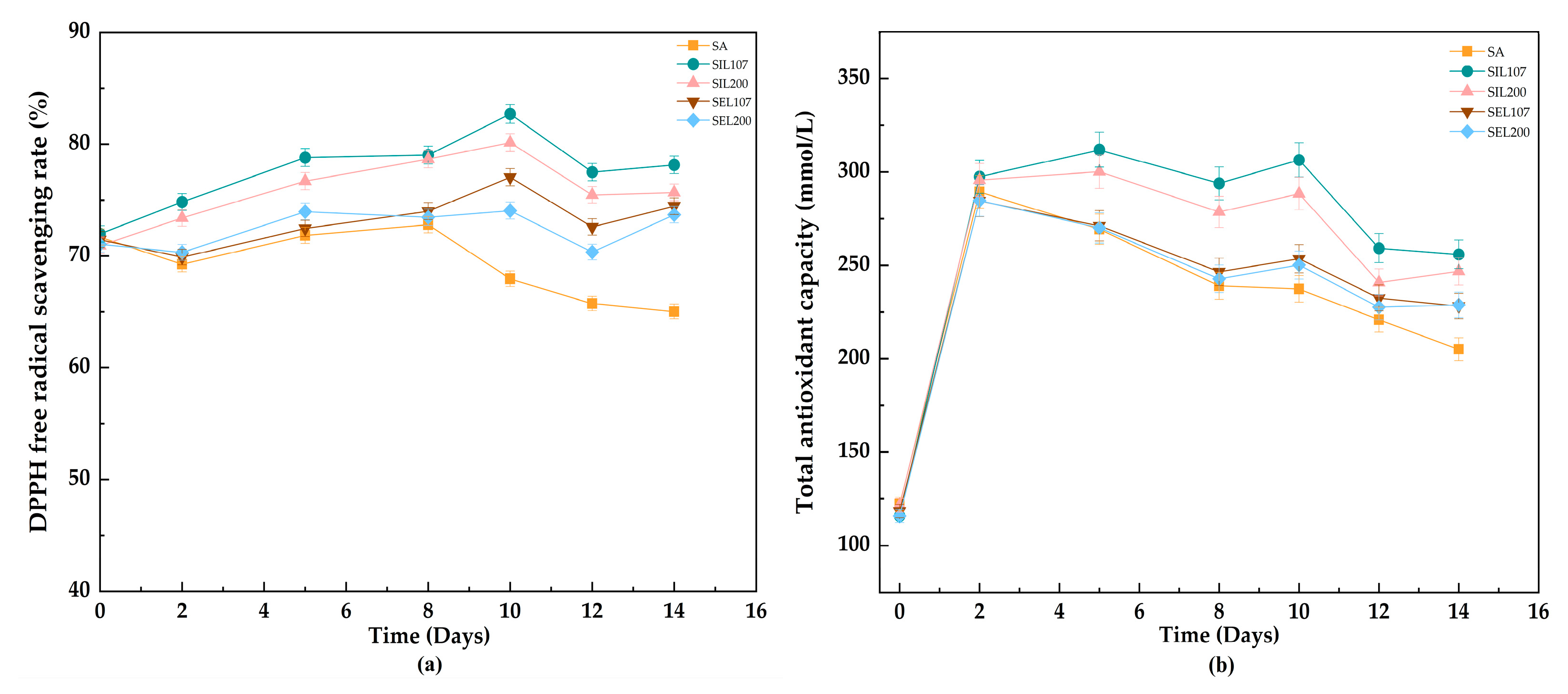
3.3. Evolution of Antioxidant Activity
3.4. Analysis of Volatiles
3.5. Analysis of Non-Volatiles
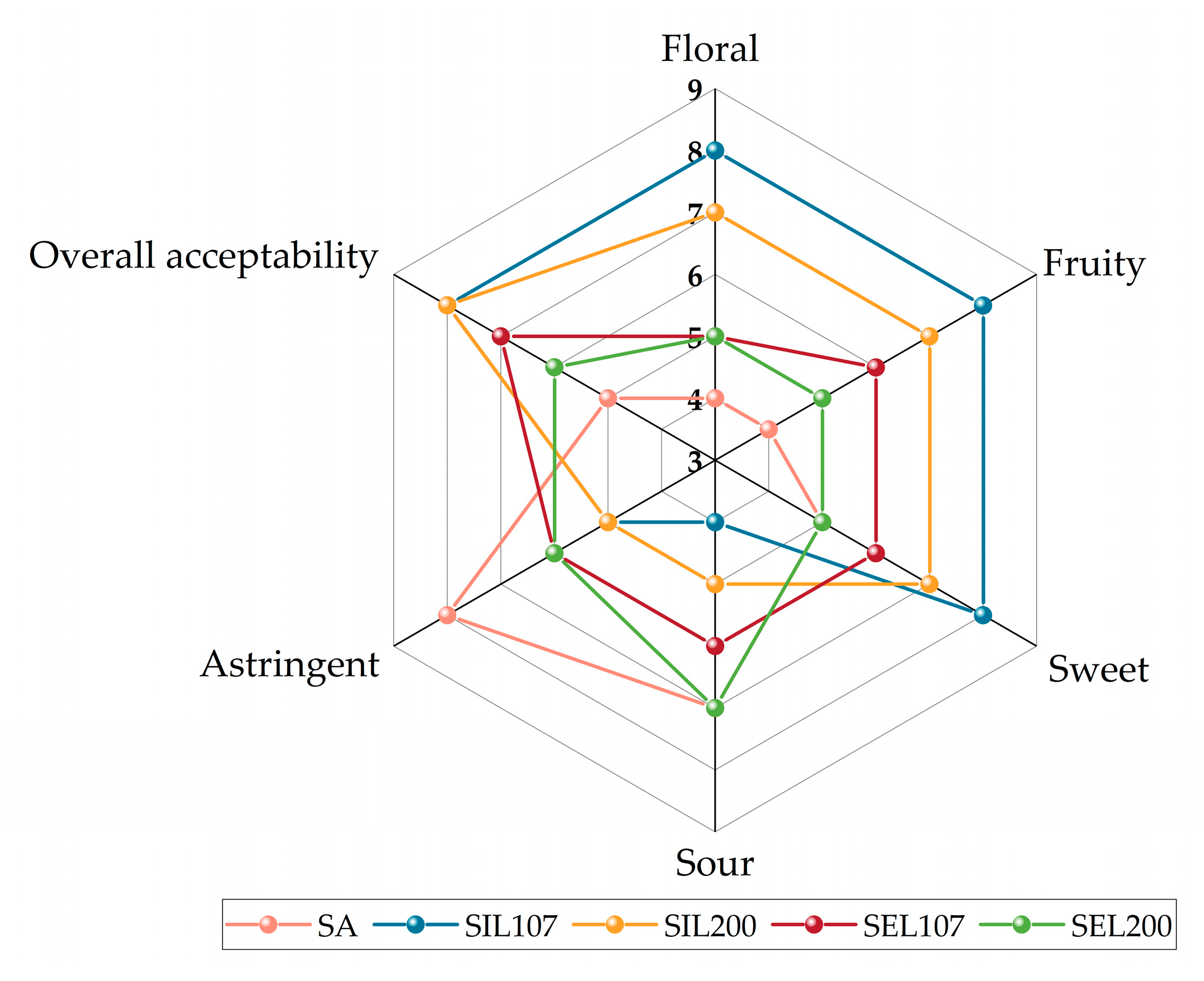
3.6. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Ma, F.; Zhang, M.; Pu, F. Distribution and metabolism of ascorbic acid in apple fruits (Malus domestica Borkh cv. Gala). Plant Sci. 2008, 174, 606–612. [Google Scholar] [CrossRef]
- Argenta, L.C.; Do Amarante, C.V.T.; de Freitas, S.T.; Brancher, T.L.; Nesi, C.N.; Mattheis, J.P. Fruit quality of ‘Gala’ and ‘Fuji’ apples cultivated under different environmental conditions. Sci. Hortic. 2022, 303, 111195. [Google Scholar] [CrossRef]
- Wei, J.P.; Zhang, Y.X.; Qiu, Y.; Guo, H.; Ju, H.M.; Wang, Y.W.; Yuan, Y.H.; Yue, T.L. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhao, H.; Kang, X.; Ge, X.; Zheng, M.; Hu, Z.; Tao, Y. Fruity aroma modifications in Merlot wines during simultaneous alcoholic and malolactic fermentations through mixed culture of S. cerevisiae, P. fermentans, and L. brevis. LWT-Food Sci. Technol. 2022, 154, 112711. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. The effects of simultaneous and sequential inoculation of yeast and autochthonous Oenococcus oeni on the chemical composition of red-fleshed apple cider. LWT-Food Sci. Technol. 2020, 124, 109184. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Changes in the concentration of yeast-derived volatile compounds of red wine during malolactic fermentation with four commercial starter cultures of Oenococcus oeni. J. Agric. Food Chem. 2005, 53, 10134–10139. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Che, C.Y.; Sun, T.F.; Lv, Z.Z.; He, S.X.; Gu, H.N.; Shen, W.J.; Chi, D.C.; Gao, Y. Evaluation of sequential inoculation of Saccharomyces cerevisiae and Oenococcus oeni strains on the chemical and aromatic profiles of cherry wines. Food Chem. 2013, 138, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT-Food Sci. Technol. 2016, 73, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.Y.; Gong, H.S.; Liu, W.L.; Jin, C.W. Application and validation of autochthonous Lactobacillus plantarum starter cultures for controlled malolactic fermentation and its influence on the aromatic profile of cherry wines. Food Microbio. 2016, 55, 16–24. [Google Scholar] [CrossRef]
- Massera, A.; Soria, A.; Catania, C.; Krieger, S.; Combina, M. Simultaneous inoculation of Malbec musts with yeast and bacteria: Effects on fermentation performance, sensory and sanitary attributes of wines. Food Technol. Biotechnol. 2009, 47, 192–201. [Google Scholar]
- Jussier, D.; Morneau, A.L.D.; de Ordun A, R.N.M. Effect of simultaneous inoculation with yeast and bacteria on fermentation kinetics and key wine parameters of cool-climate chardonnay. Appl. Environ. Microbio. 2006, 72, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniasuri, F.; Lee, P.; Liu, S. Induction of simultaneous and sequential malolactic fermentation in durian wine. Int. J. Food Microbio. 2016, 230, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, S. Transformation of chemical constituents of lychee wine by simultaneous alcoholic and malolactic fermentations. Food Chem. 2016, 196, 988–995. [Google Scholar] [CrossRef]
- Nehme, N.; Mathieu, F.; Taillandier, P. Quantitative study of interactions between Saccharomyces cerevisiae and Oenococcus oeni strains. J. Ind. Microbio. Biotechnol. 2008, 35, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Liu, S.; Heponiemi, P.; Heinonen, M.; Marsol-Vall, A.; Ma, X.; Yang, B.; Laaksonen, O. Effect of Saccharomyces cerevisiae and Schizosaccharomyces pombe strains on chemical composition and sensory quality of ciders made from Finnish apple cultivars. Food Chem. 2021, 345, 128833. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, J.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef]
- Trinh, T.; Woon, W.Y.; Yu, B.; Curran, P.; Liu, S. Growth and fermentation kinetics of a mixed culture of Saccharomyces cerevisiae var. bayanus and Williopsis saturnus var. saturnus at different ratios in longan juice (Dimocarpus longan Lour.). Int. J. Food Sci. Technol. 2011, 46, 130–137. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.; Yin, P.; Ma, B.; Ma, C.; Xu, C.; Wang, J.; Wang, Z.; Yin, D.; Xia, T. Impact of prolonged withering on phenolic compounds and antioxidant capability in white tea using LC-MS-based metabolomics and HPLC analysis: Comparison with green tea. Food Chem. 2022, 368, 130855. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, F.; Yang, L.; Li, J.; Zhu, X. Enhancement of the aroma in low-alcohol apple-blended pear wine mixed fermented with Saccharomyces cerevisiae and non-Saccharomyces yeasts. LWT-Food Sci. Technol. 2022, 155, 112994. [Google Scholar] [CrossRef]
- Braga, C.M.; Zielinski, A.A.F.; Silva, K.M.D.; de Souza, F.K.F.; Pietrowski, G.D.A.M.; Couto, M.; Granato, D.; Wosiacki, G.; Nogueira, A. Classification of juices and fermented beverages made from unripe, ripe and senescent apples based on the aromatic profile using chemometrics. Food Chem. 2013, 141, 967–974. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef]
- Wei, J.P.; Wang, S.Y.; Zhang, Y.X.; Yuan, Y.H.; Yue, T.L. Characterization and screening of non-Saccharomyces yeasts used to produce fragrant cider. LWT-Food Sci. Technol. 2019, 107, 191–198. [Google Scholar] [CrossRef]
- Li, S.; Bi, P.; Sun, N.; Gao, Z.; Chen, X.; Guo, J. Characterization of different non-Saccharomyces yeasts via mono-fermentation to produce polyphenol-enriched and fragrant kiwi wine. Food Microbio. 2022, 103, 103867. [Google Scholar] [CrossRef]
- Devi, A.; Anu-Appaiah, K.A.; Lin, T. Timing of inoculation of Oenococcus oeni and Lactobacillus plantarum in mixed malo-lactic culture along with compatible native yeast influences the polyphenolic, volatile and sensory profile of the Shiraz wines. LWT-Food Sci. Technol. 2022, 158, 113130. [Google Scholar] [CrossRef]
- Verón, H.E.; Gauffin Cano, P.; Fabersani, E.; Sanz, Y.; Isla, M.I.; Fernández Espinar, M.T.; Gil Ponce, J.V.; Torres, S. Cactus pear (Opuntia ficus-indica) juice fermented with autochthonous Lactobacillus plantarum S-811. Food Funct. 2019, 10, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.L.D.S.; Freitas, H.V.; Rodrigues, S.; Abreu, V.K.G.; Lemos, T.D.O.; Gomes, W.F.; Narain, N.; Pereira, A.L.F. Production and stability of probiotic cocoa juice with sucralose as sugar substitute during refrigerated storage. LWT-Food Sci. Technol. 2019, 99, 371–378. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Ratchadaporn, K.; Orapin, K.; Natta, L.; Dipayan, S.; Kalidas, S. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017, 59, 141–149. [Google Scholar]
- Zhang, M.; Zhong, T.; Heygi, F.; Wang, Z.; Du, M. Effects of inoculation protocols on aroma profiles and quality of plum wine in mixed culture fermentation of Metschnikowia pulcherrima with Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2022, 161, 113338. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Guo, Z.; Guo, X.; Zhang, Q.; Zhao, X.; Ning, M.; Shan, C. Effects of pretreatment methods and leaching methods on jujube wine quality detected by electronic senses and HS-SPME-GC-MS. Food Chem. 2020, 330, 127330. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of co-inoculation of candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the industrial production of negroamaro wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.G.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Relationships between Godello white wine sensory properties and its aromatic fingerprinting obtained by GC–MS. Food Chem. 2011, 129, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, L.; Chen, X.; Ding, C.; Hao, M.; Peng, X.; Zhang, Y.; Zhu, H.; Liu, W. Anti-aging effect of phlorizin on D-galactose–induced aging in mice through antioxidant and anti-inflammatory activity, prevention of apoptosis, and regulation of the gut microbiota. Exp. Gerontol. 2022, 163, 111769. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A new approach to the use of apple pomace in cider making for the recovery of phenolic compounds. LWT-Food Sci. Technol. 2020, 126, 109316. [Google Scholar] [CrossRef]
- Tian, T.; Sun, J.; Wu, D.; Xiao, J.; Lu, J. Objective measures of greengage wine quality: From taste-active compound and aroma-active compound to sensory profiles. Food Chem. 2021, 340, 128179. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; La Hens, D.V.; Hollmann, A.; Delfederico, L.; Caballero, A.; Tymczyszyn, E.E.; Semorile, L. Comparative vinification assays with selected patagonian strains of Oenococcus oeni and Lactobacillus plantarum. LWT-Food Sci. Technol. 2017, 77, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Li, Y.; Nguyen, T.T.H.; Jin, J.H.; Lim, J.; Lee, J.; Piao, M.; Mok, I.; Kim, D. Brewing of glucuronic acid-enriched apple cider with enhanced antioxidant activities through the co-fermentation of yeast (Saccharomyces cerevisiae and Pichia kudriavzevii) and bacteria (Lactobacillus plantarum). Food Sci. Biotechnol. 2021, 30, 555–564. [Google Scholar] [CrossRef]
- Martínez Leal, J.; Valenzuela Suárez, L.; Jayabalan, R.; Huerta Oros, J.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA-J. Food. 2018, 16, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Kanter, J.; Benito, S.; Brezina, S.; Beisert, B.; Fritsch, S.; Patz, C.; Rauhut, D. The impact of hybrid yeasts on the aroma profile of cool climate Riesling wines. Food Chem. X 2020, 5, 100072. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Zhang, Y.X.; Wang, Y.W.; Ju, H.M.; Niu, C.; Song, Z.H.; Yuan, Y.H.; Yue, T.L. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbio. 2020, 318, 108471. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT-Food Sci. Technol. 2020, 122, 109064. [Google Scholar] [CrossRef]
- Han, M.Z.; Wang, X.W.; Zhang, M.N.; Ren, Y.P.; Yue, T.L.; Gao, Z.P. Effect of mixed Lactobacillus on the physicochemical properties of cloudy apple juice with the addition of polyphenols-concentrated solution. Food Biosci. 2021, 41, 101049. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Z.; Rehman, R.; Shen, T.; Riaz, S.; Li, X.; Hua, E.; Zhao, J. Apple phlorizin supplementation attenuates oxidative stress in hamsters fed a high-fat diet. J. Food Biochem. 2018, 42, 12445. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef]
- Wang, X.W.; Han, M.Z.; Zhang, M.N.; Wang, Y.; Ren, Y.P.; Yue, T.L.; Gao, Z.P. In vitro evaluation of the hypoglycemic properties of lactic acid bacteria and its fermentation adaptability in apple juice. LWT-Food Sci. Technol. 2021, 136, 110363. [Google Scholar] [CrossRef]
- Seyed, M.B.H.; Dornoush, J. Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: Chemical composition, antioxidant activity and sensorial properties. LWT-Food Sci. Technol. 2020, 131, 109803. [Google Scholar]
- Gao, H.; Wen, J.; Hu, J.; Nie, Q.; Chen, H.; Nie, S.; Xiong, T.; Xie, M. Momordica charantia juice with Lactobacillus plantarum fermentation: Chemical composition, antioxidant properties and aroma profile. Food Biosci. 2019, 29, 62–72. [Google Scholar] [CrossRef]



| Number | Compounds | GL | Treatment (n = 3) (μg/L) | ||||
|---|---|---|---|---|---|---|---|
| SA | SIL107 | SIL200 | SEL107 | SEL200 | |||
| Esters | |||||||
| 1 | 4-hydroxybutyrate | - | 434.33 ± 37.88 d | 1733.15 ± 60.60 a | 1707.32 ± 72.40 a | 1422.87 ± 38.86 b | 1050.73 ± 14.54 c |
| 2 | Isoamyl acetate | 1.68 ± 0.59 e | 138.48 ± 3.62 d | 288.25 ± 9.52 a | 217.59 ± 15.96 b | 184.03 ± 11.24 c | 146.72 ± 5.76 d |
| 3 | Methyl trans-cinnamate | - | 123.39 ± 10.03 d | 829.81 ± 29.42 a | 459.11 ± 29.9 c | 612.09 ± 7.77 b | 570.31 ± 41.22 b |
| 4 | Ethyl hexanoate | 2.44 ± 0.43 f | 802.73 ± 10.26 e | 1154.57 ± 68.18 a | 1017.18 ± 6.17 b | 949.13 ± 35.65 c | 873.85 ± 12.94 d |
| 5 | Hexyl acetate | 22.80 ± 0.88 c | 50.76 ± 1.19 b | 64.11 ± 1.91 a | 63.35 ± 1.00 a | 52.14 ± 0.28 b | 52.60 ± 0.61 b |
| 6 | Dioctyl phthalate | 368.91 ± 331.12 d | 804.12 ± 58.97 c | 1244.64 ± 26.22 ab | 839.41 ± 17.64 c | 1418.40 ± 8.85 a | 1148.51 ± 10.17 b |
| 7 | Diethylphosphate | 9.43 ± 0.99 c | 81.88 ± 8.15 ab | 84.43 ± 16.10 ab | 95.46 ± 4.30 a | 74.60 ± 1.10 b | 88.76 ± 3.20 ab |
| 8 | Ethyl butanoate | 23.11 ± 1.11 f | 103.38 ± 1.26 e | 164.52 ± 8.45 a | 147.86 ± 6.36 b | 117.08 ± 6.71 d | 136.37 ± 2.97 c |
| 9 | 2-Isopropylphenyl methylcarbamate | 64.47 ± 0.22 b | 191.39 ± 3.49 a | 184.17 ± 6.12 a | 179.56 ± 9.62 a | 188.93 ± 15.12 a | 193.38 ± 3.13 a |
| 10 | Methyl 3-(methylthio)propanoate | 4.91 ± 1.13 e | 12.23 ± 0.48 d | 13.18 ± 0.47 cd | 16.86 ± 0.88 a | 14.56 ± 0.26 b | 13.91 ± 0.23 bc |
| 11 | Monoethyl malonic acid | 4.41 ± 0.27 e | 36.27 ± 3.08 c | 53.69 ± 1.93 a | 44.73 ± 4.97 b | 44.56 ± 0.63 b | 25.05 ± 2.28 d |
| 12 | D-glucurono-6,3-lactone | 94.60 ± 7.38 e | 1039.44 ± 21.81 c | 1656.37 ± 28.00 a | 1142.89 ± 21.33 b | 734.44 ± 94.57 d | 167.39 ± 14.77 e |
| 13 | Phenylphosphoric acid | - | 90.61 ± 56.00 bc | 250.92 ± 95.84 a | 112.21 ± 55.70 b | 133.14 ± 40.92 b | 111.95 ± 30.92 b |
| 14 | Ethyl caprylate | 4.65 ± 0.38 e | 526.29 ± 14.97 d | 838.80 ± 18.93 a | 716.03 ± 4.20 b | 734.29 ± 4.65 b | 658.41 ± 4.89 c |
| 15 | Methyl cinnamate | 896.57 ± 1.05 f | 1786.83 ± 11.91 e | 2570.10 ± 100.18 a | 2230.46 ± 36.37 c | 2469.53 ± 16.42 b | 2040.02 ± 12.40 d |
| 16 | 4-(Trimethylammonio)butanoate | 177.56 ± 8.90 c | 222.37 ± 3.65 b | 264.95 ± 10.27 a | 176.14 ± 2.54 c | 174.90 ± 3.90 c | 123.43 ± 4.90 d |
| 17 | Methyl 2-ethyl malonate | 359.91 ± 13.87 d | 1340.49 ± 25.42 a | 967.25 ± 20.82 b | 1001.41 ± 32.72 b | 601.28 ± 9.93 c | 272.83 ± 2.49 e |
| 18 | Triscarbamate | 9.50 ± 0.09 e | 346.91 ± 27.15 d | 534.95 ± 11.04 b | 674.07 ± 15.60 a | 463.11 ± 31.39 c | - |
| 19 | Caffeic acid, ethyl ester | 83.95 ± 2.84 e | 934.28 ± 15.44 d | 1253.29 ± 7.49 b | 1087.00 ± 6.82 c | 1283.01 ± 13.14 a | - |
| 20 | Bisphthalate | 3.40 ± 0.36 e | 990.32 ± 0.15 c | 1156.81 ± 27.84 a | 1047.93 ± 10.06 b | 1148.51 ± 10.17 a | 941.49 ± 27.31 d |
| Ʃ(Sum) | 2132.31 ± 320.96 e | 10,056.48 ± 52.56 c | 15,07.95 ± 376.28 a | 12,976.55 ± 173.18 b | 12,820.59 ± 28.58 b | 8615.71 ± 62.94 d | |
| Higher alcohols | |||||||
| 1 | 2,3-Butanediol | 53.18 ± 24.23 d | 166.65 ± 2.29 c | 227.62 ± 23.38 b | 274.33 ± 27.07 a | 215.15 ± 26.69 b | 149.51 ± 32.30 c |
| 2 | Isobutanol | 1.29 ± 0.26 f | 49.22 ± 0.06 d | 68.49 ± 0.78 a | 59.13 ± 0.19 c | 66.87 ± 1.47 b | 41.74 ± 0.29 e |
| 3 | Phenethyl alcohol | 3.54 ± 0.09 b | 23.11 ± 0.59 b | 333.71 ± 247.27 a | 40.48 ± 0.26 b | 34.52 ± 0.22 b | 32.01 ± 0.54 b |
| 4 | Glycerin | 5.79 ± 0.06 f | 402.48 ± 1.01 e | 547.90 ± 5.88 a | 442.02 ± 0.16 d | 484.35 ± 4.11 b | 462.35 ± 0.52 c |
| 5 | 3-Methyl-1-butanol | 22.83 ± 2.29 f | 3803.68 ± 1.04 e | 4184.73 ± 7.69 a | 4046.17 ± 4.05 b | 3994.67 ± 0.71 c | 3666.11 ± 45.21 d |
| 6 | 2-Amino-3-methyl-1-butanol | - | 1528.87 ± 21.93 d | 4399.72 ± 150.79 a | 1895.32 ± 37.67 b | 1479.70 ± 25.45 e | 1664.05 ± 90.44 c |
| 7 | 3-Methylamino-1,2-Propanediol | - | 1262.27 ± 15.07 b | 2812.11 ± 1287.54 a | 1308.75 ± 512.95 b | 1468.32 ± 383.83 b | 1418.26 ± 650.62 b |
| 8 | 2-Butyne-1,4-diol | - | 299.05 ± 9.34 b | 443.61 ± 16.72 a | 228.22 ± 15.87 b | 444.19 ± 8.17 a | 259.65 ± 12.85 b |
| 9 | 2-Deoxyerythritol | - | 118.47 ± 4.63 d | 516.08 ± 7.18 a | 178.50 ± 5.33 c | 182.31 ± 8.74 c | 225.93 ± 14.90 b |
| 10 | 1-Indanol | - | 120.35 ± 9.99 d | 422.29 ± 5.19 a | 216.80 ± 10.72 b | 139.04 ± 19.57 cd | 166.70 ± 34.37 c |
| 11 | Estriol | 1128.46 ± 51.22 a | 233.33 ± 13.16 e | 988.64 ± 9.00 b | 204.44 ± 0.52 e | 331.82 ± 2.58 c | 290.35 ± 4.12 d |
| 12 | Diglycerol | 54.47 ± 4.26 e | 111.86 ± 2.53 d | 422.83 ± 18.71 a | 129.25 ± 8.82 c | 172.68 ± 0.99 b | 123.81 ± 2.85 cd |
| Ʃ(Sum) | 1269.56 ± 40.01 c | 8119.32 ± 34.57 b | 15,367.73 ± 1484.83 a | 9023.40 ± 533.74 b | 9013.62 ± 418.84 b | 8500.48 ± 690.86 b | |
| Ketones | |||||||
| 1 | Acetophenone | - | 206.66 ± 3.31 d | 624.05 ± 7.72 a | 308.66 ± 15.25 b | 276.75 ± 14.6 c | 199.6 ± 6.77 d |
| 2 | Dihydroxyacetone | - | 1402.92 ± 77.31 e | 4545.63 ± 175.26 b | 3205.09 ± 20.11 d | 5262.09 ± 37.45 a | 3713.40 ± 62.40 c |
| 3 | 1,3-Cyclohexanedione | - | 373.97 ± 14.43 c | 1251.82 ± 16.19 a | 634.82 ± 14.78 b | 375.81 ± 5.06 c | 392.25 ± 2.28 c |
| 4 | Acetol | - | 985.30 ± 27.78 c | 3246.30 ± 64.58 a | 861.95 ± 26.27 d | 1215.16 ± 3.96 b | 865.50 ± 18.43 d |
| 5 | 21-Hydroxypregnenolone 2 | 65.73 ± 4.56 c | 75.96 ± 3.41 b | 63.73 ± 0.76 c | 53.00 ± 3.55 d | 64.35 ± 3.32 c | 280.41 ± 10.38 a |
| 6 | 3,4-Dihydroxyphenylacetone | 843.71 ± 21.16 c | 993.74 ± 19.29 b | 1017.23 ± 88.70 b | 2794.66 ± 52.82 a | 963.30 ± 37.07 b | 1001.97 ± 14.58 b |
| Ʃ(Sum) | 909.44 ± 25.54 f | 4038.54 ± 57.91 e | 10,748.77 ± 324.99 a | 7858.17 ± 51.67 c | 8157.46 ± 80.42 b | 6453.14 ± 94.39 d | |
| Ʃ(Sum: GC-MS) | 4311.31 ± 151.29 e | 22,214.33 ± 159.23 d | 41,424.45 ± 522.29 a | 29,858.12 ± 614.05b | 29,991.67 ± 227.51 c | 23,569.32 ± 223.77 d | |
| Number | Compounds | GL | Treatment (n = 3) (μg/L) | ||||
|---|---|---|---|---|---|---|---|
| SA | SIL107 | SIL200 | SEL107 | SEL200 | |||
| Organic acids | |||||||
| 1 | Pyruvic acid | 1643.22 ± 403.03 e | 34,544.94 ± 1802.35 d | 65,988.87 ± 2956.05 a | 42,247.69 ± 3048.87 c | 51,645.63 ± 1133.67 b | 37,289.65 ± 1349.1 d |
| 2 | Glycolic acid | 4682.42 ± 316.75 f | 11,768.34 ± 763.92 c | 19,482.25 ± 261.03 b | 7502.83 ± 414.52 e | 23,995.42 ± 1182.98 a | 10,445.44 ± 54.93 d |
| 3 | 2-Ketobutyric acid | - | 1471.06 ± 33.79 b | 2923.11 ± 45.68 a | 853.64 ± 39.00 d | 1143.15 ± 20.33 c | 1214.12 ± 122.55 c |
| 4 | 2-Keto-Isovaleric acid 1 | 2669.08 ± 185.05a | - | 1649.82 ± 145.71 b | 700.35 ± 91.75 d | 944.51 ± 35.40 c | 1049.31 ± 98.08 c |
| 5 | 2-Hydroxybutanoic acid | 2039.9 5± 49.50 e | 3437.46 ± 73.10 c | 5471.35 ± 57.65 a | 3368.03 ± 162.31 c | 4570.30 ± 236.40 b | 2646.51 ± 265.04 d |
| 6 | 2-Ketovaleric acid | - | 355.72 ± 17.59 e | 956.80 ± 13.54 a | 750.99 ± 25.47 b | 467.63 ± 20.07 c | 384.60 ± 52.33 d |
| 7 | Malonic acid | 427.89 ± 501.62 b | 6390.49 ± 1152.74 ab | 10,176.54 ± 6317.14 a | 5247.16 ± 1941.62 ab | 7472.61 ± 2390.47 ab | 7479.89 ± 5415.91 ab |
| 8 | Maleic acid | 520.07 ± 450.58 c | 1030.07 ± 32.18 b | 3358.77 ± 297.45 a | 1072.22 ± 56.77 b | 1318.69 ± 7.18 b | 1156.80 ± 18.62 b |
| 9 | Succinic acid | 763.15 ± 29.46 e | 1634.35 ± 34.82 b | 2806.17 ± 36.80 a | 51.90 ± 16.60 f | 1252.58 ± 38.22 d | 1399.44 ± 33.04 c |
| 10 | Fumaric acid | 437.32 ± 31.09 e | 5844.83 ± 328.14 d | 16,132.72 ± 527.39 a | 5830.70 ± 522.47 d | 8256.59 ± 360.08 c | 9765.62 ± 119.63 b |
| 11 | Tartronic acid | 182.08 ± 14.71d | 832.18 ± 20.17f | 1111.95 ± 30.39b | 755.57 ± 19.35e | 1346.42 ± 42.58a | 1047.37 ± 21.83c |
| 12 | Citric acid | - | 429.83 ± 0.99 d | 3409.68 ± 50.03 b | 1616.14 ± 146.73 e | 821.13 ± 9.98 a | 1882.10 ± 65.29 c |
| 13 | Glucuronic acid | - | 11,245.81 ± 978.41 e | 27,205.79 ± 1485.87 b | 36,176.00 ± 1445.20 a | 17,370.89 ± 796.59 d | 20,830.75 ± 310.45 c |
| 14 | Galactonic acid | 1081.12 ± 173.97 f | 3556.68 ± 248.23 d | 21,015.36 ± 340.93 a | 6100.63 ± 764.19 e | 17,181.97 ± 168.89 b | 7055.90 ± 677.71 c |
| 15 | Gluconic acid | - | 287.56 ± 42.40 c | 119.33 ± 7.08 d | 569.81 ± 15.64 a | 144.04 ± 8.50 d | 455.60 ± 19.50 b |
| 16 | 3,4-Dihydroxycinnamic acid | - | 18,195.70 ± 544.37 cd | 65,767.89 ± 3253.46 a | 19,246.32 ± 586.68 bc | 21,507.88 ± 1329.01 b | 16,399.49 ± 357.20 d |
| 17 | Lactobionic Acid | 24.78 ± 5.77 e | 157.11 ± 26.62 c | 735.45 ± 14.84 a | 157.03 ± 25.44 c | 364.76 ± 6.89 b | 111.01 ± 3.68 d |
| 18 | PyrrolidonecArboxylic acid | 7933.47 ± 753.72 a | 1060.88 ± 61.77 d | 1939.55 ± 21.47 c | 2306.35 ± 77.21 ab | 2240.34 ± 67.65 ab | 2557.59 ± 152.64 b |
| 19 | Vanillic acid | 3949.58 ± 164.38 c | 4803.67 ± 219.31 a | 3633.50 ± 74.49 d | 4421.21 ± 209.10 b | 2743.35 ± 54.80 e | |
| 20 | Daucic acid | - | 627.97 ± 26.30 c | 911.26 ± 8.14 a | 549.61 ± 27.06 d | 656.01 ± 6.88 c | 770.45 ± 38.57 b |
| 21 | cis-Aconitic acid | 312.34 ± 18.21 f | 10,222.02 ± 138.27 e | 31,658.31 ± 326.97 a | 19,734.22 ± 280.34 c | 20,820.07 ± 687.71 b | 11,128.07 ± 131.68 d |
| 22 | Phenylpyruvic acid | 264.77 ± 14.56 e | 4014.22 ± 163.71 c | 5401.94 ± 118.88 a | 4343.76 ± 177.75 b | 2174.58 ± 35.33 d | 2211.44 ± 119.28 d |
| 23 | L-Malic acid | 343.57 ± 47.41 a | 277.17 ± 11.72 b | 40.75 ± 40.36 e | 89.45 ± 8.16 cd | 118.91 ± 21.87 c | 84.18 ± 1.96 cd |
| 24 | L-Lactic acid | 1135.49 ± 115.13 f | 28,972.88 ± 497.55 e | 76,585.35 ± 1348.07 a | 54,613.28 ± 2720.42 c | 67,019.63 ± 1113.69 b | 48,011.79 ± 4721.11 d |
| 25 | D-Malic acid | 342.52 ± 23.64 e | 967.27 ± 11.31 d | 1076.49 ± 73.14 c | 1023.7 ± 58.99 cd | 1175.94 ± 86.57 b | 1662.27 ± 26.25 a |
| 26 | 2-Furoic acid | 166.08 ± 6.66 d | 834.71 ± 2.64 c | 999.41 ± 64.44 b | 1121.64 ± 68.71 ab | 1186.84 ± 102.98 a | 1021.07 ± 139.13 b |
| 27 | 2-Aminoacrylic acid | 13,729.5 ± 1250.61 a | 471.77 ± 25.60 b | 532.23 ± 9.28 b | 489.64 ± 24.05 b | 338.04 ± 7.42 b | 553.41 ± 21.59 b |
| 28 | 2-Methyl-3-Hydroxybutyric acid | 1573.30 ± 390.68 c | 1764.95 ± 107.63 c | 4121.93 ± 117.92 a | 2185.38 ± 122.29 b | 2130.52 ± 58.63 b | 2224.56 ± 139.98 b |
| 29 | 5-Aminolevulinic acid | 359.20 ± 14.68 f | 724.47 ± 13.67 d | 1748.97 ± 27.14 a | 656.44 ± 35.02 e | 934.22 ± 8.85 b | 794.13 ± 4.38 c |
| Ʃ(Sum) | 40631.33 ± 2010.48 f | 154,833.6 ± 2377.27 e | 378,368.09 ± 10396.61 a | 222,993.98 ± 5596.48 c | 263,020.50 ± 2066.40 b | 194,375.91 ± 4694.26 d | |
| Polyphenols | |||||||
| 1 | Epicatechin | 138.37 ± 6.66 f | 29,012.60 ± 527.35 e | 62154.47 ± 808.11 a | 55728.44 ± 1522.04 b | 42622.34 ± 1829.52 c | 33762.44 ± 2022.90 d |
| 2 | Catechin | 1791.49 ± 15.50 a | 749.43 ± 40.98 c | 922.09 ± 13.10 b | 525.76 ± 27.42 d | 351.95 ± 17.41 e | 238.26 ± 19.05 f |
| 3 | Ptelatoside B | 132.21 ± 8.11 e | 843.49 ± 25.56 b | 854.88 ± 16.46 b | 1096.14 ± 32.68 a | 378.16 ± 13.04 c | 309.31 ± 8.14 d |
| 4 | Quercetin | 36,602.35 ± 117.15 a | 7768.82 ± 171.04 c | 26,310.33 ± 435.00 b | 6612.57 ± 274.95 d | 15,974.51 ± 188.12 e | 5508.39 ± 257.74 f |
| 5 | Eugenol | 118.37 ± 0.83 f | 1649.13 ± 26.13 c | 2886.94 ± 91.05 a | 1542.27 ± 42.66 d | 1788.13 ± 65.06 b | 1149.86 ± 25.34 e |
| 6 | Cinnamaldehyde | 11.72 ± 10.31 c | 2067.41 ± 19.25 b | 3019.70 ± 81.41 a | 2192.72 ± 38.53 b | 2892.84 ± 545.90 a | 1880.87 ± 4.29 b |
| 7 | (-)-Epiafzelechin | 99.78 ± 15.87 e | 942.86 ± 20.25 c | 1146.97 ± 14.58 a | 1004.60 ± 41.05 b | 922.55 ± 11.33 c | 711.65 ± 61.52 d |
| 8 | (-)-Catechin | 19.68 ± 8.16 e | 288.55 ± 3.36 c | 463.75 ± 25.69 a | 324.54 ± 21.94 b | 233.74 ± 10.40 d | 281.37 ± 15.45 c |
| 9 | Hyperoside | 11.18 ± 0.59 f | 69.67 ± 4.55 e | 121.14 ± 21.95 d | 172.81 ± 12.55 c | 207.78 ± 3.37 b | 294.36 ± 7.12 a |
| 10 | Hesperidin | 92.91 ± 1.97 e | 3282.23 ± 29.48 d | 4641.71 ± 121.51 a | 4191.15 ± 163.05 b | 3860.34 ± 30.78 c | 3405.00 ± 39.70 d |
| 11 | Procyanidin B2 | 231.43 ± 19.18 a | 4.26 ± 0.35 c | 82.49 ± 7.15 b | 5.65 ± 0.07 c | 7.23 ± 0.42 c | 5.63 ± 0.33 c |
| 12 | Phloretin | 219.09 ± 6.21 e | 654.06 ± 31.86 c | 859.18 ± 24.19 a | 757.28 ± 20.78 b | 455.00 ± 11.52 d | 486.41 ± 22.19 d |
| 13 | Isorhamnetin | 2.13 ± 3.68 e | 70.83 ± 4.05 d | 208.07 ± 0.80 a | 105.62 ± 4.11 b | 86.46 ± 9.43 d | 88.63 ± 7.14 d |
| 14 | Quercetin-3-O-sophoroside | 252.36 ± 26.56 a | 5.17 ± 0.60 b | 8.56 ± 0.24 b | 3.36 ± 0.43 b | 2.74 ± 0.12 b | 8.02 ± 0.49 b |
| 15 | Lutein | 178.85 ± 6.60 e | 354.35 ± 17.01 d | 462.54 ± 26.66 c | 516.53 ± 14.88 a | 489.08 ± 1.36 b | 440.70 ± 1.70 c |
| 16 | 3-O-Caffeoyl-4-O-methylquinic acid | 119.06 ± 8.52 e | 316.22 ± 3.97 c | 530.25 ± 3.53 a | 431.13 ± 5.00 b | 317.87 ± 15.44 c | 276.09 ± 1.74 d |
| 17 | Vanilloside | 14.45 ± 1.55 d | 378.17 ± 14.39 c | 639.13 ± 19.65 a | 455.20 ± 29.84 b | 437.92 ± 6.54 b | 353.95 ± 10.92 c |
| 18 | Isoquercitrin | 211.78 ± 7.65 e | 527.81 ± 26.90 c | 584.29 ± 12.80 b | 530.25 ± 17.56 c | 615.02 ± 16.60 a | 396.14 ± 2.04 d |
| 19 | Curcumin I | 180.07 ± 8.03 bc | 192.57 ± 6.11 b | 226.54 ± 24.03 a | 171.73 ± 6.45 bc | 192.49 ± 3.71 b | 163.42 ± 1.37 c |
| 20 | 3,4-Dihydroxycinnamic acid | 3166.35 ± 11.72 d | 2652.23 ± 190.40 e | 4715.93 ± 211.34 a | 4064.15 ± 30.78 b | 3838.03 ± 8.89 c | 1469.48 ± 116.02 f |
| 21 | Sinapic acid | 131.03 ± 6.70 d | 163.29 ± 0.66 c | 429.03 ± 8.14 a | 130.06 ± 6.14 d | 215.13 ± 3.10 b | 23.33 ± 1.42 e |
| 22 | Ferulic acid | 19.24 ± 4.98 a | 18.74 ± 0.83 b | 14.52 ± 2.70 ab | 2.60 ± 4.50 c | 11.71 ± 4.17 b | 0.01 ± 0.00 c |
| 23 | 1,5-Dicaffeoylquinic acid | 8.65 ± 0.47 a | 9.24 ± 1.69 a | 7.91 ± 1.54 ab | 7.21 ± 0.93 ab | 6.01 ± 0.97 b | 0.10 ± 0.15 c |
| 24 | 3,4-Dihydroxybenzaldehyde | 67.19 ± 4.59 c | 79.10 ± 4.87 b | 106.11 ± 0.60 a | 60.83 ± 3.37 d | 58.01 ± 1.55 d | 55.65 ± 2.56 d |
| 25 | Phlorizin | 22,867.47 ± 183.84 f | 30,977.57 ± 176.39 c | 39,529.56 ± 371.01 a | 32,671.37 ± 209.63 b | 25,666.46 ± 309.77 e | 30,017.00 ± 2.56 d |
| 26 | ( + )-Catechin | 661.55 ± 27.93 d | 625.79 ± 6.22 d | 887.70 ± 45.31 c | 1330.51 ± 32.38 a | 1179.10 ± 0.92 b | 17.54 ± 1.13 e |
| 27 | Bergapten | 480.31 ± 11.58 e | 2508.01 ± 1.85d | 6138.47 ± 21.30 c | 15,134.16 ± 13.60 c | 5186.70 ± 45.03 c | 28,092.95 ± 1549.78 a |
| 28 | 3,4-Dihydroxybenzoic acid | 117.92 ± 6.93 b | 90.59 ± 8.51 c | 192.99 ± 5.65 a | 80.24 ± 12.41 c | 106.60 ± 0.30 b | 8.81 ± 0.46 d |
| 29 | Genistein | 104.41 ± 3.64 a | 107.62 ± 10.69 a | 123.38 ± 92.30 a | 108.50 ± 0.36 a | 86.12 ± 4.78 a | 0.01 ± 0.00b |
| 30 | Gallic acid | 7160.50 ± 393.28 c | 8244.61 ± 57.80 b | 9439.28 ± 211.72 a | 8233.66 ± 336.09 b | 7565.90 ± 182.82 c | 5015.77 ± 1.00d |
| 31 | Succinate semialdehyde | - | 4475.85 ± 63.88 c | 11453.38 ± 304.85 a | 5399.67 ± 337.68 b | 5161.43 ± 63.86 b | 4525.48 ± 222.44 c |
| 32 | Quinic acid | - | 51,940.93 ± 244.52 d | 83,310.55 ± 1575.54 a | 67,309.36 ± 785.89 b | 62,524.53 ± 466.61 c | 51,833.43 ± 265.98 d |
| 33 | Caffeic acid | - | 352.40 ± 41.62 e | 1363.67 ± 64.91 a | 521.05 ± 5.52 c | 807.39 ± 6.19 b | 443.16 ± 3.23 d |
| 34 | Neohesperidin | - | 60.38 ± 3.89 c | 420.93 ± 14.70 a | - | 116.02 ± 4.49 b | - |
| 35 | Epigallocatechin | - | 64.17 ± 2.89 d | 54.42 ± 2.69 b | 73.01 ± 1.15 a | 45.26 ± 1.91 c | 53.63 ± 0.10 b |
| 36 | Chlorogenic Acid | 537.27 ± 16.86 f | 1030.01 ± 14.68 e | 4738.72 ± 11.54 a | 1819.99 ± 16.85 d | 2077.14 ± 13.51 b | 1967.71 ± 33.55 c |
| 37 | Naringin | 5611.94 ± 212.36 a | 657.26 ± 37.78 e | 2856.04 ± 119.23 b | 941.61 ± 7.86 d | 1834.92 ± 8.68 c | 752.68 ± 10.75 e |
| 38 | Trans-Ferulic acid | 3532.12 ± 116.74 a | 855.91 ± 18.32 b | 1914.69 ± 50.29 c | 961.64 ± 27.19 c | 879.13 ± 52.76 c | 916.22 ± 53.37 c |
| 39 | 3,5-Dihydroxybenzaldehyde | 1387.60 ± 118.06 f | 2239.00 ± 45.79 e | 7432.99 ± 142.22 b | 4291.46 ± 55.72 d | 7770.18 ± 123.72 a | 5673.13 ± 11.63 c |
| Ʃ(Sum) | 86,280.84 ± 566.02 f | 156,330.35 ± 784.93 e | 281,253.29 ± 1384.79 a | 219,508.87 ± 1854.71 b | 196,971.89 ± 2843.26 c | 180,626.58 ± 3008.34 d | |
| Terpenoids | |||||||
| 1 | Ginkgolide j | 1.40 ± 0.99 e | 197.94 ± 13.15 c | 240.43 ± 9.01 c | 117.71 ± 8.53 d | 426.54 ± 2.80 b | 489.18 ± 56.05 a |
| 2 | Xanthoxylin | 46.74 ± 1.87 e | 2049.57 ± 24.76 c | 2861.51 ± 109.67 a | 2371.83 ± 3.06 d | 2937.94 ± 18.10 a | 2616.75 ± 22.22 b |
| 3 | (-)-Dihydrocarveol | 34.99 ± 10.57 d | 102.58 ± 20.08 bc | 402.47 ± 15.18 a | 84.45 ± 2.80 c | 118.09 ± 5.76 b | 87.71 ± 5.74 c |
| 4 | 1,4-Cyclohexanedione 2 | 1330.41 ± 24.34 f | 1611.56 ± 168.71 cd | 1737.61 ± 10.01 c | 1985.92 ± 10.16 b | 1513.64 ± 17.81 de | 2492.55 ± 221.21 a |
| 5 | Celastrol | 354.89 ± 25.20 a | 85.80 ± 10.11 b | 57.38 ± 2.98 c | 74.54 ± 3.05 ab | 82.52 ± 5.50 b | 74.57 ± 2.18 ab |
| 6 | Millefin | 287.32 ± 4.06 a | 143.15 ± 10.68 f | 167.81 ± 2.67 b | 148.85 ± 6.84 de | 155.55 ± 2.57 cd | 164.90 ± 3.72 bc |
| 7 | Perillyl acetate | 276.15 ± 26.68 c | 2358.09 ± 107.42 b | 3668.55 ± 133.02 a | 3567.11 ± 43.51 a | 2269.88 ± 9.63 b | 2385.89 ± 9.65 b |
| 8 | Osmundalactone | 20765.76 ± 164.82 a | 8122.54 ± 301.35 c | 8063.08 ± 13.24 c | 8709.71 ± 165.83 b | 7396.50 ± 353.08 d | 7604.66 ± 295.83 d |
| Ʃ(Sum) | 23,097.65 ± 134.86 a | 14,671.22 ± 155.73 d | 17,198.83 ± 74.01 b | 17,060.12 ± 126.10 b | 14,900.66 ± 367.71 d | 15,916.21 ± 143.33 c | |
| Ʃ(Sum: LC-MS) | 150,009.83 ± 2411.58 f | 325,835.16 ± 2377.11 e | 676,820.21 ± 9550.59 a | 459,562.96 ± 4288.97 c | 474,893.06 ± 1788.72 b | 390,918.69 ± 7542.29 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Lin, M.; Hu, L.; Xu, T.; Xiong, D.; Li, L.; Zhao, Z. Research on the Effect of Simultaneous and Sequential Fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum on Antioxidant Activity and Flavor of Apple Cider. Fermentation 2023, 9, 102. https://doi.org/10.3390/fermentation9020102
Chen X, Lin M, Hu L, Xu T, Xiong D, Li L, Zhao Z. Research on the Effect of Simultaneous and Sequential Fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum on Antioxidant Activity and Flavor of Apple Cider. Fermentation. 2023; 9(2):102. https://doi.org/10.3390/fermentation9020102
Chicago/Turabian StyleChen, Xiaodie, Man Lin, Lujun Hu, Teng Xu, Dake Xiong, Li Li, and Zhifeng Zhao. 2023. "Research on the Effect of Simultaneous and Sequential Fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum on Antioxidant Activity and Flavor of Apple Cider" Fermentation 9, no. 2: 102. https://doi.org/10.3390/fermentation9020102






