Hydrolase Production via Food Waste Fermentation and Its Application to Enhance Anaerobic Digestion of Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Inoculation
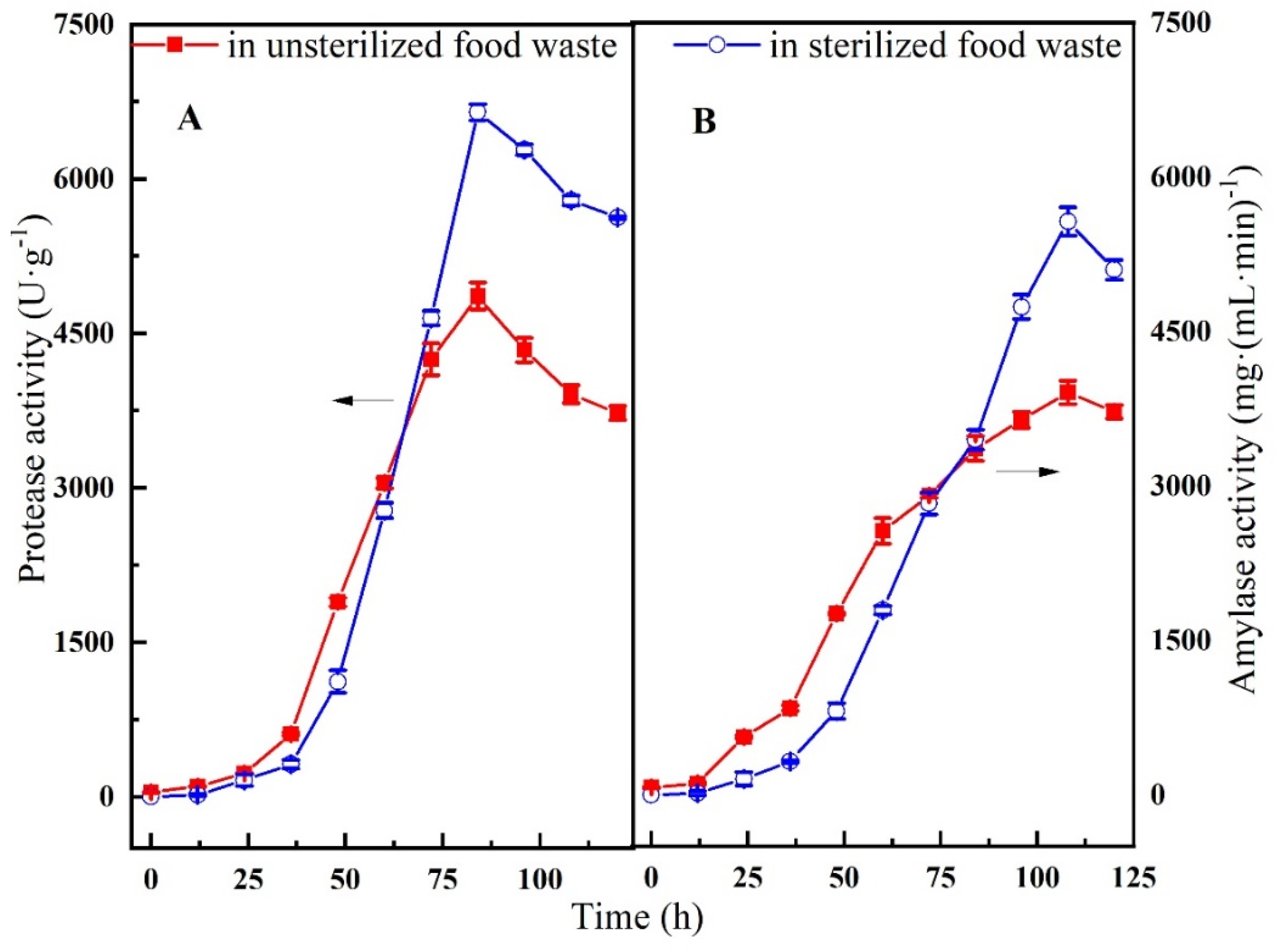
2.3. Hydrolase Production from Food Waste Fermentation
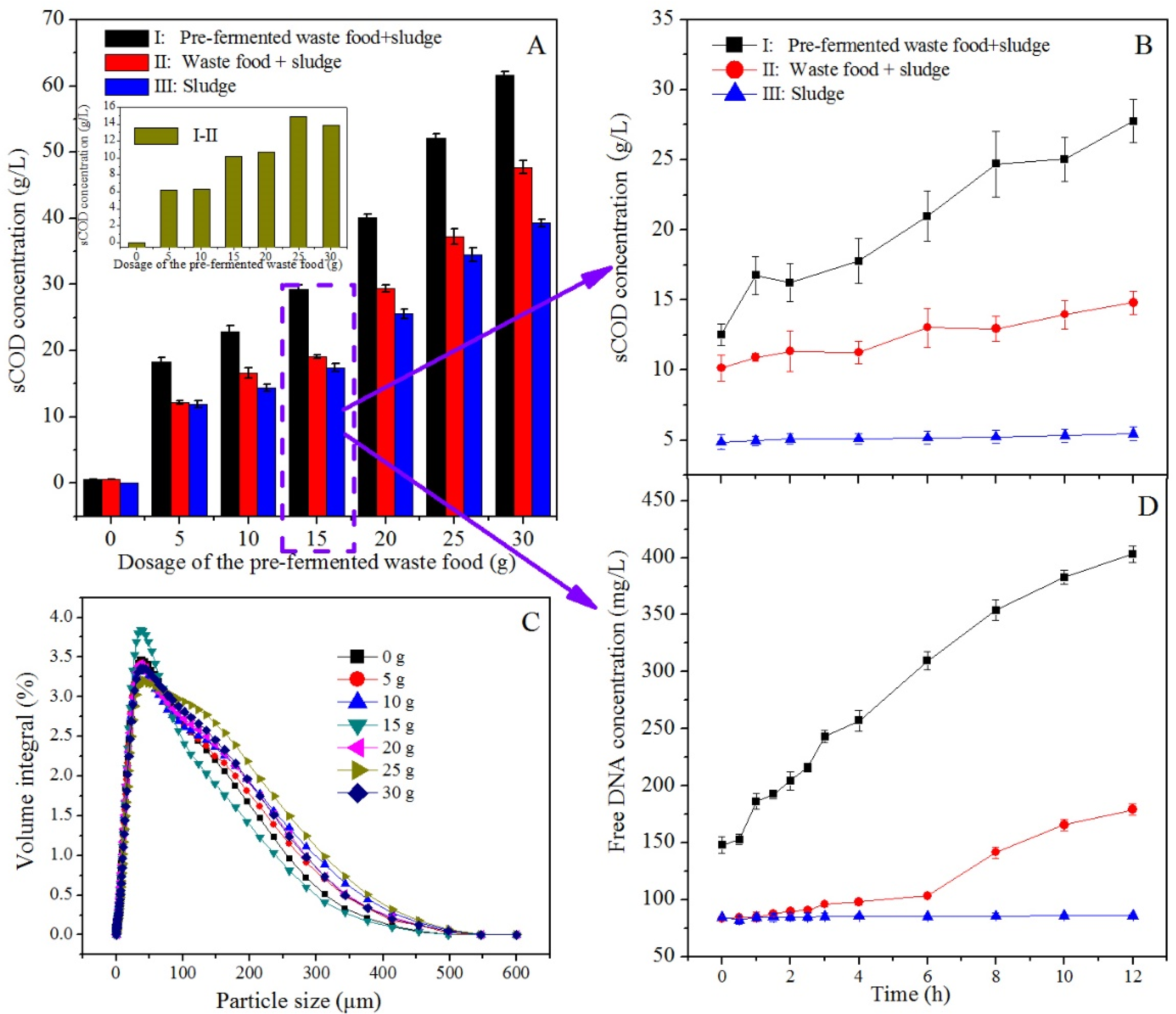
2.4. Sludge Pretreatment by Hydrolase in Pre-Fermented Food Waste
2.5. Anaerobic Digestion for Methane Production
2.6. Analytical Methods
3. Results
3.1. Hydrolase Production from Food Waste Fermentation
3.2. Optimal Conditions of the Hydrolase
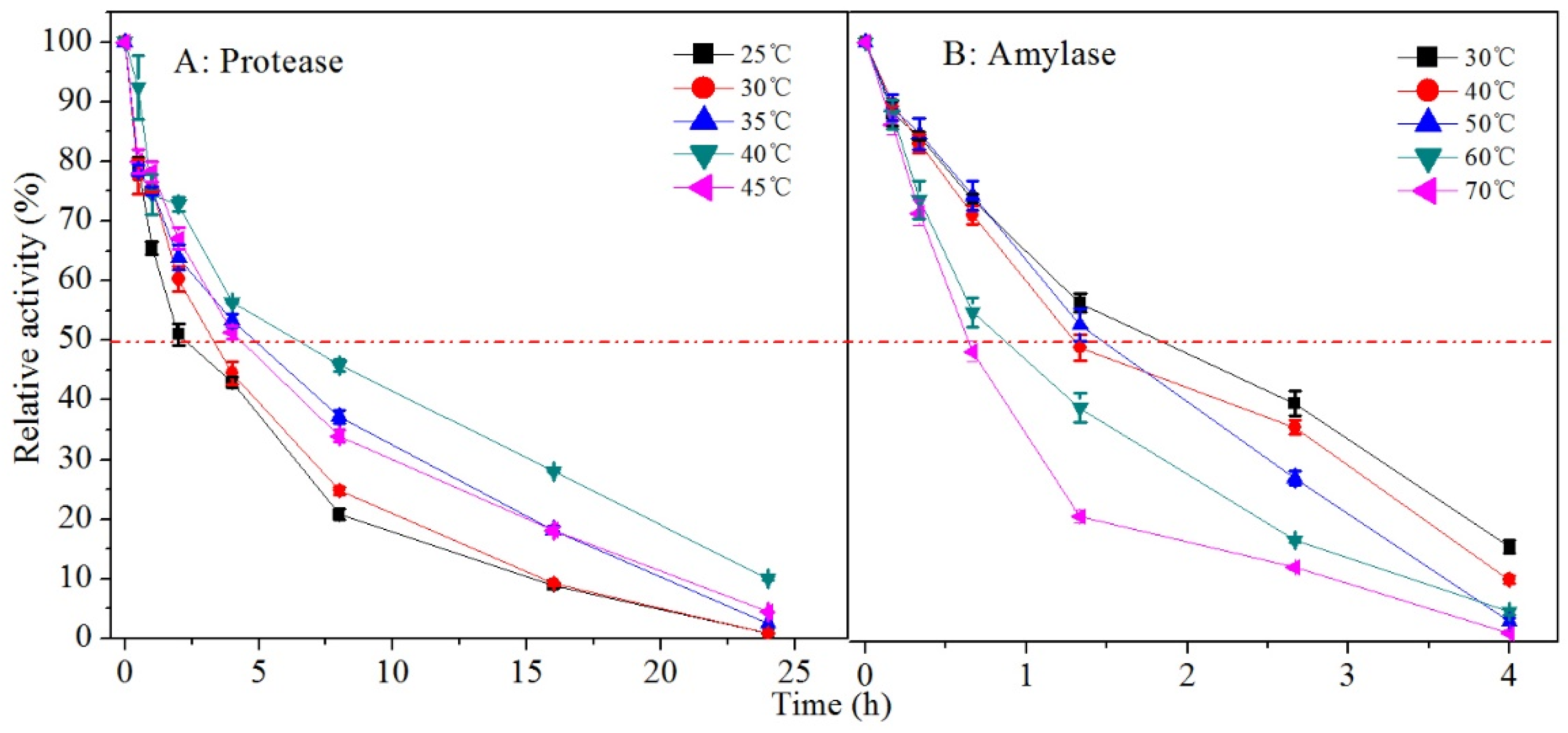
3.3. Stability of Hydrolase
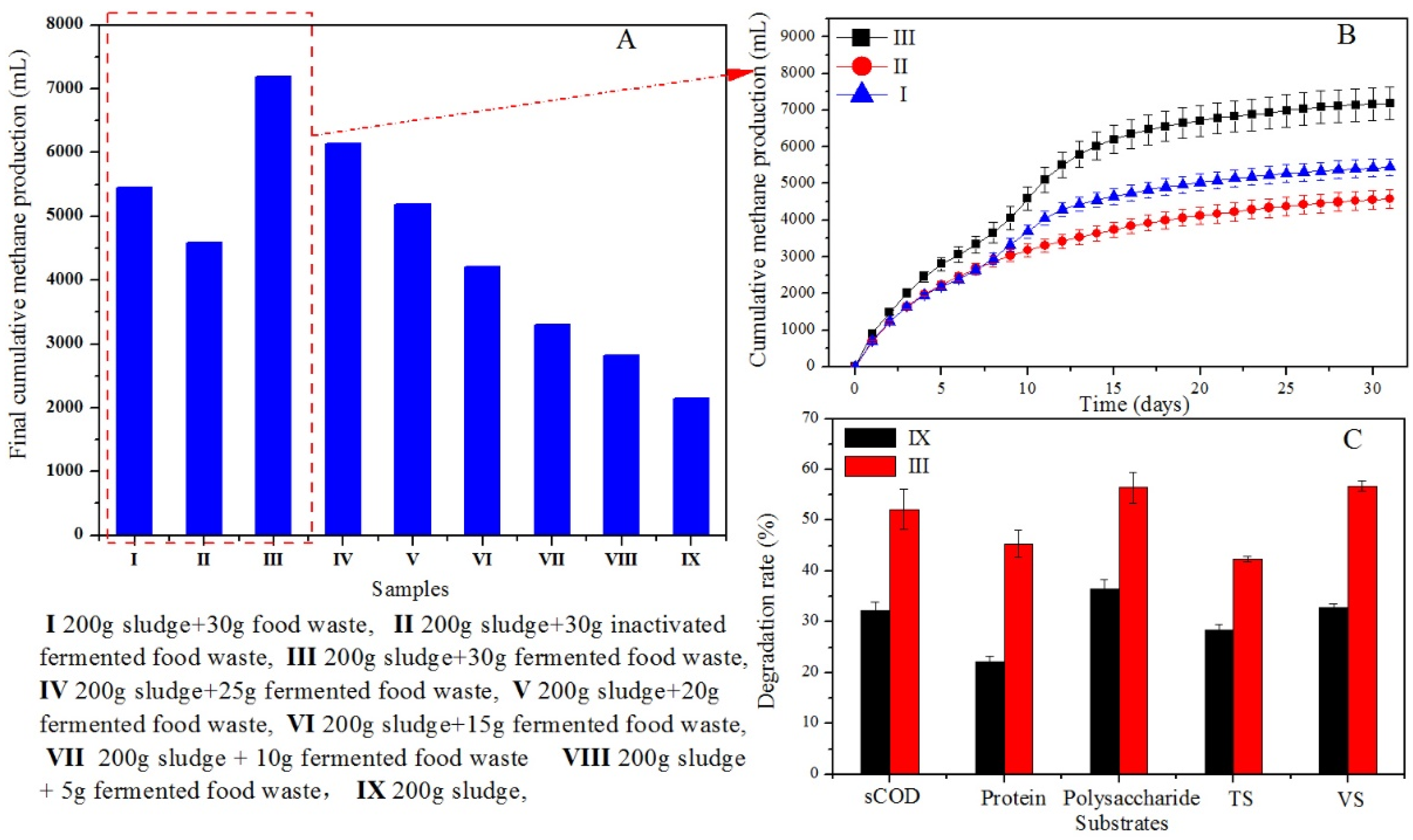
3.4. Performance of Hydrolase in Sludge Pretreatment
3.4.1. Solubilization of Organic Matter
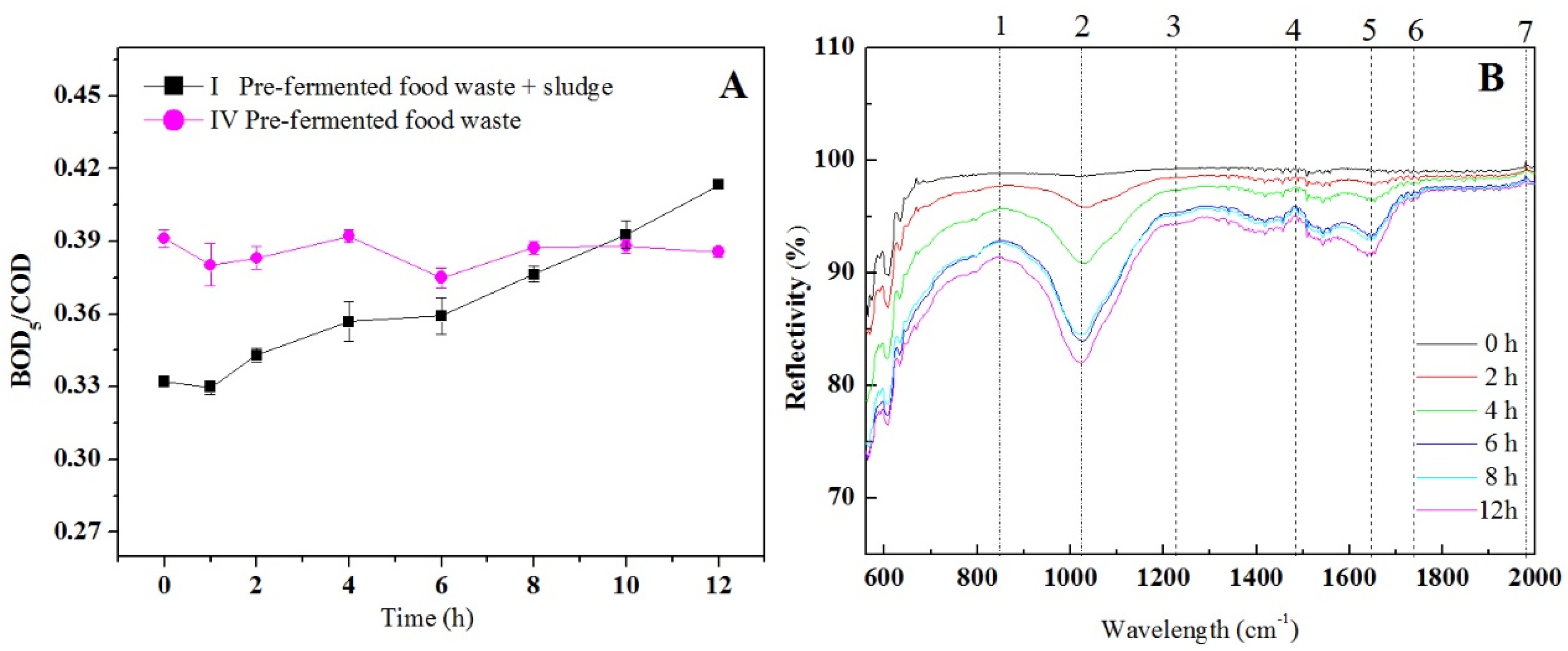
3.4.2. Improved Biodegradability of the Pretreated Sludge
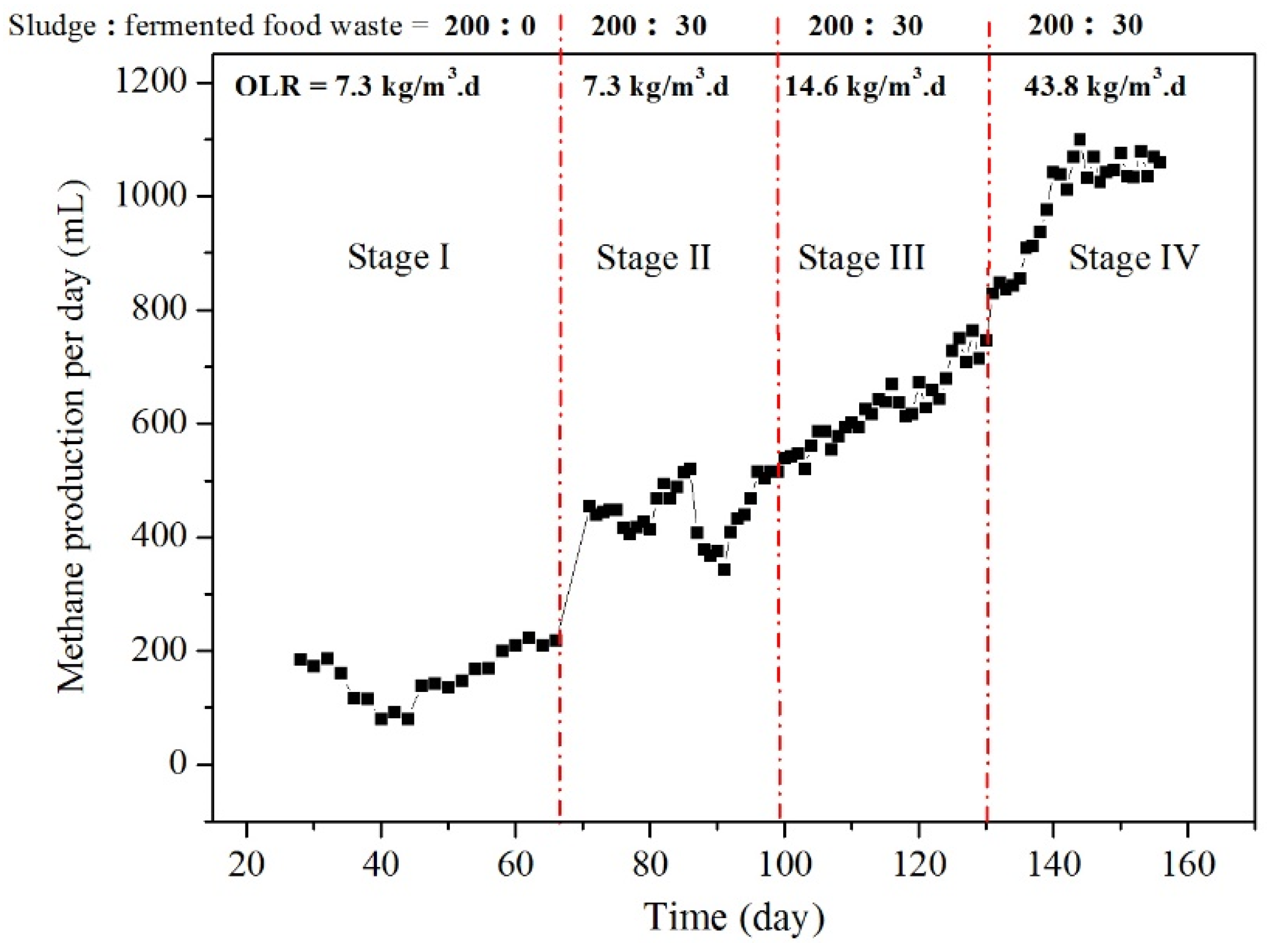
3.4.3. Enhanced methane production from sludge digestion by hydrolase pretreatment
4. Discussion
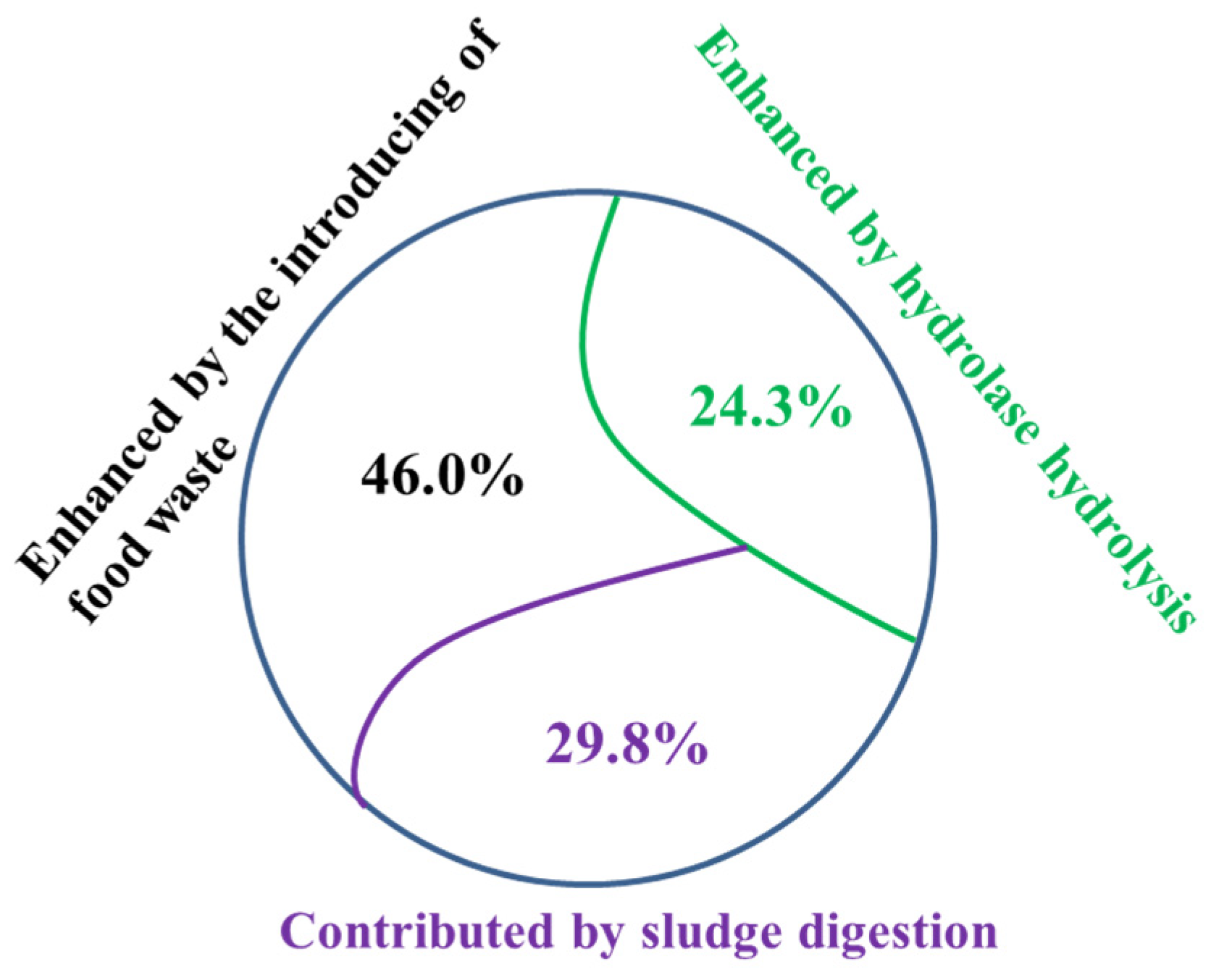
4.1. Contributors to the Enhanced Methane Production
4.2. Economic Evaluation
4.3. Application of Conventional Co-Digestion Optimization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.P.; Zhai, Y.B.; Xu, Z.X.; Zhu, Y.; Zhou, Y.; Wang, Z.X.; Liu, L.M.; Liang, F.S.; Ren, W.Y.; Xie, Y.; et al. One-pot production of 5-methylfurfural (5-MF) and enhanced dewaterability of waste activated sludge by hydrothermal treatment with natural deep eutectic solvents (NADES): Experimental and theoretical studies. Chem. Eng. J. 2023, 464, 142575. [Google Scholar] [CrossRef]
- Liu, X.R.; Huang, X.D.; Wu, Y.X.; Xu, Q.X.; Du, M.T.; Wang, D.B.; Yang, Q.; Liu, Y.W.; Ni, B.J.; Yang, G.J.; et al. Activation of nitrite by freezing process for anaerobic digestion enhancement of waste activated sludge: Performance and mechanisms. Chem. Eng. J. 2020, 387, 124147. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, R.; Wang, Q.P.; Zhang, Y.R.; Yang, Z.H. Sludge anaerobic digestion with high concentrations of tetracyclines and sulfonamides: Dynamics of microbial communities and change of antibiotic resistance genes. Bioresour. Technol. 2019, 276, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.X.; Yuan, Z.W.; Wang, R.M.; Angelidaki, I.; Zhu, G.F. Syntrophy mechanism, microbial population, and process optimization for volatile fatty acids metabolism in anaerobic digestion. Chem. Eng. J. 2023, 452, 139137. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.K.; Geng, H.; Liu, R.; Dai, X.H. Enhancing acidogenic fermentation of waste activated sludge via isoelectric-point pretreatment: Insights from physical structure and interfacial thermodynamics. Water Res. 2020, 185, 116237. [Google Scholar] [CrossRef] [PubMed]
- Deaver, J.A.; Diviesti, K.I.; Soni, M.N.; Campbell, B.J.; Finneran, K.T.; Popat, S.C. Palmitic acid accumulation limits methane production in anaerobic co-digestion of fats, oils and grease with municipal wastewater sludge. Chem. Eng. J. 2020, 396, 125235. [Google Scholar] [CrossRef]
- Li, W.; Fang, A.R.; Liu, B.F.; Xie, G.J.; Lou, Y.; Xing, D.F. Effect of different co-treatments of waste activated sludge on biogas production and shaping microbial community in subsequent anaerobic digestion. Chem. Eng. J. 2019, 378, 122098. [Google Scholar] [CrossRef]
- Pang, H.L.; Li, L.; He, J.G.; Yan, Z.S.; Ma, Y.Q.; Nan, J.; Liu, Y. New insight into enhanced production of short -chain fatty acids from waste activated sludge by cation exchange resin-induced hydrolysis. Chem. Eng. J. 2020, 388, 124235. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Monlau, F.; Abdelouahdi, K.; Oukarroum, A.; Barakat, A. Pretreatment and co-digestion of wastewater sludge for biogas production: Recent research advances and trends. Renew. Sustain. Energy Rev. 2019, 114, 109287. [Google Scholar] [CrossRef]
- Zheng, M.; Ping, Q.; Wang, L.; Dai, X.H.; Li, Y.M.; Snyder, S.A. Pretreatment using UV combined with CaO2 for the anaerobic digestion of waste activated sludge: Mechanistic modeling for attenuation of trace organic contaminants. J. Hazard. Mater. 2021, 402, 123484. [Google Scholar] [CrossRef]
- Chen, H.; Yi, H.; Li, H.C.; Guo, X.S.; Xiao, B.Y. Effects of thermal and thermal-alkaline pretreatments on continuous anaerobic sludge digestion: Performance, energy balance and, enhancement mechanism. Renew. Energ. 2020, 147, 2409–2416. [Google Scholar] [CrossRef]
- Liu, H.B.; Wang, Y.Y.; Wang, L.; Yu, T.T.; Fu, B.; Liu, H. Stepwise hydrolysis to improve carbon releasing efficiency from sludge. Water Res. 2017, 119, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Xiao, H.; Yin, B.; Zu, Y.P.; Liu, H.; Fu, B.; Ma, H.J. Enhanced volatile fatty acid production by a modified biological pretreatment in anaerobic fermentation of waste activated sludge. Chem. Eng. J. 2016, 284, 194–201. [Google Scholar] [CrossRef]
- Zhang, J.X.; Li, W.L.; Lee, J.; Loh, K.C.; Dai, Y.J.; Tong, Y.W. Enhancement of biogas production in anaerobic co-digestion of food waste and waste activated sludge by biological co-pretreatment. Energy 2017, 137, 479–486. [Google Scholar] [CrossRef]
- Wawrzynczyk, J.; Szewczyk, E.; Norrlow, O.; Dey, E.S. Application of enzymes, sodium tripolyphosphate and cation exchange resin for the release of extracellular polymeric substances from sewage sludge—Characterization of the extracted polysaccharides/glycoconjugates by a panel of lectins. J. Biotechnol. 2007, 130, 274–281. [Google Scholar] [CrossRef]
- Gyuranova, D.; Krasnan, V.; Spanik, I.; Rebros, M. Elimination of biodiesel contaminants by recombinant glucoside hydrolase produced from crude glycerol. Fuel 2022, 330, 125550. [Google Scholar] [CrossRef]
- Ding, H.H.H.; Chang, S.; Liu, Y. Biological hydrolysis pretreatment on secondary sludge: Enhancement of anaerobic digestion and mechanism study. Bioresour. Technol. 2017, 244, 989–995. [Google Scholar] [CrossRef]
- Paul, J.S.; Beliya, E.; Tiwari, S.; Patel, K.; Gupta, N.; Jadhav, S.K. Production of biocatalyst alpha-amylase from agro-waste ‘rice bran’ by using Bacillus tequilensis TB5 and standardizing its production process. Biocatal. Agric. Biotechnol. 2020, 26, 101648. [Google Scholar] [CrossRef]
- Albuquerque, K.K.S.A.; Albuquerque, W.W.C.; Costa, R.M.P.B.; Batista, J.M.S.; Marques, D.A.V.; Bezerra, R.P.; Herculano, P.N.; Porto, A.L.F. Biotechnological potential of a novel tannase-acyl hydrolase from Aspergillus sydowii using waste coir residue: Aqueous two-phase system and chromatographic techniques. Biocatal. Agric. Biotechnol. 2020, 23, 101453. [Google Scholar] [CrossRef]
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.J.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138. [Google Scholar] [CrossRef]
- Ichinose, S.; Tanaka, M.; Shintani, T.; Gomi, K. Increased production of biomass-degrading enzymes by double deletion of creA and creB genes involved in carbon catabolite repression in Aspergillus oryzae. J. Biosci. Bioeng. 2018, 125, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Melnichuk, N.; Braia, M.J.; Anselmi, P.A.; Meini, M.R.; Romanini, D. Valorization of two agroindustrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Manag. 2020, 106, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Watanabe, M.; Nakamichi, Y.; Fujimoto, Z.; Yaoi, K. Crystal structure and substrate recognition mechanism of Aspergillus oryzae isoprimeverose-producing enzyme. J. Struct. Biol. 2019, 205, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Cheng, H.; Guo, G.Z.; Zhang, T.; Qin, Y.; Li, Y.Y. High solid mono-digestion and co-digestion performance of food waste and sewage sludge by a thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2020, 310, 123433. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, R.; Cheng, Y.; Stanisavljevic, N.; Li, L.; Djatkov, D.; Peng, X.Y.; Wang, X.M. Anaerobic co-digestion of food waste and sewage sludge under mesophilic and thermophilic conditions: Focusing on synergistic effects on methane production. Bioresour. Technol. 2020, 301, 122765. [Google Scholar] [CrossRef]
- State Environmental Protection Administration of China. Standard Methods for the Analysis of Water and Wastewater; China Environmental Science Publishing House: Beijing, China, 2002. [Google Scholar]
- Abouelenien, F.; Nakashimada, Y.; Nishio, N. Dry mesophilic fermentation of chicken manure for production of methane by repeated batch culture. J. Biosci. Bioeng. 2009, 107, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Zhang, B.; He, P.J.; Lu, F.; Shao, L.M.; Wang, P. Extracellular enzyme activities during regulated hydrolysis of high-solid organic wastes. Water Res. 2007, 41, 4468–4478. [Google Scholar] [CrossRef]
- Yin, B.; Liu, H.; Wang, Y.; Bai, J.; Liu, H.; Fu, B. Improving volatile fatty acids production by exploiting the residual substrates in post-fermented sludge: Protease catalysis of refractory protein. Bioresour. Technol. 2016, 203, 124–131. [Google Scholar] [CrossRef]

| Items | Conventional Sludge Digestion (CSD) | Conventional Co-Digestion of Sludge and Food Waste (CDSF) | Modified Co-Digestion of Sludge and Food Waste (MCSF) |
|---|---|---|---|
| Volume of digester (m3) | 5000 | 5750 | Co-digestion: 5750 Hydrolase production: 94 |
| Mass of treated sludge or waste food (ton/d) | 200 (sludge) | 200 (sludge) +30 (food waste) | 200 (sludge) +30 (fermented food waste) |
| Cost of biogas purification (USD/m3 sludge) a | 2.68 | 5.92 | 7.81 |
| Cost for digester operation (USD/m3 sludge) b | 10.5 | 10.5 | 10.5 + 1.97 |
| Total cost (USD/m3 sludge) | 13.18 | 16.42 | 20.28 |
| Amount of biogas (m3/m3 sludge) | 17.84 | 39.45 | 52.08 |
| Price of products (USD/m3 biogas) | 0.44 | 0.44 | 0.44 |
| Gross Profit (USD/m3 sludge) | 7.85 | 17.36 | 22.92 |
| Net profit (USD/m3 sludge) | −5.35 | 0.94 | 2.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, G.; Dong, J.; Chen, M.; He, Y.; Liu, H.; Li, Y.; Liu, H. Hydrolase Production via Food Waste Fermentation and Its Application to Enhance Anaerobic Digestion of Sewage Sludge. Fermentation 2023, 9, 526. https://doi.org/10.3390/fermentation9060526
Zhang X, Wang G, Dong J, Chen M, He Y, Liu H, Li Y, Liu H. Hydrolase Production via Food Waste Fermentation and Its Application to Enhance Anaerobic Digestion of Sewage Sludge. Fermentation. 2023; 9(6):526. https://doi.org/10.3390/fermentation9060526
Chicago/Turabian StyleZhang, Xuedong, Ganghui Wang, Jian Dong, Min Chen, Yanhua He, He Liu, Yajie Li, and Hongbo Liu. 2023. "Hydrolase Production via Food Waste Fermentation and Its Application to Enhance Anaerobic Digestion of Sewage Sludge" Fermentation 9, no. 6: 526. https://doi.org/10.3390/fermentation9060526





