Mimicking the Fungal Decay Strategy for Promoting the Bacterial Production of Polyhydroxyalkanoate from Kraft Lignin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pretreatment of Kraft Lignin by Fenton-like Reaction
2.2. Analysis of Hydroxyl Radical and Lignin Concentration
2.3. Bacterial Strains and Cultivation
2.4. Extraction of PHA Form Cells
2.5. Kraft Lignin Characterizations
2.6. Detection of Lignin Depolymerized Products
2.7. Black Liquor Fermentation
3. Results
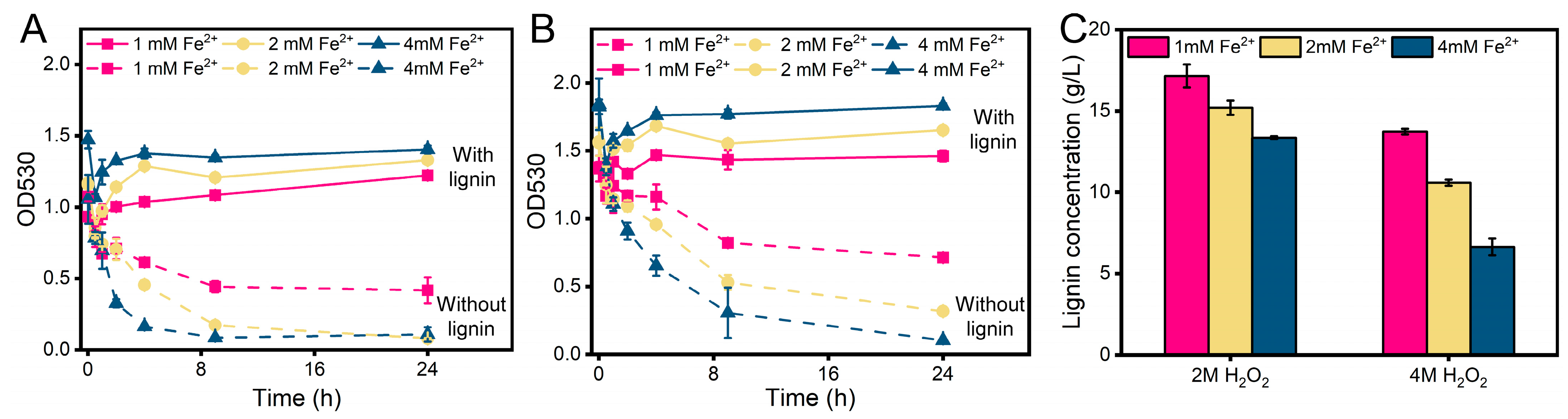
3.1. Fenton-like Reaction Treatment
3.2. PHA Production by P. putida KT2440 Fermentation
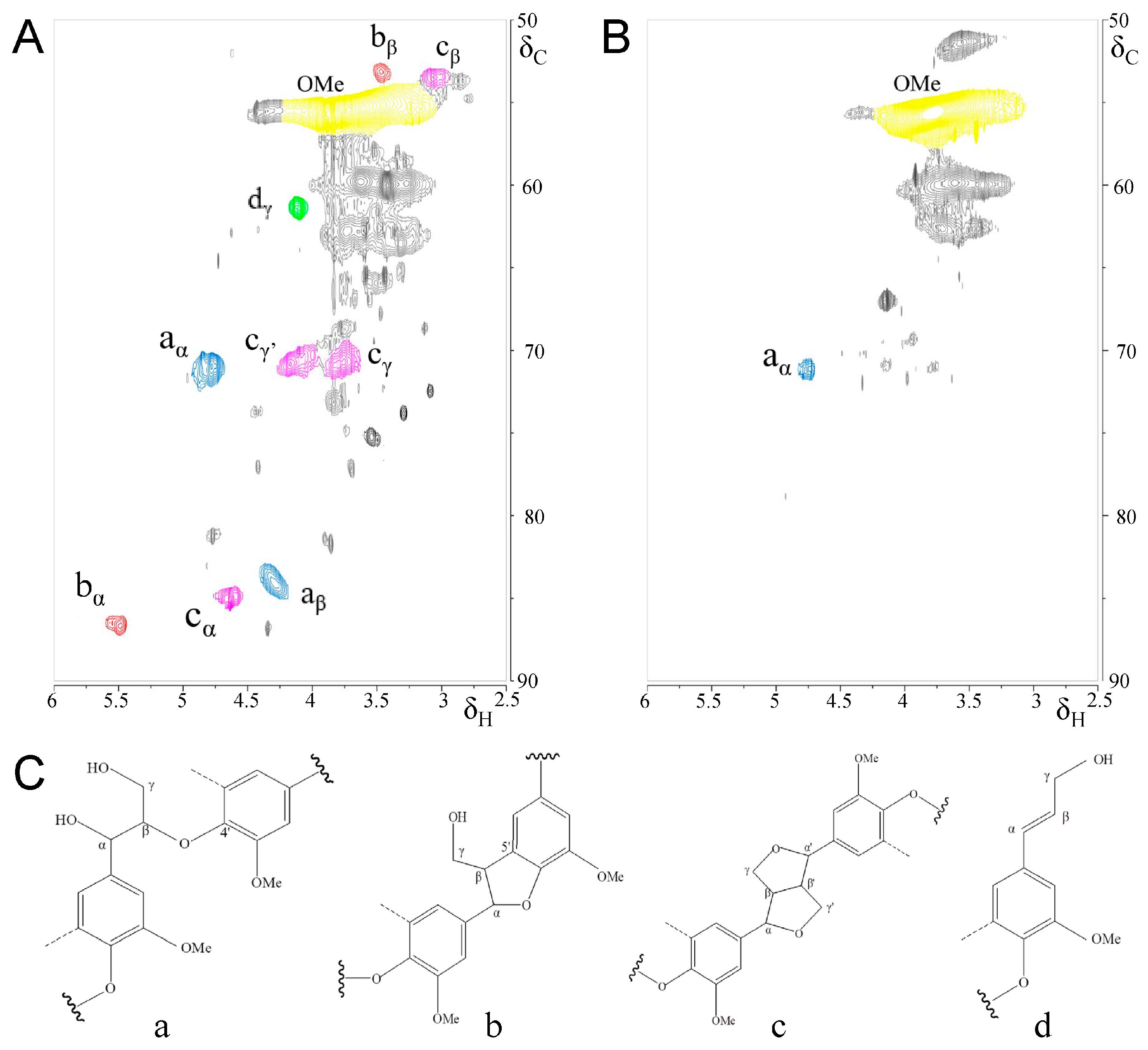
3.3. Characterization of Kraft Lignin
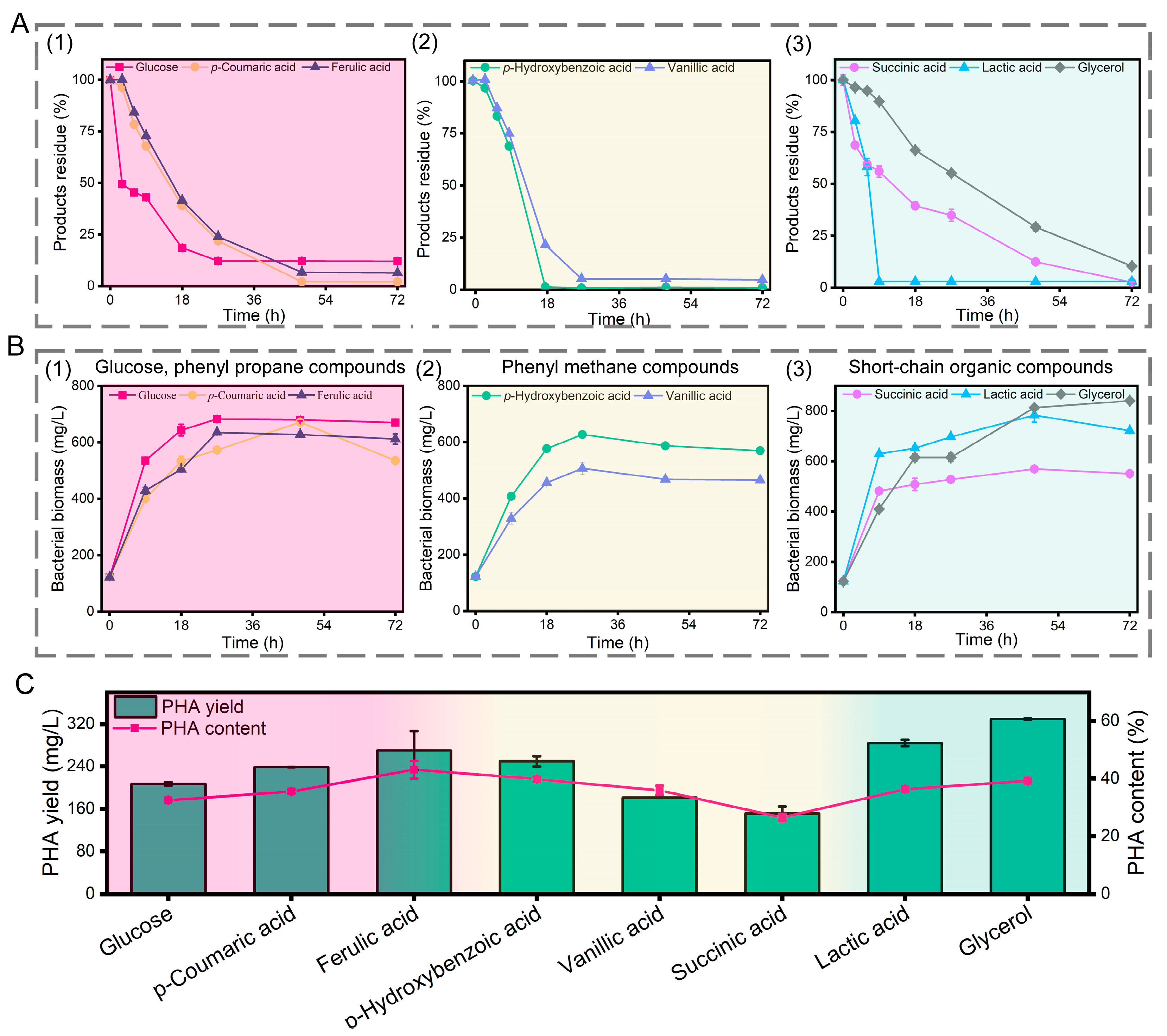
3.4. Growth of P. putida KT2440 and PHA Accumulation on Lignin Depolymerized Products
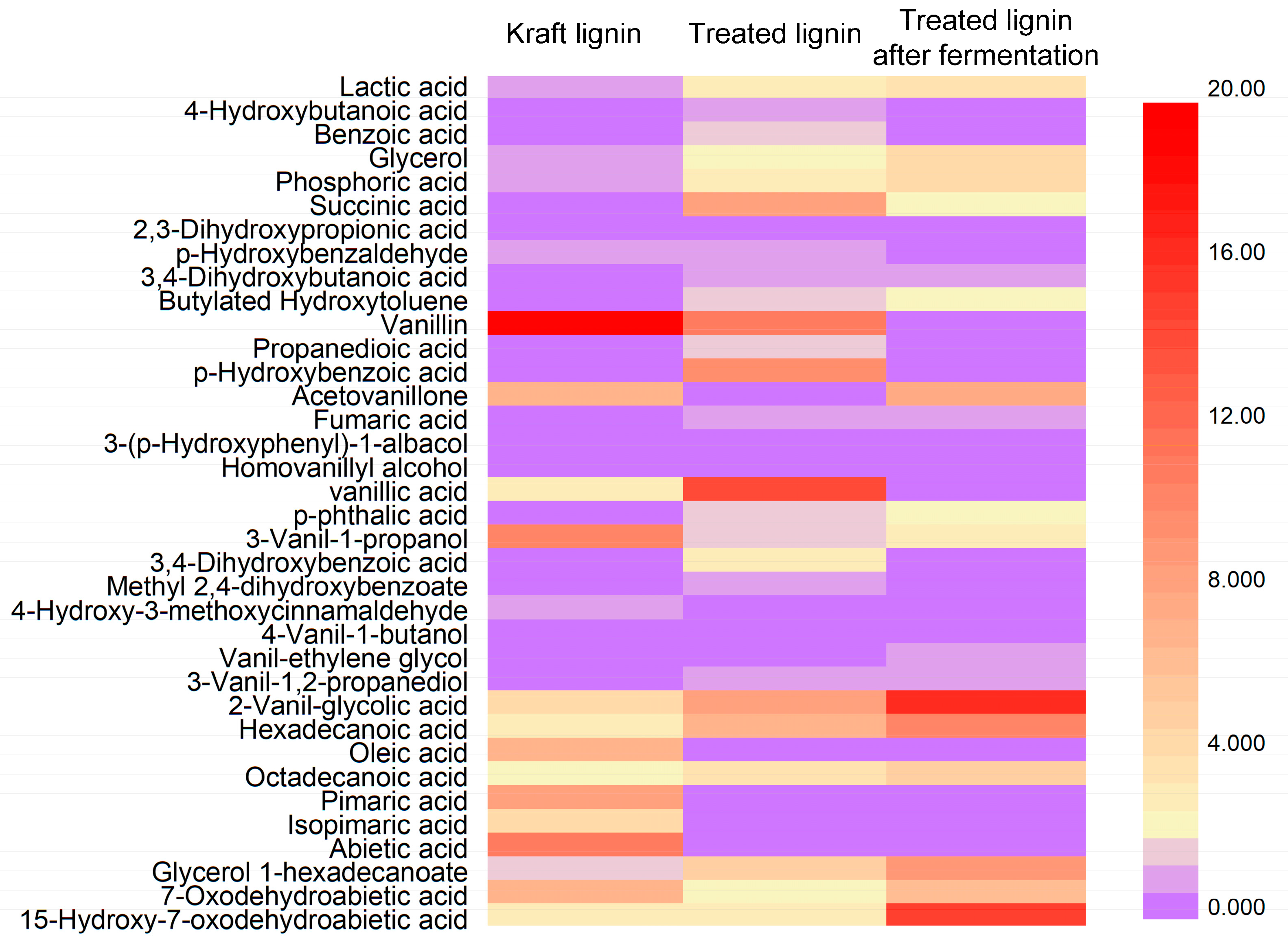
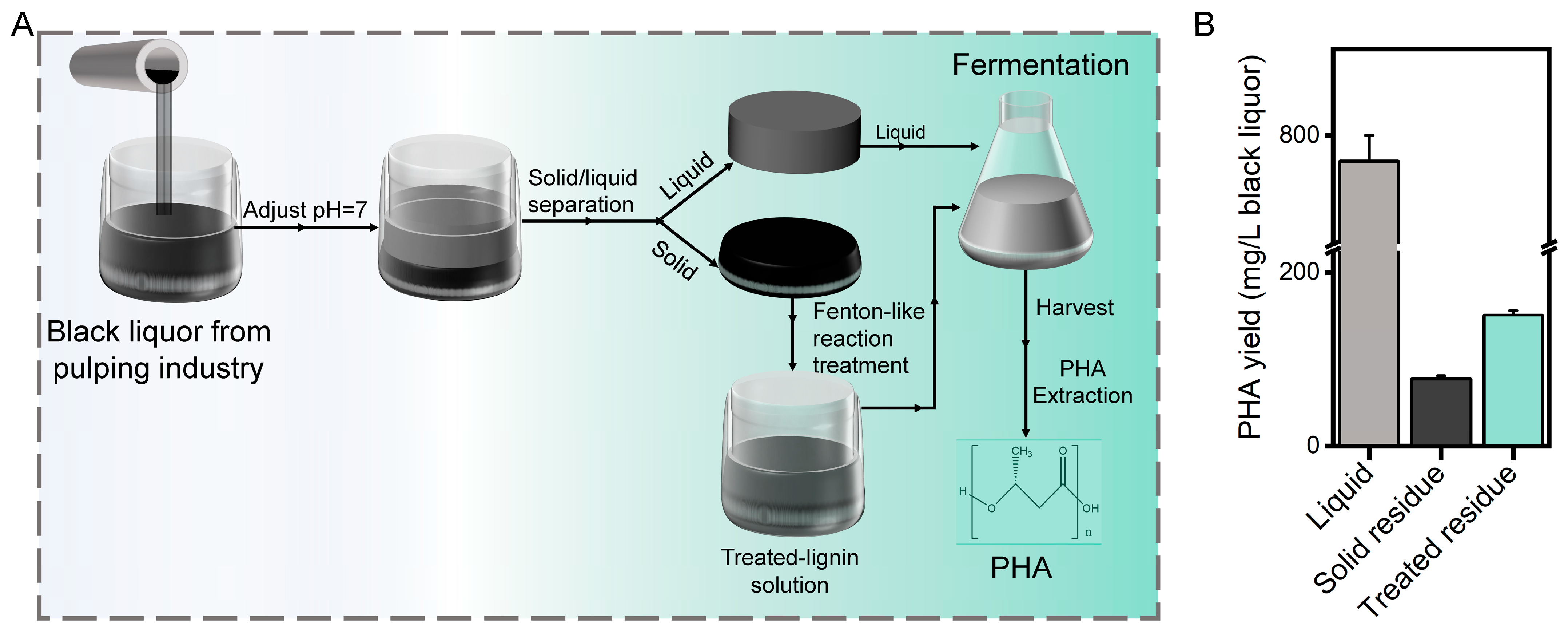
3.5. Fenton-like Reaction Treatment of Black Liquor for PHA Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Xie, S.; Pu, Y.; Zhang, R.; Huang, F.; Ragauskas, A.J.; Yuan, J.S. Synergistic enzymatic and microbial lignin conversion. Green Chem. 2016, 18, 1306–1312. [Google Scholar] [CrossRef]
- Bajwa, D.; Pourhashem, G.; Ullah, A.H.; Bajwa, S. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Velez, J.; Kelkar, S.; Saffron, C.M.; Thies, M.C.; Hodge, D.B. Correlating lignin structural features to phase partitioning behavior in a novel aqueous fractionation of softwood Kraft black liquor. Green Chem. 2013, 15, 2904–2912. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Linger, J.G.; Vardon, D.R.; Guarnieri, M.T.; Karp, E.M.; Hunsinger, G.B.; Franden, M.A.; Johnson, C.W.; Chupka, G.; Strathmann, T.J.; Pienkos, P.T. Lignin valorization through integrated biological funneling and chemical catalysis. Natl. Acad. Sci. 2014, 111, 12013–12018. [Google Scholar] [CrossRef]
- Liu, Z.H.; Olson, M.L.; Shinde, S.; Wang, X.; Hao, N.; Yoo, C.G.; Bhagia, S.; Dunlap, J.R.; Pu, Y.; Kao, K.C. Synergistic maximization of the carbohydrate output and lignin processability by combinatorial pretreatment. Green Chem. 2017, 19, 4939–4955. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, X.; Li, Q.; Wang, X.; Liu, M.; Xie, S.; Chai, L.; Yuan, J.S. Directed bioconversion of Kraft lignin to polyhydroxyalkanoate by Cupriavidus basilensis B-8 without any pretreatment. Process. Biochem. 2017, 52, 238–242. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Raj, A.; Reddy, M.K.; Chandra, R. Identification of low molecular weight aromatic compounds by gas chromatography–mass spectrometry (GC–MS) from kraft lignin degradation by three Bacillus sp. Int. Biodeterior. Biodegrad. 2007, 59, 292–296. [Google Scholar] [CrossRef]
- Lin, L.; Cheng, Y.; Pu, Y.; Sun, S.; Li, X.; Jin, M.; Pierson, E.A.; Gross, D.C.; Dale, B.E.; Dai, S.Y.; et al. Systems biology-guided biodesign of consolidated lignin conversion. Green Chem. 2016, 18, 5536–5547. [Google Scholar] [CrossRef]
- Xie, S.; Ragauskas, A.J.; Yuan, J.S. Lignin conversion: Opportunities and challenges for the integrated biorefinery. Ind. Biotechnol. 2016, 12, 161–167. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Milagres, A.M.; Filley, T.R.; Goodell, B. Lignocellulosic polysaccharides and lignin degradation by wood decay fungi: The relevance of nonenzymatic Fenton-based reactions. J. Ind. Microbiol. Biotechnol. 2011, 38, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Hammel, K.E.; Kapich, A.N.; Jensen, K.A., Jr.; Ryan, Z.C. Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb. Technol. 2002, 30, 445–453. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Y.Z.; Yu, H.Q. Degradation of lignin in pulp mill wastewaters by white-rot fungi on biofilm. Bioresour. Technol. 2005, 96, 1357–1363. [Google Scholar] [CrossRef]
- Walter, K.; Paulsson, M.; Engstrand, P. Acid hydrogen peroxide treatment of Norway spruce TMP: The effect of an extended pH range when catalyzed by free ferrous and free or EDG/EDTA-chelated ferric ions. J. Wood Chem. Technol. 2014, 34, 135–155. [Google Scholar] [CrossRef]
- Aruoma, O.I. Deoxyribose assay for detecting hydroxyl radicals. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 233, pp. 57–66. [Google Scholar]
- Kulkarni, S.; Kanekar, P.; Nilegaonkar, S.; Sarnaik, S.; Jog, J. Production and characterization of a biodegradable poly (hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) copolymer by moderately haloalkalitolerant Halomonas campisalis MCM B-1027 isolated from Lonar Lake, India. Bioresour. Technol. 2010, 101, 9765–9771. [Google Scholar] [CrossRef]
- Ma, F.; Huang, X.; Ke, M.; Shi, Q.; Chen, Q.; Shi, C.; Zhang, J.; Zhang, X.; Yu, H. Role of selective fungal delignification in overcoming the saccharification recalcitrance of bamboo culms. ACS Sustain. Chem. Eng. 2017, 5, 8884–8894. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, J.; Gu, X.; Yu, H.; Chen, S. A comprehensive study of the promoting effect of manganese on white rot fungal treatment for enzymatic hydrolysis of woody and grass lignocellulose. Biotechnol. Biofuels 2021, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ni, H.; Zhang, J.; Shi, Q.; Zhang, R.; Yu, H.; Li, M. Enzymatic treatment improves fast pyrolysis product selectivity of softwood and hardwood lignin. Sci. Total Environ. 2020, 717, 137241. [Google Scholar] [CrossRef]
- Xie, S.; Sun, Q.; Pu, Y.; Lin, F.; Sun, S.; Wang, X.; Ragauskas, A.J.; Yuan, J.S. Advanced chemical design for efficient lignin bioconversion. ACS Sustain. Chem. Eng. 2017, 5, 2215–2223. [Google Scholar] [CrossRef]
- Mottweiler, J.; Rinesch, T.; Besson, C.; Buendia, J.; Bolm, C. Iron-catalysed oxidative cleavage of lignin and β-O-4 lignin model compounds with peroxides in DMSO. Green Chem. 2015, 17, 5001–5008. [Google Scholar] [CrossRef] [Green Version]
- Ohra-aho, T.; Tenkanen, M.; Tamminen, T. Direct analysis of lignin and lignin-like components from softwood kraft pulp by Py-GC/MS techniques. J. Anal. Appl. Pyrolysis 2005, 74, 123–128. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, K.; Huang, M.; Hector, S.B.; Liu, B.; Tong, C.; Liu, Q.; Zeng, J.; Gao, Y.; Xu, T. Involvement of Fenton chemistry in rice straw degradation by the lignocellulolytic bacterium Pantoea ananatis Sd-1. Biotechnol. Biofuels 2016, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Filley, T.; Cody, G.; Goodell, B.; Jellison, J.; Noser, C.; Ostrofsky, A. Lignin demethylation and polysaccharide decomposition in spruce sapwood degraded by brown rot fungi. Org. Geochem. 2002, 33, 111–124. [Google Scholar] [CrossRef]
- Bao, H.; Sagues, W.J.; Wang, Y.; Peng, W.; Zhang, L.; Yang, S.; Xiao, D.; Tong, Z. Depolymerization of lignin into monophenolics by ferrous/persulfate reagent under mild conditions. ChemSusChem 2020, 13, 6582–6593. [Google Scholar] [CrossRef] [PubMed]
- Van Erven, G.; Nayan, N.; Sonnenberg, A.S.; Hendriks, W.H.; Cone, J.W.; Kabel, M.A. Mechanistic insight in the selective delignification of wheat straw by three white-rot fungal species through quantitative 13C-IS py-GC–MS and whole cell wall HSQC NMR. Biotechnol. Biofuels 2018, 11, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Pérez-Boada, M.; Fernández, C.; Rencoret, J.; Del Río, J.C.; Jiménez-Barbero, J.; Li, J.; Gutiérrez, A.; Martínez, A.T. Analysis of lignin–carbohydrate and lignin–lignin linkages after hydrolase treatment of xylan–lignin, glucomannan–lignin and glucan–lignin complexes from spruce wood. Planta 2014, 239, 1079–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.M.; Liu, Z.H.; Pu, Y.; Meng, X.; Xu, J.; Yuan, J.S.; Ragauskas, A.J. Emerging strategies for modifying lignin chemistry to enhance biological lignin valorization. ChemSusChem 2020, 13, 5423–5432. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Liao, Y.; Lv, W.; Ma, L.; Wang, C. Degradation of vanillin during lignin valorization under alkaline oxidation. In Lignin Chemistry; Serrano, L., Luque, R., Sels, B.F., Eds.; Springer Nature Switzerland AG: Basel, Switzerland, 2020; pp. 33–51. [Google Scholar]
- Morya, R.; Kumar, M.; Singh, S.S.; Thakur, I.S. Genomic analysis of Burkholderia sp. ISTR5 for biofunneling of lignin-derived compounds. Biotechnol. Biofuels 2019, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Morya, R.; Kumar, M.; Kumar, V.; Thakur, I.S. Biovalorization of lignin derived compounds with molasses as co-substrate for polyhydroxyalkanoate production. Environ. Technol. Innov. 2021, 23, 101695. [Google Scholar] [CrossRef]
- Li, F.; Zhao, Y.; Xue, L.; Ma, F.; Dai, S.Y.; Xie, S. Microbial lignin valorization through depolymerization to aromatics conversion. Trends Biotechnol. 2022, 40, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zheng, Z.; Sha, Z.; Cao, H.; Yuan, Q.; Yu, H.; Li, Q. Biorefining waste into nano-biotechnologies can revolutionize sustainable agriculture. Trends Biotechnol. 2022, 40, 1503–1518. [Google Scholar] [CrossRef] [PubMed]

| Kraft Lignin | Treated Lignin | |
|---|---|---|
| Lignin subunits (%) | ||
| H | 6.49 | 43.56 |
| G | 80.02 | 56.44 |
| G/H | 12.33 | 1.30 |
| Structural moieties (%) | ||
| Ph-C1 | 27.19 | 34.24 |
| Ph-C2 | 20.80 | 14.77 |
| Ph-C3 | 6.28 | 2.35 |
| Ph-C1-2/ph-C3 | 7.64 | 20.84 |
| Kraft Lignin | Treated Lignin | |
|---|---|---|
| Lignin inter-unit linkages 1 | ||
| β-O-4′ | 1.23 | 0.64 |
| β-5′ | 0.26 | ND |
| β-β′ | 0.53 | ND |
| Lignin end-group 2 | 0.60 | ND |
| Condensation degree 3 | 0.43 | ND |
| OMe (%) | 21.62 | 14.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Gong, Q.; Liu, X.; Zheng, Z.; Zhang, X.; Ma, F.; Yu, H.; Xie, S. Mimicking the Fungal Decay Strategy for Promoting the Bacterial Production of Polyhydroxyalkanoate from Kraft Lignin. Fermentation 2023, 9, 649. https://doi.org/10.3390/fermentation9070649
Fu X, Gong Q, Liu X, Zheng Z, Zhang X, Ma F, Yu H, Xie S. Mimicking the Fungal Decay Strategy for Promoting the Bacterial Production of Polyhydroxyalkanoate from Kraft Lignin. Fermentation. 2023; 9(7):649. https://doi.org/10.3390/fermentation9070649
Chicago/Turabian StyleFu, Xiao, Qing Gong, Xuan Liu, Ze Zheng, Xiaoyu Zhang, Fuying Ma, Hongbo Yu, and Shangxian Xie. 2023. "Mimicking the Fungal Decay Strategy for Promoting the Bacterial Production of Polyhydroxyalkanoate from Kraft Lignin" Fermentation 9, no. 7: 649. https://doi.org/10.3390/fermentation9070649











