Joint Multi-Optimization of an Extremophilic Microbial Bioanode for Mitigation of Mixed Hazardous Azo Dyes in Textile Synthetic Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Hypersaline Sediment and Textile Dyes
2.2. Combined Effects of Working Electrode Material, Working Electrode Surface Area, Glucose Co-Substrate Concentration, and Applied Polarization Potential on the Performance of the BES
2.3. Bioelectrochemical System Construction, Inoculation, and Operation
2.4. Decolorization and Biodegradation Analysis
2.5. Microscopic Characterization of Biofilms
2.6. Bactrial Community Analyses
3. Results and Discussion
3.1. Analyses of Concomitant Effects of Anode Material, Anode Surface, Glucose Concentration, and Applied Potential on Current Production, Dye Decolorization, and COD Removal
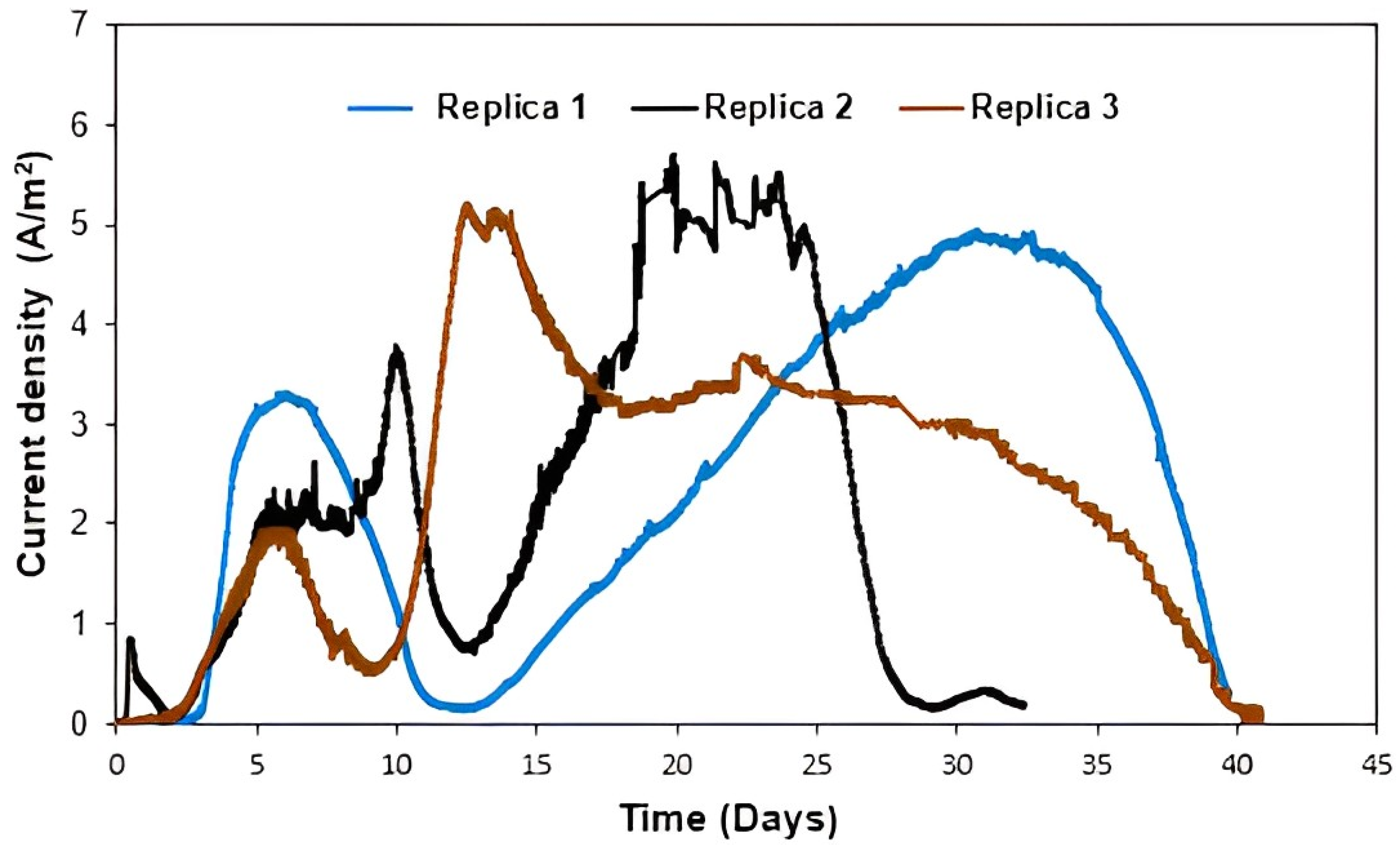
3.1.1. Concomitant Effects of Anode Material, Anode Surface, Glucose Concentration, and Applied Potential on Current Production
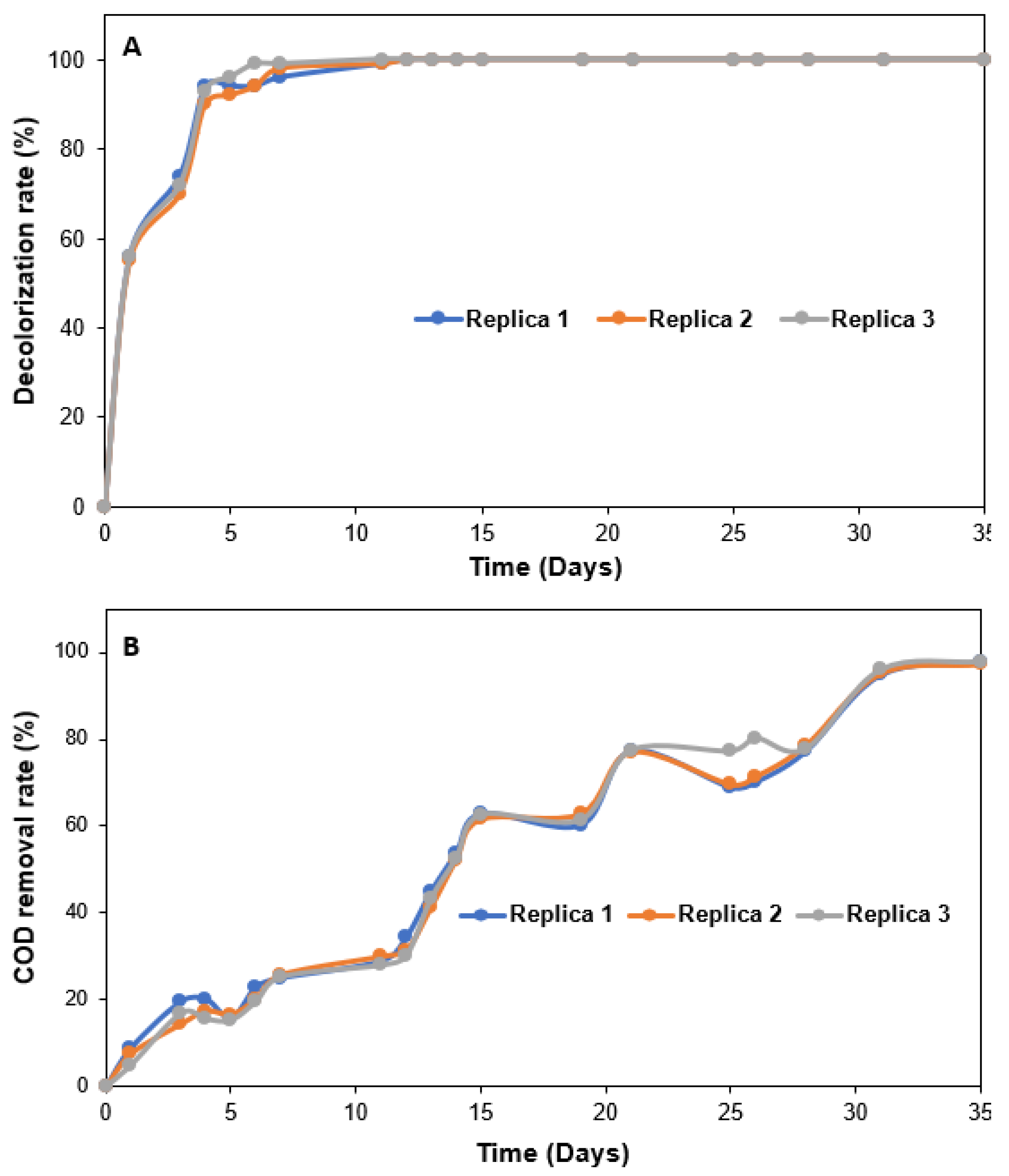
3.1.2. Concomitant Effects of Anode Material, Anode Surface, Glucose Concentration, and Applied Potential on Dye Decolorization
3.1.3. Concomitant Effects of Anode Material, Anode Surface, Glucose Concentration and Applied Potential on COD Removal Rate
3.2. Experimental Validation of the Model
3.3. FTIR Analysis
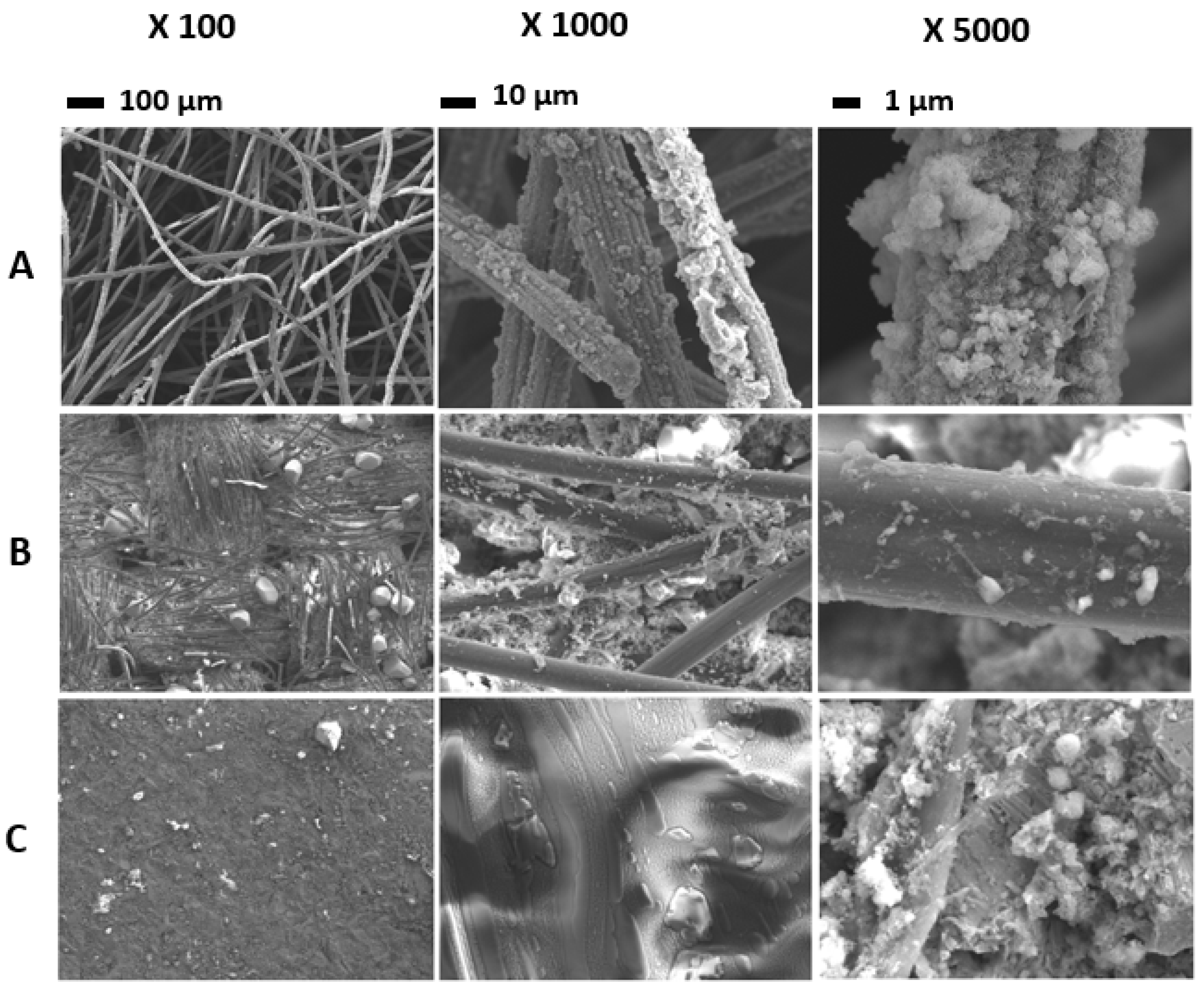
3.4. Biofilm Architecture
3.5. Comparison of Bacterial Communities in Biofilms Colonising Electrode Materials and Synthetic Media Containing Azo Dye Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.-H.; Rai, B.N.; Singh, R.S. Recent Advancements in Bioremediation of Dye: Current Status and Challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Chittal, V.; Gracias, M.; Anu, A.; Saha, P.; Bhaskara Rao, K.V. Biodecolorization and Biodegradation of Azo Dye Reactive Orange-16 by Marine Nocardiopsis sp. Iran. J. Biotechnol. 2019, 17, 18–26. [Google Scholar] [CrossRef]
- Saadaoui, S.; Erable, B.; Saidi, N.; Etcheverry, L.; Neifar, M.; Masmoudi, A.S.; Driouech, R.; Cherif, A.; Chouchane, H. Saline Sediments as a Suitable Source for Halophilic Inoculums to Degrade Azo Dyes in Synthetic and Real Textile Wastewaters by Microbial Electrochemical Systems. Appl. Sci. 2023, 13, 5581. [Google Scholar]
- Cui, M.-H.; Liu, W.-Z.; Tang, Z.-E.; Cui, D. Recent Advancements in Azo Dye Decolorization in Bio-Electrochemical Systems (BESs): Insights into Decolorization Mechanism and Practical Application. Water Res. 2021, 203, 117512. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Crowley, D.E.; Khalid, A.; Hussain, S.; Mumtaz, M.W.; Arshad, M. Microbial Biotechnology for Decolorization of Textile Wastewaters. Rev. Environ. Sci. Bio/Technol. 2015, 14, 73–92. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Garud, H.B.; Patil, A.H.; Patil, G.D.; Patil, C.R.; Dongale, T.D.; Patil, P.S. Recent Advancements in Silica Nanoparticles Based Technologies for Removal of Dyes from Water. Colloid Interface Sci. Commun. 2019, 30, 100181. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Solanki, K.; Subramanian, S.; Basu, S. Microbial Fuel Cells for Azo Dye Treatment with Electricity Generation: A Review. Bioresour. Technol. 2013, 131, 564–571. [Google Scholar]
- Askri, R.; Erable, B.; Etcheverry, L.; Saadaoui, S.; Neifar, M.; Cherif, A.; Chouchane, H. Allochthonous and autochthonous halothermotolerant bioanodes from hypersaline sediment and textile wastewater: A promising microbial electrochemical process for energy recovery coupled with real textile wastewater treatment. Front. Bioeng. Biotechnol. 2020, 8, 609446. [Google Scholar]
- Saidi, N.; Erable, B.; Saadaoui, S.; Driouech, R.; Zaouak, A.; Jelassi, H.; Neifar, M.; Masmoudi, A.S.; Cherif, A.; Chouchane, H. Tunisian Hypersaline Sediments to Set up Suitable Halotolerant Microbial Bioanodes for Electrostimulated Biodegradation of Thiabendazole. Front. Energy Res. 2022, 10, 981802. [Google Scholar]
- Cruz Viggi, C.; Casale, S.; Chouchane, H.; Askri, R.; Fazi, S.; Cherif, A.; Zeppilli, M.; Aulenta, F. Magnetite nanoparticles enhance the bioelectrochemical treatment of municipal sewage by facilitating the syntrophic oxidation of volatile fatty acids. J. Chem. Technol. Biotechnol. 2019, 94, 3134–3146. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Jamal, A.; Ilyas, M.; Zubair, M.; Khan, G.; Atieh, M.A. Bioremediation of Dyes: Current Status and Prospects. J. Water Process Eng. 2020, 38, 101680. [Google Scholar] [CrossRef]
- Sun, L.; Mo, Y.; Zhang, L. A Mini Review on Bio-Electrochemical Systems for the Treatment of Azo Dye Wastewater: State-of-the-Art and Future Prospects. Chemosphere 2022, 294, 133801. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Song, H.; Wei, S.; Liu, Q.; Li, X.; Qian, X. Effect of Direct Electrical Stimulation on Decolorization and Degradation of Azo Dye Reactive Brilliant Red X-3B in Biofilm-Electrode Reactors. Biochem. Eng. J. 2015, 93, 294–302. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Liu, J.; Wang, Y.-X.; He, C.-S.; Zhang, L.-S.; Mu, Y.; Li, W.-H. Bioelectrochemical Decolorization of a Reactive Diazo Dye: Kinetics, Optimization with a Response Surface Methodology, and Proposed Degradation Pathway. Bioelectrochemistry 2019, 128, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Wei, H.; Qiu, H.; Su, Y.; Jaafry, S.W.H.; Zhan, L.; Xie, B. Power Generation and Pollutants Removal from Landfill Leachate in Microbial Fuel Cell: Variation and Influence of Anodic Microbiomes. Bioresour. Technol. 2018, 247, 434–442. [Google Scholar] [CrossRef]
- Grattieri, M.; Minteer, S.D. Microbial Fuel Cells in Saline and Hypersaline Environments: Advancements, Challenges and Future Perspectives. Bioelectrochemistry 2018, 120, 127–137. [Google Scholar] [CrossRef]
- Askri, R.; Erable, B.; Neifar, M.; Etcheverry, L.; Masmoudi, A.S.; Cherif, A.; Chouchane, H. Understanding the Cumulative Effects of Salinity, Temperature and Inoculation Size for the Design of Optimal Halothermotolerant Bioanodes from Hypersaline Sediments. Bioelectrochemistry 2019, 129, 179–188. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Zhang, M.-M.; Ding, Y.; Chang, C.-T. Feasibility Study of Simultaneous Bioelectricity Generation and Dye Decolorization Using Naturally Occurring Decolorizers. J. Taiwan Inst. Chem. Eng. 2010, 41, 682–688. [Google Scholar] [CrossRef]
- Cui, M.-H.; Cui, D.; Gao, L.; Wang, A.-J.; Cheng, H.-Y. Evaluation of Anaerobic Sludge Volume for Improving Azo Dye Decolorization in a Hybrid Anaerobic Reactor with Built-in Bioelectrochemical System. Chemosphere 2017, 169, 18–22. [Google Scholar] [CrossRef]
- Thung, W.-E.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Ridwan, F.; Oon, Y.-L.; Oon, Y.-S.; Lehl, H.K. A Highly Efficient Single Chambered Up-Flow Membrane-Less Microbial Fuel Cell for Treatment of Azo Dye Acid Orange 7-Containing Wastewater. Bioresour. Technol. 2015, 197, 284–288. [Google Scholar] [CrossRef]
- Sun, J.; Bi, Z.; Hou, B.; Cao, Y.; Hu, Y. Further Treatment of Decolorization Liquid of Azo Dye Coupled with Increased Power Production Using Microbial Fuel Cell Equipped with an Aerobic Biocathode. Water Res. 2011, 45, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Carmalin, S.A.; Bhalambaal, V.M.; Lima, E.C. Re-Use of Carbon Rods from Used Batteries as Cathode for Textile Azo Dye Degradation in a Microbial Fuel Cell. Desalin. Water Treat. 2017, 79, 322–328. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, W.; Xu, G.; Luan, X.; Li, Q.; Yin, X.; Huang, D.; Zheng, L. Marinobacter Aromaticivorans Sp. Nov., a Polycyclic Aromatic Hydrocarbon-Degrading Bacterium Isolated from Sea Sediment. Int. J. Syst. Evol. Microbiol. 2016, 66, 353–359. [Google Scholar] [CrossRef]
- Mu, Y.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Decolorization of Azo Dyes in Bioelectrochemical Systems. Environ. Sci. Technol. 2009, 43, 5137–5143. [Google Scholar] [CrossRef]
- Kalathil, S.; Lee, J.; Cho, M.H. Efficient Decolorization of Real Dye Wastewater and Bioelectricity Generation Using a Novel Single Chamber Biocathode-Microbial Fuel Cell. Bioresour. Technol. 2012, 119, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Miran, W.; Nawaz, M.; Kadam, A.; Shin, S.; Heo, J.; Jang, J.; Lee, D.S. Microbial Community Structure in a Dual Chamber Microbial Fuel Cell Fed with Brewery Waste for Azo Dye Degradation and Electricity Generation. Environ. Sci. Pollut. Res. 2015, 22, 13477–13485. [Google Scholar] [CrossRef]
- Fernando, E.; Keshavarz, T.; Kyazze, G.; Fonseka, K. Treatment of Colour Industry Wastewaters with Concomitant Bioelectricity Production in a Sequential Stacked Mono-Chamber Microbial Fuel Cells–Aerobic System. Environ. Technol. 2016, 37, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, L.; Lin, C.; Zheng, J.; Wen, Q.; Chen, Y.; Wang, Y.; Qi, L. Improved Simultaneous Decolorization and Power Generation in a Microbial Fuel Cell with the Sponge Anode Modified by Polyaniline and Chitosan. Appl. Biochem. Biotechnol. 2020, 192, 698–718. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.; Santaella, C.; Bonnafous, A.; Achouak, W.; Godon, J.-J.; Delia, M.-L.; Bergel, A. Halotolerant Bioanodes: The Applied Potential Modulates the Electrochemical Characteristics, the Biofilm Structure and the Ratio of the Two Dominant Genera. Bioelectrochemistry 2016, 112, 24–32. [Google Scholar] [CrossRef]
- Chouchane, H.; Mahjoubi, M.; Ettoumi, B.; Neifar, M.; Cherif, A. A Novel Thermally Stable Heteropolysaccharide-Based Bioflocculant from Hydrocarbonoclastic Strain KocuriaRosea BU22S and Its Application in Dye Removal. Environ. Technol. 2018, 39, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Oon, Y.-S.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Oon, Y.-L.; Lehl, H.K.; Thung, W.-E.; Nordin, N. Microbial Fuel Cell Operation Using Monoazo and Diazo Dyes as Terminal Electron Acceptor for Simultaneous Decolourisation and Bioelectricity Generation. J. Hazard. Mater. 2017, 325, 170–177. [Google Scholar] [CrossRef]
- Kang, C.S.; Eaktasang, N.; Kwon, D.-Y.; Kim, H.S. Enhanced Current Production by Desulfovibrio Desulfuricans Biofilm in a Mediator-Less Microbial Fuel Cell. Bioresour. Technol. 2014, 165, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, L.; Tao, J.; Zhou, L.; Zhang, S. Microbial Fuel Cells Coupling with the Three-Dimensional Electro-Fenton Technique Enhances the Degradation of Methyl Orange in the Wastewater. Environ. Sci. Pollut. Res. 2018, 25, 17989–18000. [Google Scholar] [CrossRef]
- Teoh, T.-P.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Lutpi, N.A.; Oon, Y.-L.; Tan, S.-M.; Ong, Y.-P.; Yap, K.-L. Insights into the Decolorization of Mono and Diazo Dyes in Single and Binary Dyes Containing Wastewater and Electricity Generation in Up-Flow Constructed Wetland Coupled Microbial Fuel Cell. Environ. Sci. Pollut. Res. 2022, 30, 17546–17563. [Google Scholar] [CrossRef]
- Miran, W.; Jang, J.; Nawaz, M.; Shahzad, A.; Lee, D.S. Sulfate-Reducing Mixed Communities with the Ability to Generate Bioelectricity and Degrade Textile Diazo Dye in Microbial Fuel Cells. J. Hazard. Mater. 2018, 352, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Lin, J.; Han, S.; Lei, L. Azo Dye Treatment with Simultaneous Electricity Production in an Anaerobic–Aerobic Sequential Reactor and Microbial Fuel Cell Coupled System. Bioresour. Technol. 2010, 101, 4440–4445. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Oon, Y.-S.; Lehl, H.K.; Thung, W.-E.; Nordin, N. Up-Flow Constructed Wetland-Microbial Fuel Cell for Azo Dye, Saline, Nitrate Remediation and Bioelectricity Generation: From Waste to Energy Approach. Bioresour. Technol. 2018, 266, 97–108. [Google Scholar]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Shahi, A.; Rai, B.N.; Singh, R.S. Biodegradation of Reactive Orange 16 Dye in Microbial Fuel Cell: An Innovative Way to Minimize Waste Along with Electricity Production. Appl. Biochem. Biotechnol. 2020, 192, 196–210. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Hu, Y.; Hou, B.; Xu, Q.; Zhang, Y.; Li, S. Enlargement of Anode for Enhanced Simultaneous Azo Dye Decolorization and Power Output in Air-Cathode Microbial Fuel Cell. Biotechnol. Lett. 2012, 34, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Ilamathi, R.; Jayapriya, J. Microbial Fuel Cells for Dye Decolorization. Environ. Chem. Lett. 2018, 16, 239–250. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Cao, X.; Huang, X. A New Insight into Potential Regulation on Growth and Power Generation of Geobacter Sulfurreducens in Microbial Fuel Cells Based on Energy Viewpoint. Environ. Sci. Technol. 2010, 44, 3187–3191. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Shen, F.; Yin, A.; Liu, J.; Wu, B.; Li, Y.; Xiao, Y.; Hai, J.; Xu, B. Biodegradation and Detoxification of Azo Dyes by Halophilic/Halotolerant Microflora Isolated From the Salt Fields of Tibet Autonomous Region China. Front. Microbiol. 2022, 13, 877151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, L.; Li, H.; Wang, Y.; Chen, G.; Zhang, Q. Biodegradation and Detoxification of Direct Black G Textile Dye by a Newly Isolated Thermophilic Microflora. Bioresour. Technol. 2018, 250, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, E.; Erable, B.; De Solan, M.-L.; Bergel, A. Two-Dimensional Carbon Cloth and Three-Dimensional Carbon Felt Perform Similarly to Form Bioanode Fed with Food Waste. Electrochem. Commun. 2016, 66, 38–41. [Google Scholar] [CrossRef]
- Dong, Y.; Sanford, R.A.; Boyanov, M.I.; Kemner, K.M.; Flynn, T.M.; O’Loughlin, E.J.; Chang, Y.; Locke, R.A.; Weber, J.R.; Egan, S.M.; et al. Orenia Metallireducens Sp. Nov. Strain Z6, a Novel Metal-Reducing Member of the Phylum Firmicutes from the Deep Subsurface. Appl. Environ. Microbiol. 2016, 82, 6440–6453. [Google Scholar] [CrossRef]
- Diniz, P.E.; Lopes, A.T.; Lino, A.R.; Serralheiro, M.L. Anaerobic Reduction of a Sulfonated Azo Dye, Congo Red, by Sulfate-Reducing Bacteria. Appl. Biochem. Biotechnol. 2002, 97, 147–164. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, S.; Liu, H.; Huang, J.; Li, L. Sulfide-Mediated Azo Dye Degradation and Microbial Community Analysis in a Single-Chamber Air Cathode Microbial Fuel Cell. Bioelectrochemistry 2020, 131, 107349. [Google Scholar] [CrossRef]
- Selvaraj, V.; Swarna Karthika, T.; Mansiya, C.; Alagar, M. An over Review on Recently Developed Techniques, Mechanisms and Intermediate Involved in the Advanced Azo Dye Degradation for Industrial Applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Joshi, A.U.; Hinsu, A.T.; Kotadiya, R.J.; Rank, J.K.; Andharia, K.N.; Kothari, R.K. Decolorization and Biodegradation of Textile Di-Azo Dye Acid Blue 113 by Pseudomonas Stutzeri AK6. 3 Biotech 2020, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.P.; Tank, S.K. Screening and Identification of Newly Isolated Pseudomonas Sp. for Biodegrading the Textile Azo Dye C.I. Procion Red H-3B. J. Appl. Microbiol. 2021, 130, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Garg, A.; Mukherji, S. Eco-Friendly Decolorization and Degradation of Reactive Yellow 145 Textile Dye by Pseudomonas Aeruginosa and Thiosphaera Pantotropha. J. Environ. Manag. 2020, 263, 110383. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Li, X.; Tian, F.; Liu, T.; Yang, F.; Ding, K.; Liu, C.; Chen, J.; Wang, C. Azo Dye Decolorization by a Halotolerant Consortium under Microaerophilic Conditions. Chemosphere 2020, 244, 125510. [Google Scholar] [CrossRef]

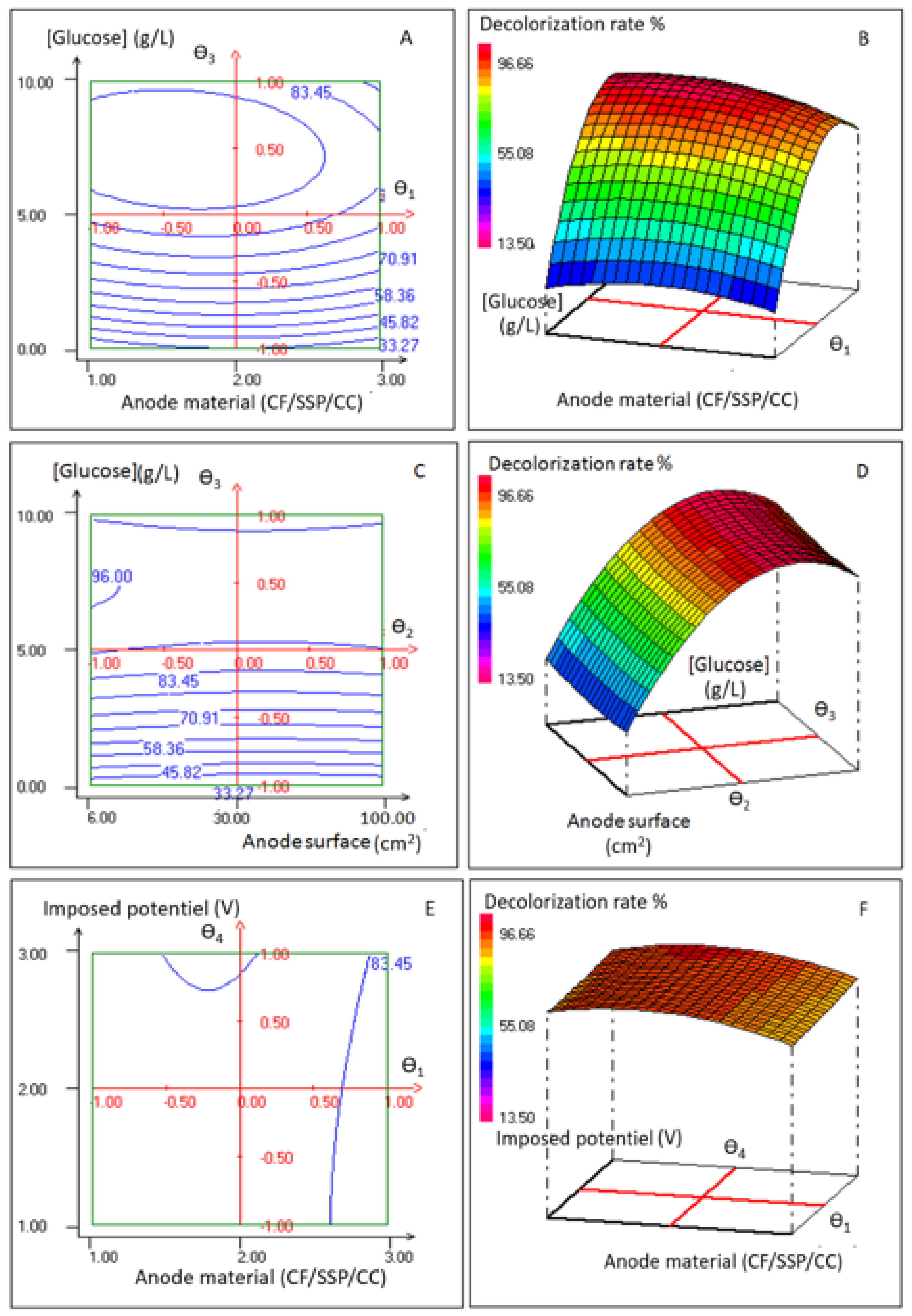
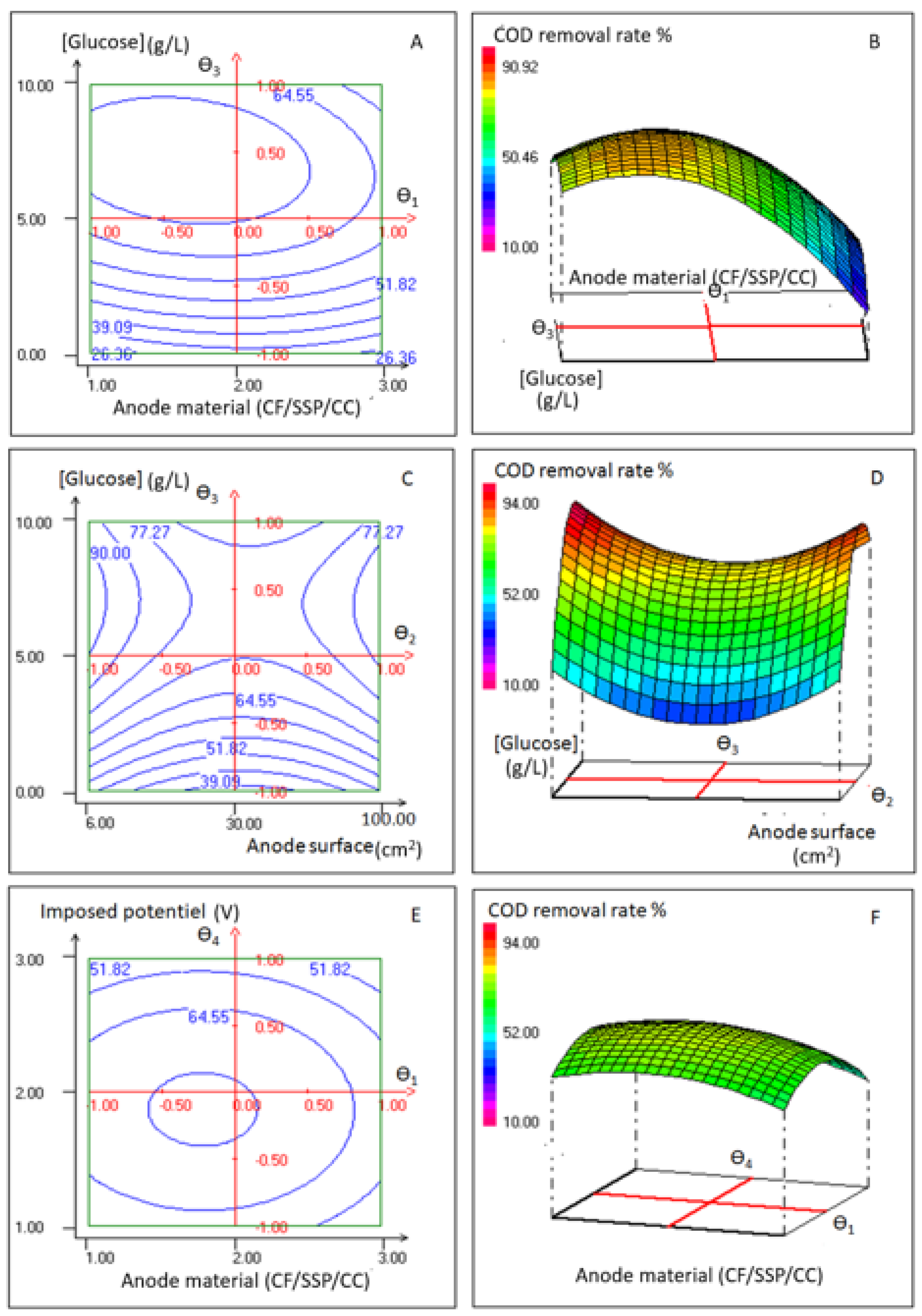


| Variables | −1 | 0 | 1 |
|---|---|---|---|
| Working electrode material (θ1) | Carbon cloth | Carbon felt | Stainless steel plate |
| Working electrode surface area (θ2) (cm2) | 6 | 30 | 100 |
| Glucose co-substrate concentration (θ3) (g/L) | 5 | 0 | 10 |
| Applied polarization potential (θ4) (V/SCE) | −0.3 | −0.1 | +0.2 |
| Responses (ψ) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coded Variables | Natural Variables | Ψ1 | Ψ2 | Ψ3 | ||||||||||
| Reactors | θ1 | θ2 | θ3 | θ4 | Anode Material | Anode Surface | [Glucose] (g/L) | Applied Potential (V) | Exp (A/m2) | Pred (A/m2) | Exp (%) | Pred (%) | Exp (%) | Pred (%) |
| R1 | −1 | −1 | −1 | −1 | CF | 6 | 0 | −0.3 | 0.00 | −0.02 | 29.00 | 25.02 | 30.00 | 28.40 |
| R2 | 1 | −1 | −1 | −1 | SSP | 6 | 0 | −0.3 | 0.00 | −0.59 | 28.00 | 22.89 | 27.00 | 25.86 |
| R3 | −1 | 1 | −1 | −1 | CF | 100 | 0 | −0.3 | 0.00 | 0.23 | 20.00 | 29.23 | 29.00 | 29.92 |
| R4 | 1 | 1 | −1 | −1 | SSP | 100 | 0 | −0.3 | 0.00 | 0.75 | 25.00 | 36.85 | 27.00 | 31.13 |
| R5 | −1 | −1 | 1 | −1 | CF | 6 | 10 | −0.3 | 3.30 | 3.97 | 72.00 | 75.56 | 90.00 | 86.58 |
| R6 | 1 | −1 | 1 | −1 | SSP | 6 | 10 | −0.3 | 0.23 | 0.53 | 42.00 | 56.69 | 64.00 | 72.29 |
| R7 | −1 | 1 | 1 | −1 | CF | 100 | 10 | −0.3 | 3.50 | 2.51 | 80.00 | 75.02 | 89.00 | 88.85 |
| R8 | 1 | 1 | 1 | −1 | SSP | 100 | 10 | −0.3 | 0.42 | 0.17 | 75.00 | 65.89 | 82.00 | 78.31 |
| R9 | −1 | −1 | −1 | 1 | CF | 6 | 0 | +0.2 | 0.02 | 0.82 | 25.00 | 33.23 | 33.00 | 33.97 |
| R10 | 1 | −1 | −1 | 1 | SSP | 6 | 0 | +0.2 | 0.02 | 0.43 | 26.00 | 30.35 | 31.00 | 33.18 |
| R11 | −1 | 1 | −1 | 1 | CF | 100 | 0 | +0.2 | 0.05 | −0.82 | 30.00 | 14.69 | 32.00 | 25.74 |
| R12 | 1 | 1 | −1 | 1 | SSP | 100 | 0 | +0.2 | 0.00 | −0.12 | 26.00 | 21.56 | 28.00 | 28.69 |
| R13 | −1 | −1 | 1 | 1 | CF | 6 | 10 | +0.2 | 7.00 | 5.67 | 90.00 | 77.52 | 96.00 | 93.91 |
| R14 | 1 | −1 | 1 | 1 | SSP | 6 | 10 | +0.2 | 2.10 | 2.41 | 68.00 | 57.89 | 85.00 | 81.36 |
| R15 | −1 | 1 | 1 | 1 | CF | 100 | 10 | +0.2 | 1.20 | 2.32 | 50.00 | 54.23 | 88.00 | 86.42 |
| R16 | 1 | 1 | 1 | 1 | SSP | 100 | 10 | +0.2 | 0.70 | 0.15 | 41.00 | 44.35 | 74.00 | 77.63 |
| R17 | −1 | 0 | 0 | 0 | CF | 30 | 5 | −0.1 | 1.14 | 1.54 | 56.00 | 67.51 | 72.00 | 85.22 |
| R18 | 1 | 0 | 0 | 0 | SSP | 30 | 5 | −0.1 | 0.40 | 0.16 | 67.00 | 61.51 | 90.00 | 79.57 |
| R19 | 0 | −1 | 0 | 0 | CC | 6 | 5 | −0.1 | 3.25 | 2.71 | 89.00 | 89.84 | 90.00 | 90.44 |
| R20 | 0 | 1 | 0 | 0 | CC | 30 | 5 | −0.1 | 1.00 | 1.70 | 80.00 | 85.17 | 87.00 | 89.33 |
| R21 | 0 | 0 | −1 | 0 | CC | 30 | 0 | −0.1 | 0.00 | −0.58 | 35.00 | 30.17 | 33.00 | 33.11 |
| R22 | 0 | 0 | 1 | 0 | CC | 30 | 10 | −0.1 | 0.80 | 1.54 | 56.00 | 66.84 | 84.00 | 86.67 |
| R23 | 0 | 0 | 0 | −1 | CC | 30 | 5 | −0.3 | 0.90 | 0.82 | 78.00 | 61.84 | 91.00 | 87.67 |
| R24 | 0 | 0 | 0 | 1 | CC | 30 | 5 | +0.2 | 1.00 | 1.24 | 33.00 | 55.17 | 84.00 | 90.11 |
| R25 | 0 | 0 | 0 | 0 | CC | 30 | 5 | −0.1 | 1.00 | 1.14 | 75.00 | 71.32 | 89.00 | 88.22 |
| R26 | 0 | 0 | 0 | 0 | CC | 30 | 5 | −0.1 | 1.50 | 1.14 | 76.00 | 71.32 | 92.00 | 88.22 |
| R27 | 0 | 0 | 0 | 0 | CC | 30 | 5 | −0.1 | 1.40 | 1.14 | 81.00 | 71.32 | 92.00 | 88.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadaoui, S.; Erable, B.; Etchevery, L.; Cherif, A.; Chouchane, H. Joint Multi-Optimization of an Extremophilic Microbial Bioanode for Mitigation of Mixed Hazardous Azo Dyes in Textile Synthetic Wastewater. Fermentation 2023, 9, 782. https://doi.org/10.3390/fermentation9090782
Saadaoui S, Erable B, Etchevery L, Cherif A, Chouchane H. Joint Multi-Optimization of an Extremophilic Microbial Bioanode for Mitigation of Mixed Hazardous Azo Dyes in Textile Synthetic Wastewater. Fermentation. 2023; 9(9):782. https://doi.org/10.3390/fermentation9090782
Chicago/Turabian StyleSaadaoui, Sirine, Benjamin Erable, Luc Etchevery, Ameur Cherif, and Habib Chouchane. 2023. "Joint Multi-Optimization of an Extremophilic Microbial Bioanode for Mitigation of Mixed Hazardous Azo Dyes in Textile Synthetic Wastewater" Fermentation 9, no. 9: 782. https://doi.org/10.3390/fermentation9090782





