Innovative Fermented Soy Drink with the Sea Buckthorn Syrup and the Probiotics Co-Culture of Lactobacillus Paracasei ssp. Paracasei (L. Casei® 431) and Bifidobacterium Animalis ssp. Lactis (Bb-12®)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Soymilk
- -
- the product obtained directly from NMG soybeans (95% soy content).
- -
- it contains natural ingredients and flavors.
- -
- it does not contain preservatives or dyes.
- -
- it does not contain lactose.
2.1.2. Sea Buckthorn Syrup
2.1.3. Probiotic Lactic Acid Bacteria
2.1.4. Preparation of Fermented Products
2.1.5. Analytical Methodology
3. Results
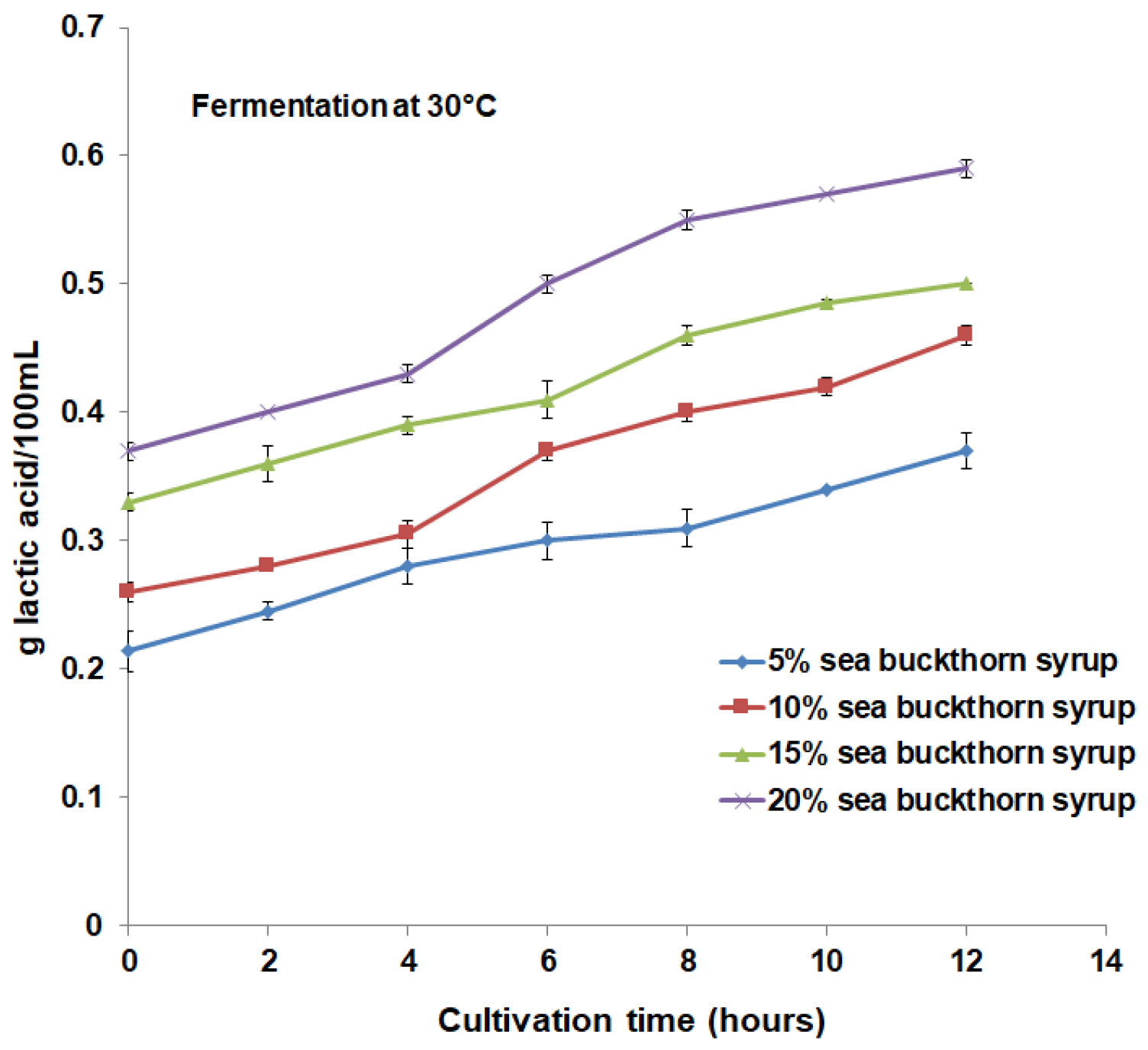
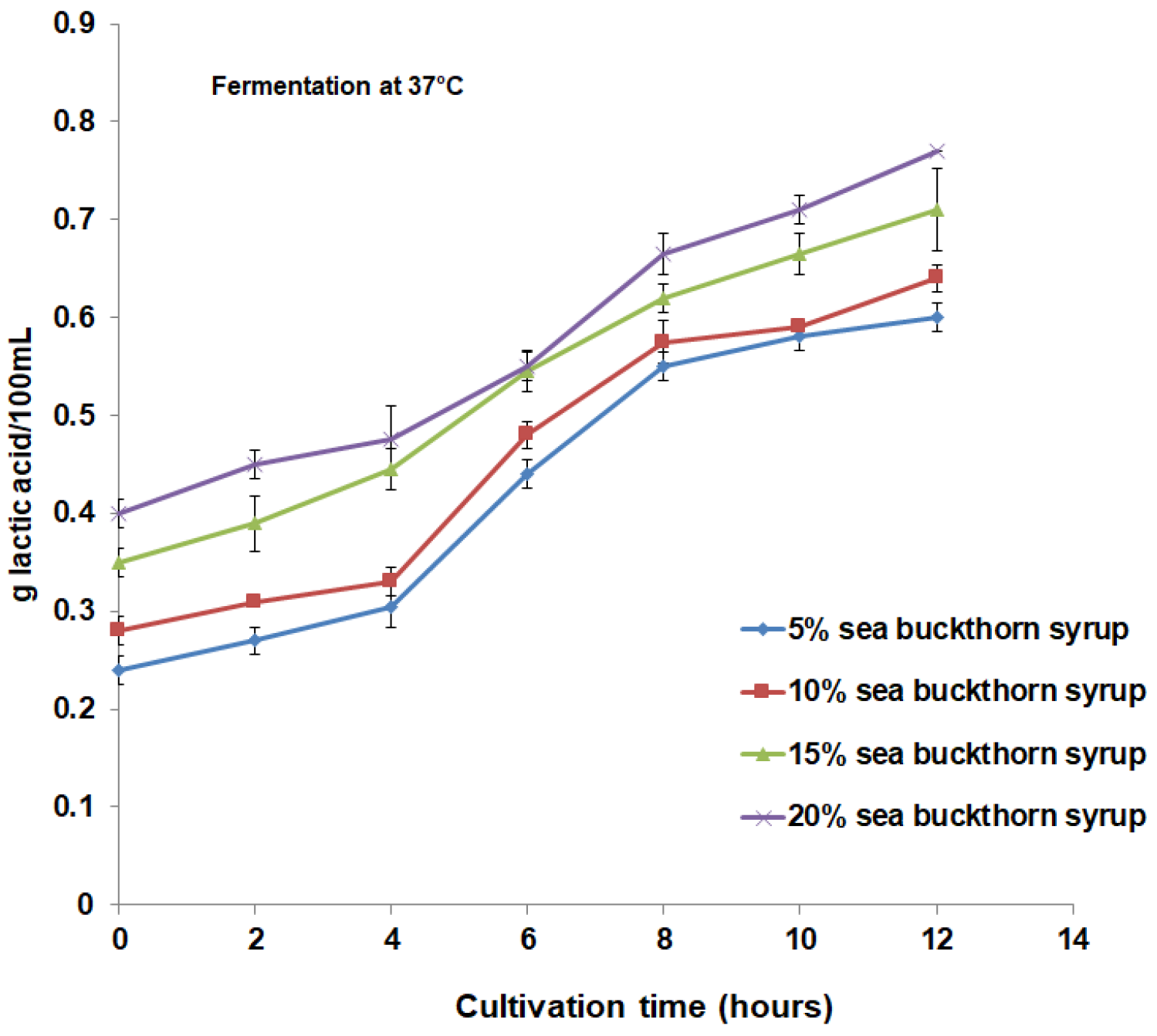
3.1. Microbial Content and Physicochemical Properties Analysis during Fermentation
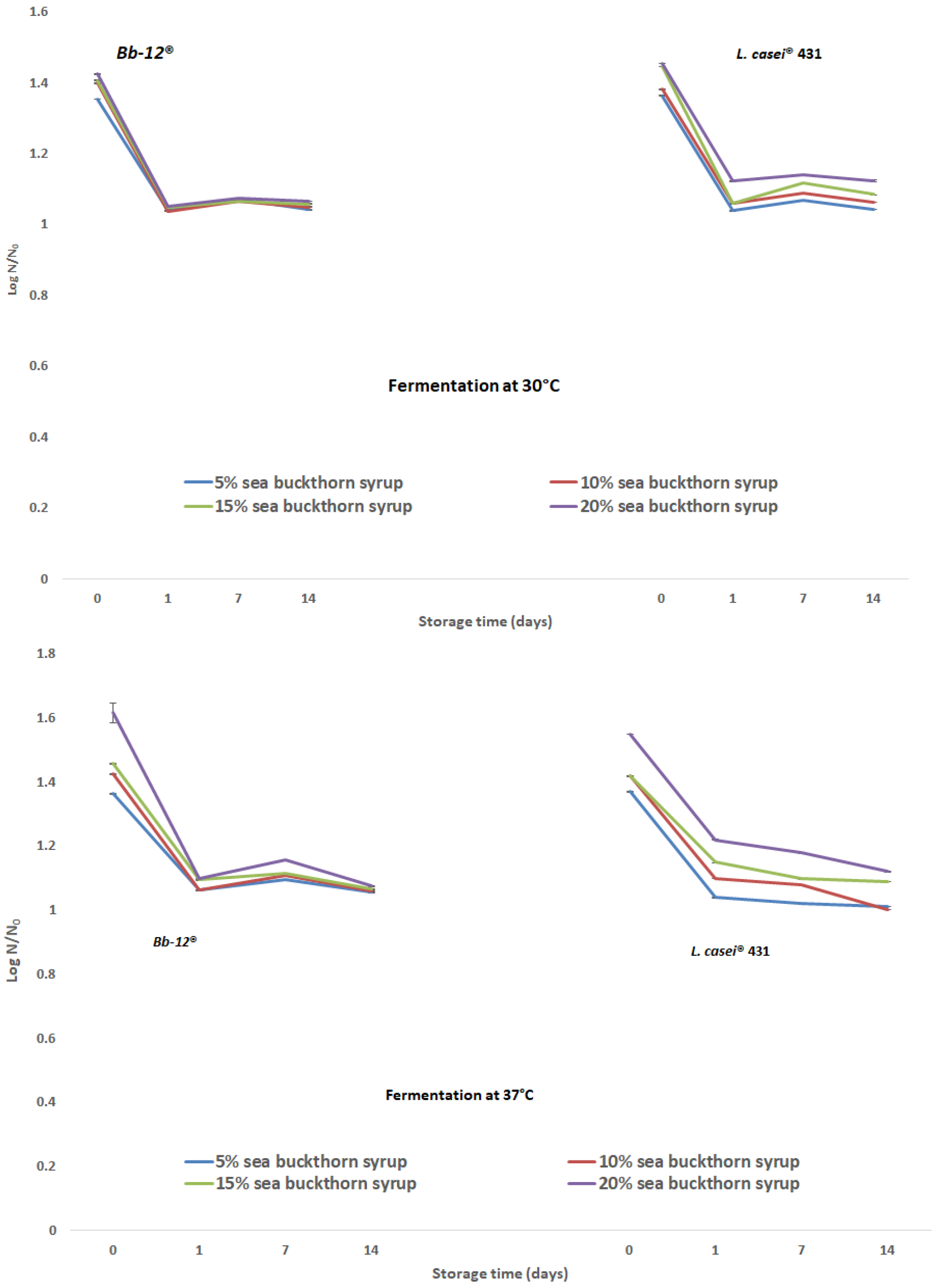
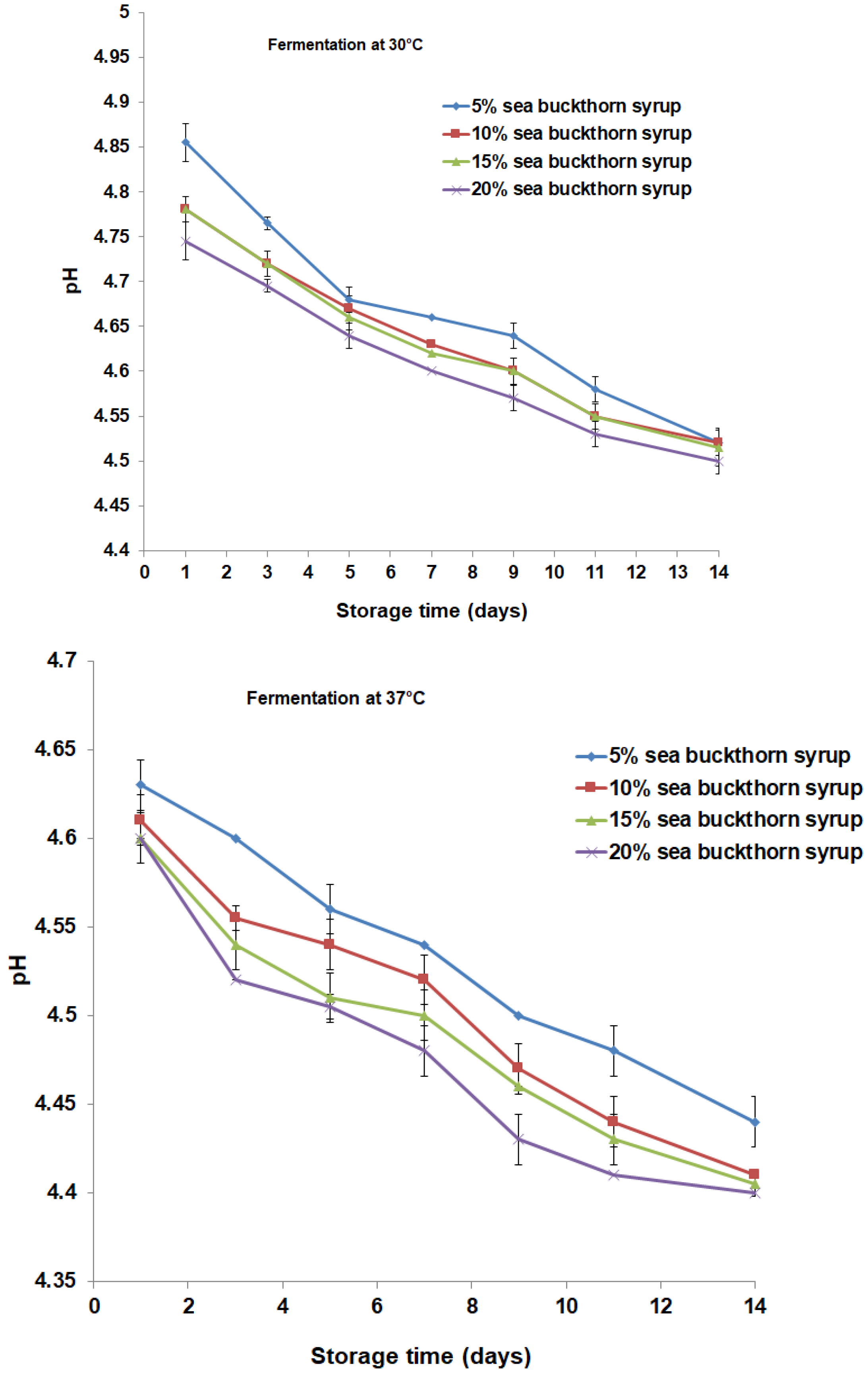
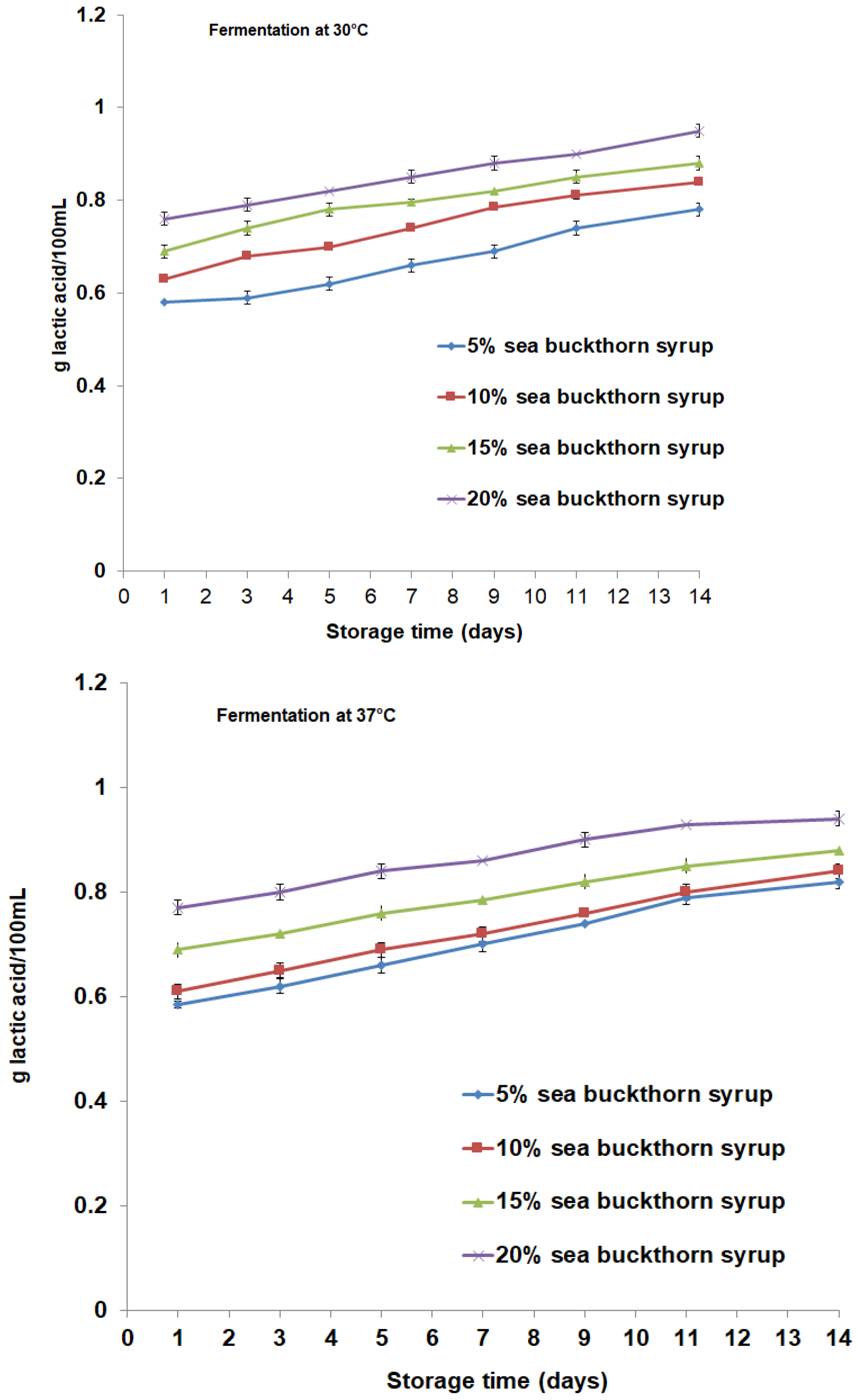
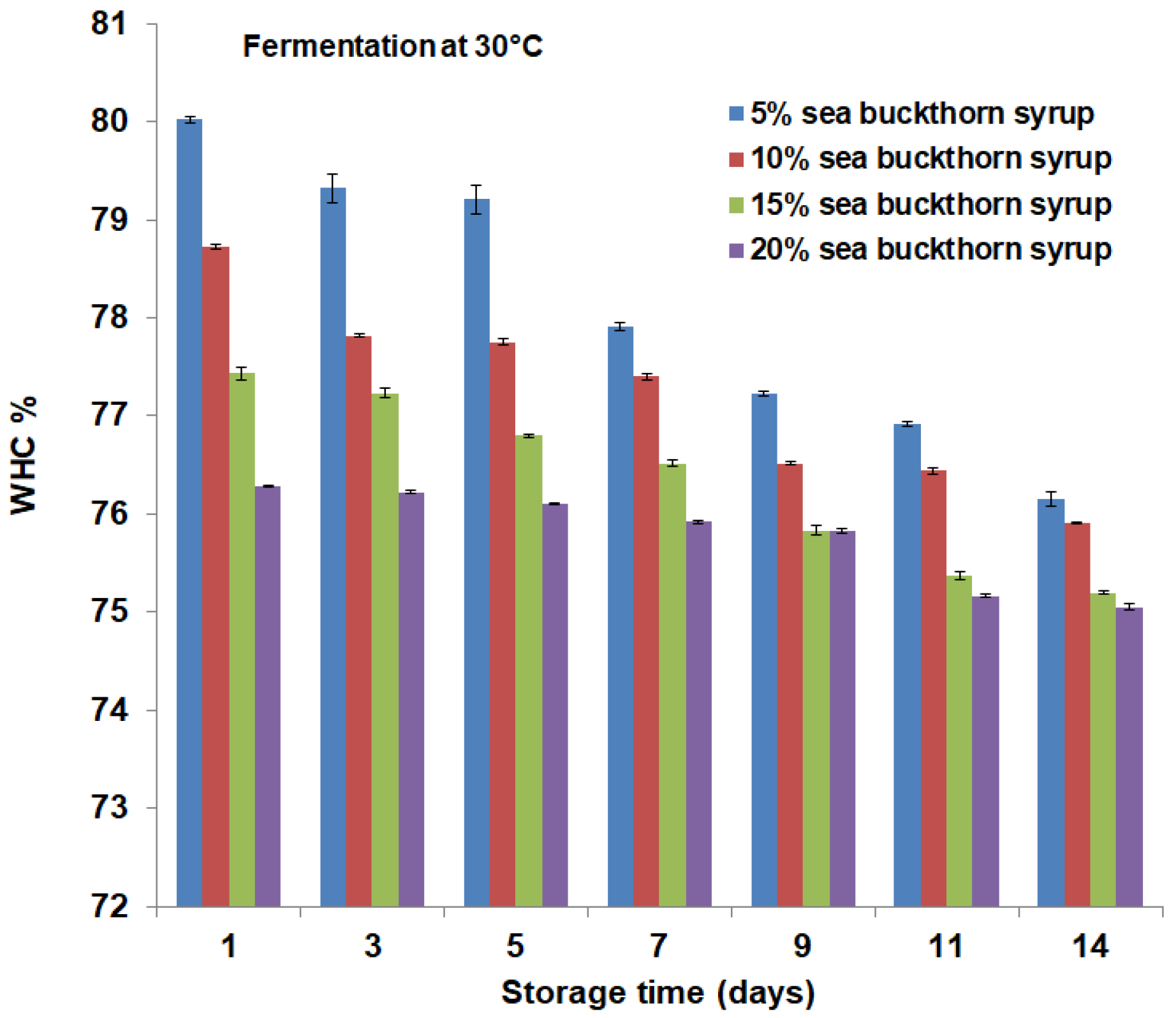
3.2. Microbial Content and Physicochemical Properties Analysis during Storage Period
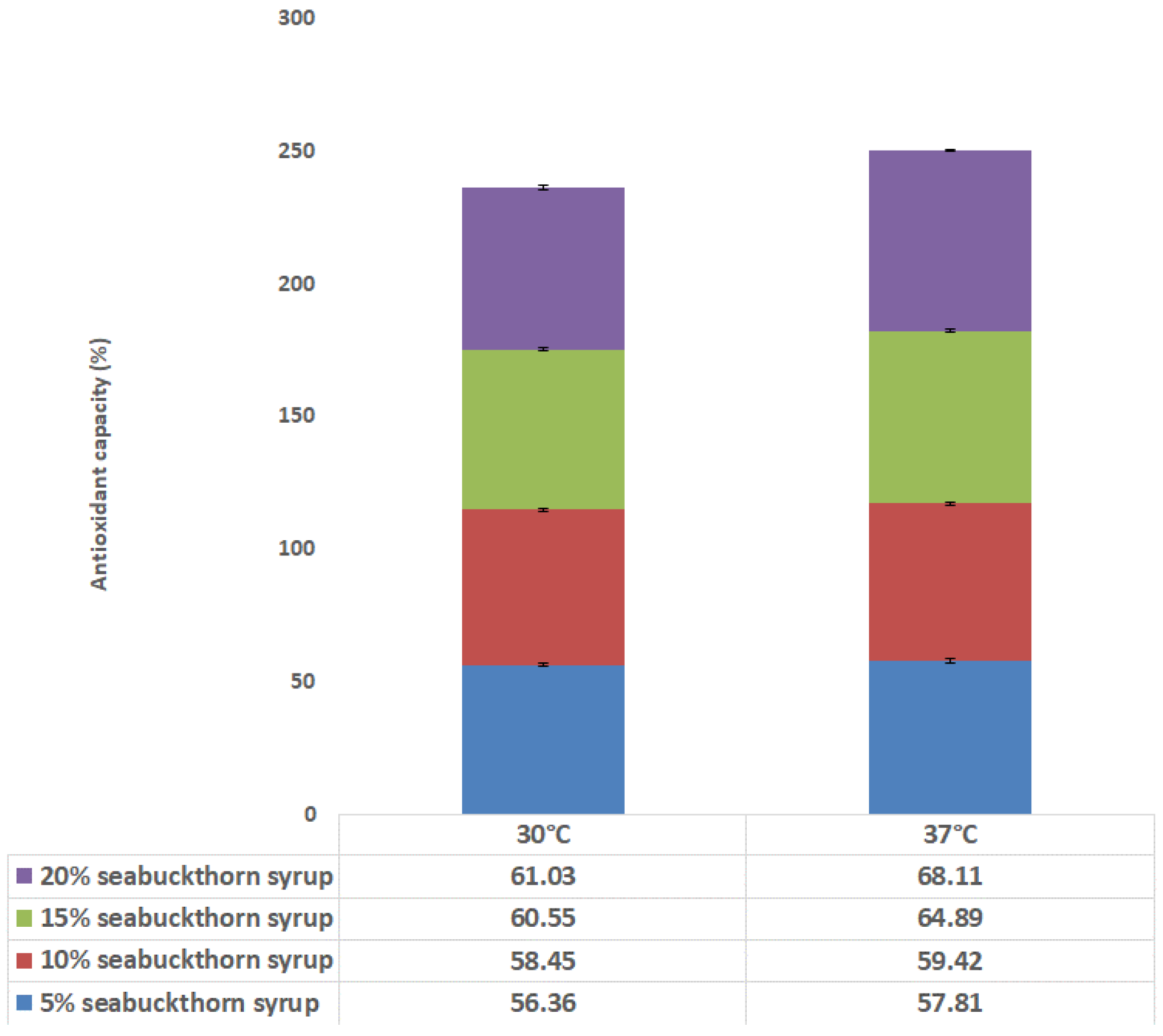
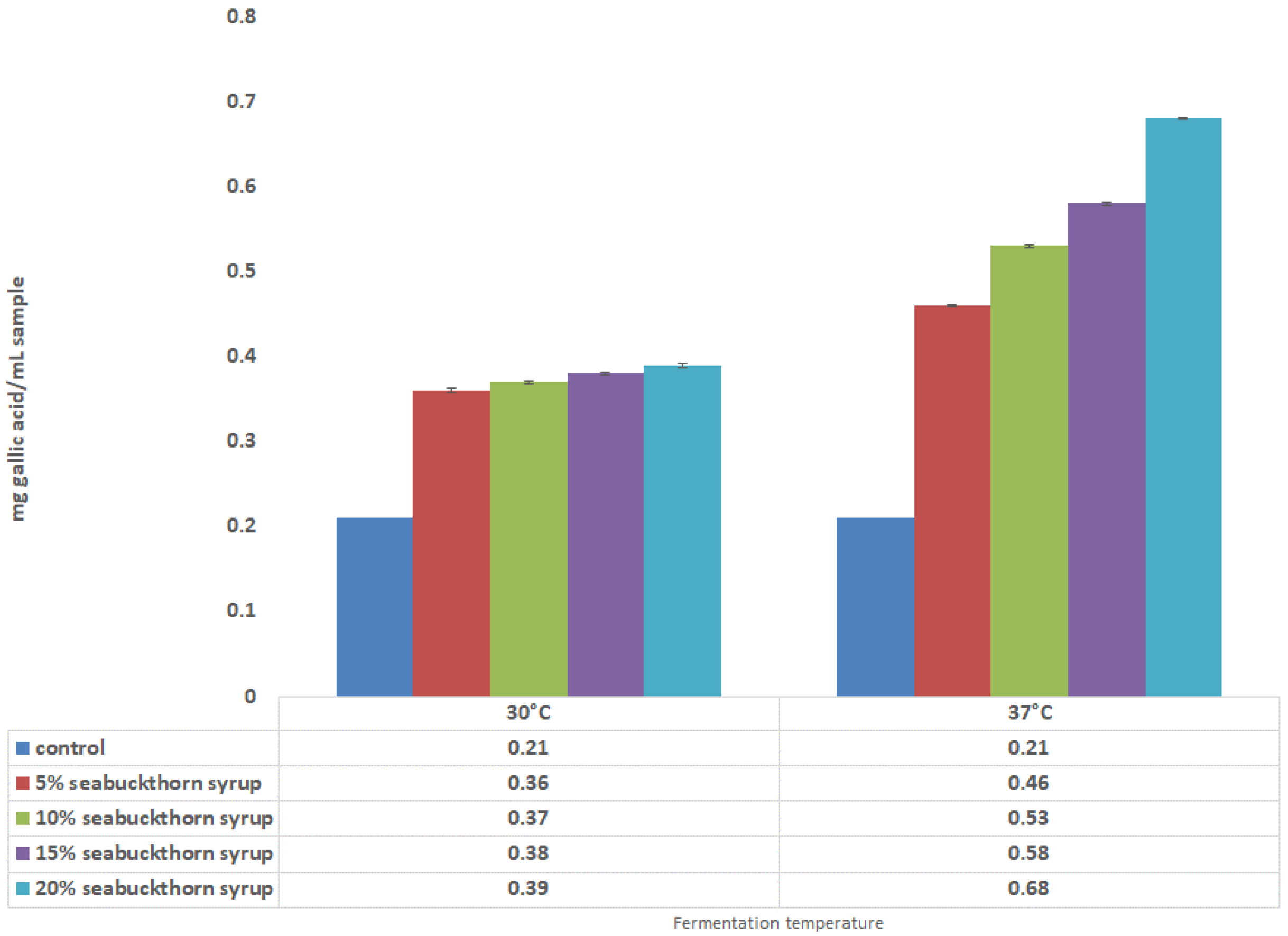
3.3. Antioxidant Properties in Fermented Drinks
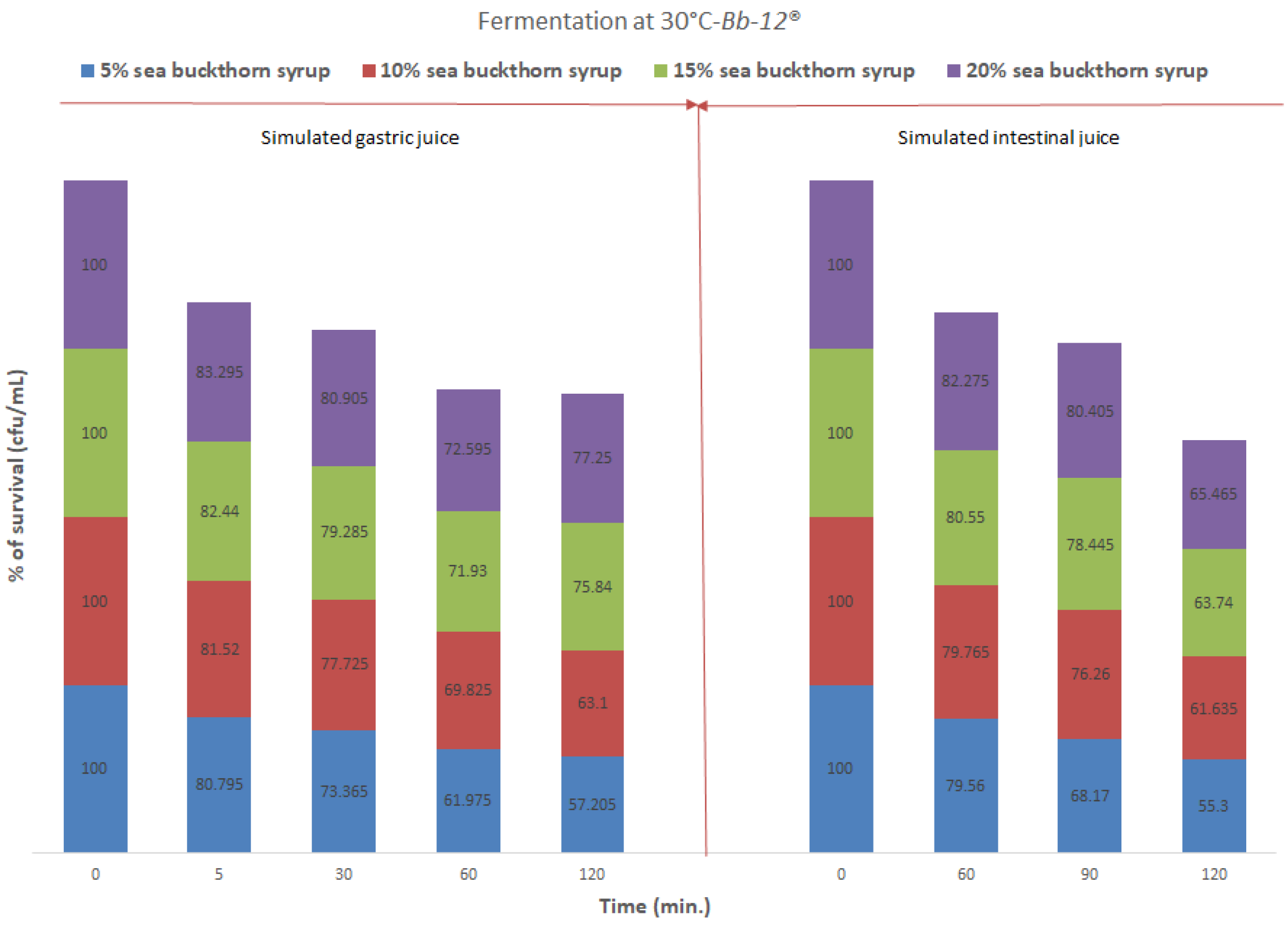
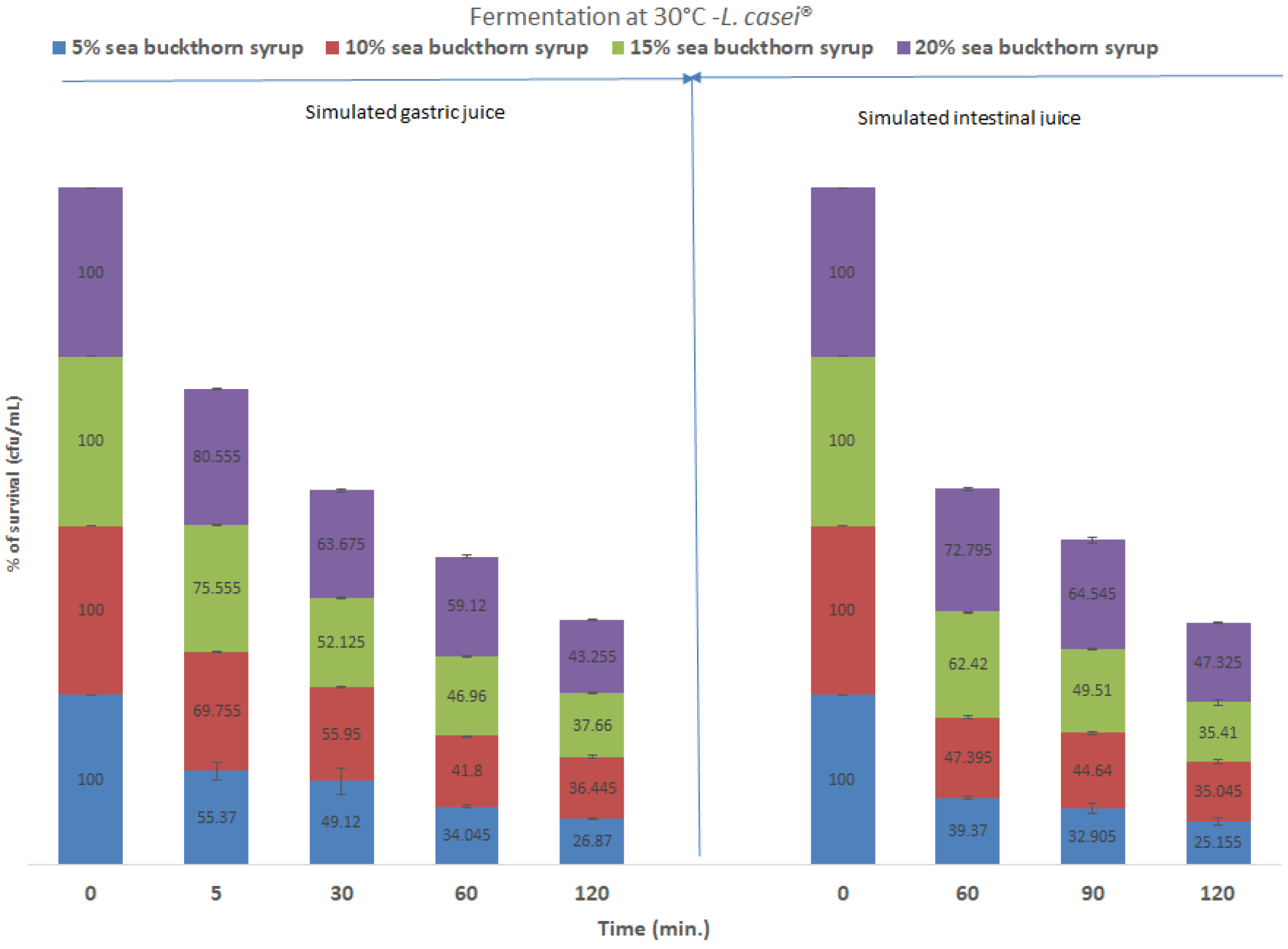
3.4. Gastrointestinal Simulation
3.5. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhang, S.; Sun, Y.; Dai, Y. ACE-inhibitory peptide isolated from fermented soybean meal as functional food. Int. J. Food Eng. 2013, 9, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Thakur, K.; Feng, J.Y.; Cai, J.S.; Zhang, J.G.; Hu, F.; Wei, Z.J. B-vitamin enriched fermented soymilk: A novel strategy for soy-based functional foods development. Trends Food Sci. Technol. 2020, 105, 43–55. [Google Scholar] [CrossRef]
- Functional Foods Market Size, Share & Trends Analysis Report by Ingredient (Carotenoids, Prebiotics & Probiotics, Fatty Acids, Dietary Fibers), by Product, by Application, by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/functional-food-market (accessed on 16 July 2023).
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.; Khaliq, A.; Chughtai, M.F.J.; Nadeem, M.; Din, A.A.; Hlebová, M.; Rebezov, M.; Khayrullin, M.; Mikolaychik, I.; Morozova, L.; et al. Functional exploration of bioactive moieties of fermented and non-fermented soy milk with reference to nutritional attributes. J. Microbiol. Biotechnol. 2020, 10, 145–149. [Google Scholar] [CrossRef]
- Cai, J.S.; Feng, J.Y.; Ni, Z.J.; Ma, R.H.; Thakur, K.; Wang, S.; Hu, F.; Zhang, J.G.; Wei, Z.J. An update on the nutritional, functional, sensory characteristics of soy products, and applications of new processing strategies. Trends Food Sci. Technol. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- He, F.; Chen, J. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: Differences between Chinese women and women in western countries and possible mechanisms. Food Sci. Hum. Wellness. 2013, 2, 146–161. [Google Scholar] [CrossRef]
- Bao, X.; Xiang, S.S.; Chen, J.; Shi, Y.; Chen, Y.G.; Wang, H.H.; Zhu, X. Effect of Lactobacillus reuteri on vitamin B12 content and microbiota composition of furu fermentation. Food Sci. Technol. 2013, 100, 138–143. [Google Scholar] [CrossRef]
- Thakur, K.; Tomar, S.K.; Wei, Z.J. Comparative mRNA expression profiles of riboflavin biosynthesis genes in lactobacilli isolated from human feces and fermented bamboo shoots. Front. Microbiol. 2017, 8, 427. [Google Scholar] [CrossRef]
- Srinivasan, P.; Khan, K.A.; Rajaperumal, U.A.; Kumar, R.V.; Suganya, K.; Srinivasan, M.R. In vitro antibacterial activity of Lactobacillus plantarum isolated from soy milk. Int. J. Pharma Bio. Sci. 2012, 3, 209–219. [Google Scholar]
- Jack, R.W.; Tagg, J.R.; Ray, B. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 1995, 59, 171–200. [Google Scholar] [CrossRef]
- Kinosita, E.; Yamakoshi, J.; Kikuchi, M. Purification and identification of an angiotensin I-converting enzyme inhibitor from soy sauce. Biosc. Biotech. Biochem. 1993, 57, 1107–1110. [Google Scholar] [CrossRef]
- Li, F.J.; Cheng, Y.Q.; Yin, L.J.; Liu, H.J.; Li, L.T. Application of electrolyzed water to improve angiotensin i-converting enzyme inhibitory activities of fermented soybeans started with Bacillus subtilis B1. Int. J. Food Prop. 2011, 14, 145–156. [Google Scholar] [CrossRef]
- Naaber, P.; Mikelsaar, R.H.; Salminen, S.; Mikelsaar, M. Bacterial translocation, intestinal microflora and morphological changes of intestinal mucosa in experimental models of Clostridium difficile infection. J. Med. Microbiol. 1998, 47, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.K.; Hati, S.; Das, S. Bio-nutritional aspects of Tungrymbai, an ethnic functional fermented soy food of Khasi Hills, Meghalaya, India. Clin. Nutr. Exp. 2019, 26, 8–22. [Google Scholar] [CrossRef]
- Pelin, A.M.; Gavat, C.C.; Balan, G.; Georgescu, C.V. Pharmacological Principles Used in Patient Monitoring with Type 2 Diabetes. Rev. Chim. 2017, 68, 378–383. [Google Scholar] [CrossRef]
- Pelin, A.M.; Matasaru, S. Metabolic syndrome in obese children and adolescents. Rev. Med. Chir. Soc. Med. Nat. 2012, 116, 957–961. [Google Scholar]
- Meca, A.D.; Mititelu-Tarțău, L.; Bogdan, M.; Dijmarescu, L.A.; Pelin, A.M.; Foia, L.G. Mycobacterium tuberculosis and Pulmonary Rehabilitation: From Novel Pharmacotherapeutic Approaches to Management of Post-Tuberculosis Sequelae. J. Pers. Med. 2022, 12, 569. [Google Scholar] [CrossRef]
- Dantas, A.; Verruck, S.; Machado Canella, M.H.; Hernandez, E.; Prudencio, E.S. Encapsulated Bifidobacterium BB-12 addition in a concentrated lactose-free yogurt: Its survival during storage and effects on the product’s properties. Food Res. Int. 2021, 150, 110742. [Google Scholar] [CrossRef]
- Wang, A.; Lin, J.; Zhong, Q. Physical and microbiological properties of powdered Lactobacillus salivarius NRRL B-30514 as affected by relative amounts of dairy proteins and lactose. LWT-Food Sci. Technol. 2020, 121, 109044. [Google Scholar] [CrossRef]
- Yang, B.; Kallio, H. Effects of harvesting time on triacylglycerols and glycerophospholipids of sea buckthorn (Hippophaë rhamnoides L.) berries of different origins. J. Food Compos. Anal. 2002, 15, 143–157. [Google Scholar] [CrossRef]
- Olas, B.; Kontek, B.; Malinowska, P.; Żuchowski, J.; Stochmal, A. Hippophae rhamnoides L. fruits reduce the oxidative stress in human blood platelets and plasma. Oxid. Med. Cell. Longev. 2016, 2016, 4692486. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Negi, P.S.; Ramteke, R.S. Antioxidant and antibacterial activities of aqueous extract of seabuckthorn (Hippophae rhamnoides) seeds. Fitoterapia 2007, 78, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R.H. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of (Hippophaë rhamnoides L.) berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. Vitamin E inadequacy in humans: Causes and consequences. Adv. Nutr. 2014, 5, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Ranard, K.M.; Erdman, J.W., Jr. Effects of dietary RRR α-tocopherol vs allracemic α-tocopherol on health outcomes. Nutr. Rev. 2017, 76, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Skalski, B.; Ulanowska, K. The anticancer activity of sea buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Front. Pharmacol. 2018, 9, 232. [Google Scholar] [CrossRef]
- Xu, H.; Hao, Q.; Yuan, F.; Gao, Y. Nonenzymatic browning criteria to sea buckthorn juice during thermal processing. J. Food Process. Eng. 2015, 38, 67–75. [Google Scholar] [CrossRef]
- Hou, D.D.; Di, Z.H.; Qi, R.Q.; Wang, H.X.; Zheng, S.; Hong, Y.; Guo, H.; Chen, H.D.; Gao, X.H. Sea buckthorn (Hippophaë rhamnoides L.) oil improves atopic dermatitis-like skin lesions via inhibition of NF-κB and STAT1 activation. Skin Pharmacol. Physiol. 2017, 30, 268–276. [Google Scholar] [CrossRef]
- Pyo, Y.H.; Song, S.M. Physicochemical and sensory characteristics of a medicinal soy yogurt containing health benefit ingredients. J. Agric. Food Chem. 2009, 57, 170–175. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.M.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 2008, 9, 272–279. [Google Scholar] [CrossRef]
- USP, National Formulary. Available online: www.uspnf.com (accessed on 12 July 2023).
- De Vuyst, L. Technology aspects related to the application of functional starter cultures. Food Technol. Biotechnol. 2000, 38, 105–112. [Google Scholar]
- Nova, E.; Pérez de Heredia, F.; Gómez-Martínez, S.; Marcos, A. The role of probiotics on the microbiota: Effect on obesity. Nutr. Clin. Pract. 2016, 31, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Sajid MaqsooddShori, A.B. The potential applications of probiotics on dairy and non-dairy foods focusing on viability during storage. Biocatal. Agric. Biotechnol. 2015, 4, 423–431. [Google Scholar]
- Tantipaibulvut, S.; Soontornsophan, C.; Luangviphusavanich, S. Fermentation of roselle juice by lactic acid bacteria. Asian J. Food Agro-Ind. 2008, 1, 213–222. [Google Scholar]
- Nionelli, L.; Coda, R.; Curiel, J.A.; Kaisa, P.; Gobetti, M.; Rizzello, C.G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of substrate composition and inoculum on the fermentation kinetics and flavor compound profiles of potentially non-dairy probiotic formulations. LWT-Food Sci. Technol. 2014, 55, 240–247. [Google Scholar] [CrossRef]
- Wang, C.; Liang, S.; Wang, H.; Guo, M. Physicochemical properties and probiotic survivability of symbiotic oat-based beverage. Food Sci. Biotechnol. 2018, 27, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Ludena Urquizo, F.E.; García Torres, S.M.; Tolonen, T.; Jaakkola, M.; Pena-Niebuhr, M.G.; von Wright, A.; Repo-Carrasco-Valencia, R.; Korhonen, H.; Plumed-Ferrer, C. Development of a fermented quinoa-based beverage. Food Sci. Nutr. 2017, 5, 602–608. [Google Scholar] [CrossRef]
- Lin, F.-M.; Chiu, C.-H.; Tzu-Ming, P. Fermentation of a milk–soymilk and Lycium chinense Miller mixture using a new isolate of Lactobacillus paracasei subsp. Paracasei NTU101 and Bifidobacterium longum. J. Ind. Microbiol. Biotechnol. 2004, 31, 559–564. [Google Scholar] [CrossRef]
- Bezerra, M.; Araujo, A.; Santos, K.; Correia, R. Caprine frozen yoghurt produced with fresh and spray dried jambolan fruit pulp (Eugenia jambolana Lam) and Bifidobacterium animalis subsp. lactis BI-07. Food Sci. Technol. 2015, 62, 1099–1104. [Google Scholar] [CrossRef]
- Kandler, O.; Weiss, N. Genus Lactobacillus. In Sneath PHA; Bergey’s manual of systematic, bacteriology; Mair, N.S., Sharpe, M.E., Eds.; William and Wilkins Co.: Baltimore, MD, USA, 1986; Volume 2, pp. 1209–1234. [Google Scholar]
- Gokavi, S.; Zhang, L.; Huang, M.K.; Zhao, X.; Guo, M. Oat-based Symbiotic Beverage Fermented by Lactobacillus plantarum, Lactobacillus paracasei ssp. casei, and Lactobacillus acidophilus. J. Food Sci. 2005, 70, M216–M223. [Google Scholar] [CrossRef]
- He, Z.; Wang, X.; Li, G.; Zhao, Y.; Zhang, J.; Niu, C.; Zhang, L.; Zhang, X.; Ying, D.; Li, S. Antioxidant activity of prebiotic ginseng polysaccharides combined with potential probiotic Lactobacillus plantarum C88. Int. J. Food Sci. Technol. 2015, 50, 1673–1682. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Rinaldoni, A.N.; Campderrós, M.E.; Padilla, A.P. Physico-chemical and sensory properties of yogurt from ultrafiltreted soymilk concentrate added with inulin. LWT-Food Sci. Technol. 2012, 45, 142–147. [Google Scholar] [CrossRef]
- Bianchi, F.; Rossi, E.A.; Gomes, R.G.; Sivieri, K. Potentially symbiotic fermented beverage with aqueous extracts of quinoa (Chenopodium quinoa Willd) and soy. Food Sci. Technol. Int. 2014, 21, 403–415. [Google Scholar] [CrossRef]
- Canaviri Paz, P.; Janny, R.J.; Håkansson, A. Safeguarding of quinoa beverage production by fermentation with Lactobacillus plantarum DSM 9843. Int. J. Food Microbiol. 2020, 324, 108630. [Google Scholar] [CrossRef]
- Isanga, J.; Zhang, G.N. Biologically active components and nutraceuticals in peanuts and related products: Review. Food Rev. Int. 2007, 23, 123–140. [Google Scholar] [CrossRef]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of Potentially Probiotic Beverages using Single and Mixed Cereal Substrates Fermented with Lactic Acid Bacteria Cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef]
- Mondragón-Bernal, O.L.; Lembi Ferreira Alves, J.G.; Teixeira, M.A.; Perina Ferreira, M.F.; Maugeri Filho, F. Stability and functionality of synbiotic soy food during shelf-life. J. Funct. Foods 2017, 35, 134–145. [Google Scholar] [CrossRef]
- Wedad, M. El-K.; Reda, A.A.; Abdel Nabey, A.A. Utilization of inulin extracted from chicory (Cichoriumintybus L.) roots toimprove the properties of low-fat synbiotic yoghurt. Ann. Agric. Sci. 2020, 65, 59–67. [Google Scholar]
- Tasşkın, B.; Bağdatlıoğlu, N. Influence of conventional fermentation on antioxidant activity and phenolic contents of two common dairy products: Yogurt and kefir. TURJFAS 2020, 8, 1277–1282. [Google Scholar]
- Fernandes de Araújo, F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Rincon, L.; Botelho, R.B.A.; de Alencar, E.R. Development of Novel Plant-Based Milk Based on Chickpea and Coconut. LWT-Food Sci. Technol. 2020, 128, 109479. [Google Scholar] [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Isoflavone phytoestrogens in soymilk fermented with β-glucosidase producing probiotic lactic acid bacteria. Int. J. Food Sci. Nutr. 2011, 62, 111–120. [Google Scholar] [CrossRef]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legumes. LWT-Food Sci. Technol. 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Pasqualone, A.; Summo, C.; Laddomada, B.; Mudura, E.; Coldea, T.E. Effect of Processing Variables on the Physico-chemical characteristics and aroma of Borş, a Tradicional Beverage Derived from Wheat Bran. Food Chem. 2018, 265, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Wijeratne, S.S.K.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant polyphenols in almond and its coproducts. J. Agric. Food Chem. 2005, 54, 312–318. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Santillo, A.; Guimarães, J.T.; Capozzi, V.; Russo, P.; Caroprese, M.; Marino, R.; Esmerino, E.A.; Raices, R.S.L.; Silva, M.C.; et al. Novel milk–juice beverage with fermented sheep milk and strawberry (Fragaria × ananassa): Nutritional and functional characterization. J. Dairy Sci. 2019, 102, 10724–10736. [Google Scholar] [CrossRef]
- Zahrani, A.J.A.L.; Shori, A.B. Viability of probiotics and antioxidant activity of soy and almond milk fermented with selected strains of probiotic Lactobacillus spp. LWT-Food Sci. Technol. 2023, 176, 114531. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Antioxidant properties of fermented soy during shelf life. Plant Foods Hum. Nutr. 2019, 74, 287–292. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1–6. J. Funct. Foods. 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Horáčková, Š.; Žaludová, K.; Plocková, M. Stability of selected lactobacilli in the conditions simulating those in the gastrointestinal tract. Czech J. Food Sci. 2011, 29, S30–S35. [Google Scholar] [CrossRef]
- Ricciardi, A.; Guidone, A.; Ianniello, R.G.; Cioffi, S.; Aponte, M.; Pavlidis, D.; Tsakalidou, E.; Zotta, T.; Parente, E. A survey of non-starter lactic acid bacteria in traditional cheeses: Culture dependent identification and survival to simulated gastrointestinal transit. Int. Dairy J. 2015, 43, 42–50. [Google Scholar] [CrossRef]
- Csatlos, N.I.; Simon, E.; Teleky, B.E.; Szabo, K.; Diaconeasa, Z.M.; Vodnar, D.C.; Ciont (Nagy), C.; Pop, O.L. Development of a Fermented Beverage with Chlorella vulgaris Powder on Soybean-Based Fermented Beverage. Biomolecules 2023, 13, 245. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Kociubinski, G.; Perez, P.; De Antoni, G. Isolation and characterization of Bifidobacterium strains for probiotic formulation. J. Food Prot. 1998, 61, 865–873. [Google Scholar] [CrossRef]
- Prasad, J.; Gill, H.; Smart, J.; Gopal, P.K. Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. Int. Dairy J. 1998, 8, 993–1002. [Google Scholar] [CrossRef]
- Dunne, C.; O’Mahony, L.; Murphy, L.; Thornton, G.; Morrissey, D.; O’Halloran, S.; Feeney, M.; Flynn, S.; Fitzgerald, G.; Daly, C.; et al. In vitro selection criteria for probiotic bacteria of human origin: Correlation with in vivo findings. Am. J. Clin. Nutr. 2001, 73, 386S–392S. [Google Scholar] [CrossRef] [PubMed]
- Canzi, E.; Guglielmetti, S.; Mora, D.; Tamagnini, I.; Parini, C. Conditions affecting cell surface properties of human intestinal bifidobacteria. Antonie Leeuwenhoek 2005, 88, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.L.; Vodnar, D.C.; Suharoschi, R.; Mudura, E.; Socaciu, C.L. Plantarum ATCC 8014 Entrapment with Prebiotics and Lucerne Green Juice and Their Behavior in Simulated Gastrointestinal Conditions. J. Food Process. Eng. 2015, 39, 433–441. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Li, F.; Jiao, X.; Ma, D.; Zhang, L.; Yang, B.; Zhao, J.; Han, J.; Li, Q. Effects of lactic acid bacteria fermentation on chemical compounds, antioxidant capacities and hypoglycemic properties of pumpkin juice. Food Biosci. 2022, 50, 102126. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, A.; Tomer, V. Process optimization for the development of a synbiotic beverage based on lactic acid fermentation of nutricereals and milk-based beverage. LWT-Food Sci. Technol. 2020, 131, 109774. [Google Scholar] [CrossRef]
- Xi, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Yin, L.; Wang, Z.; Li, X.; Zhou, J. Kombucha fermentation enhances the health-promoting properties of soymilk beverage. J. Funct. Foods 2019, 62, 103549. [Google Scholar] [CrossRef]




| Characteristics | Beverage Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| Fermented at 30 °C | Fermented at 37 °C | |||||||
| 5% | 10% | 15% | 20% | 5% | 10% | 15% | 20% | |
| Color | 5.8 c | 7.3 a,b | 7.6 a,b | 7.9 a | 5.85 c | 7.3 a,b | 7.6 a,b | 8.9 a,b |
| Flavor | 6.9 b | 7.3 a,b | 7.3 a,b | 7.8 a | 7.1 b | 7.4 a,b | 7.6 a,b | 8.8 a |
| Taste | 6.9 b | 7.4 a | 7.3 a,b | 7.4 a | 6.8 b | 7.3 a,b | 7.3 a,b | 8.6 b |
| Texture | 6.7 b | 7.3 a,b | 7.4 a | 7.5 a | 6.8 b | 7.3 a,b | 7.4 a | 8.5 a |
| Overall acceptability | 6.9 b | 7.2 b | 7.9 a | 8.1 a | 7.1 b | 7.4 b | 76.9 a | 8.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maftei, N.-M.; Bogdan, R.E.G.; Boev, M.; Marin, D.B.; Ramos-Villarroel, A.Y.; Iancu, A.-V. Innovative Fermented Soy Drink with the Sea Buckthorn Syrup and the Probiotics Co-Culture of Lactobacillus Paracasei ssp. Paracasei (L. Casei® 431) and Bifidobacterium Animalis ssp. Lactis (Bb-12®). Fermentation 2023, 9, 806. https://doi.org/10.3390/fermentation9090806
Maftei N-M, Bogdan REG, Boev M, Marin DB, Ramos-Villarroel AY, Iancu A-V. Innovative Fermented Soy Drink with the Sea Buckthorn Syrup and the Probiotics Co-Culture of Lactobacillus Paracasei ssp. Paracasei (L. Casei® 431) and Bifidobacterium Animalis ssp. Lactis (Bb-12®). Fermentation. 2023; 9(9):806. https://doi.org/10.3390/fermentation9090806
Chicago/Turabian StyleMaftei, Nicoleta-Maricica, Roxana Elena Goroftei Bogdan, Monica Boev, Denisa Batîr Marin, Ana Yndira Ramos-Villarroel, and Alina-Viorica Iancu. 2023. "Innovative Fermented Soy Drink with the Sea Buckthorn Syrup and the Probiotics Co-Culture of Lactobacillus Paracasei ssp. Paracasei (L. Casei® 431) and Bifidobacterium Animalis ssp. Lactis (Bb-12®)" Fermentation 9, no. 9: 806. https://doi.org/10.3390/fermentation9090806





