Global Perspectives on the Medicinal Implications of Green Walnut and Its Benefits: A Comprehensive Review
Abstract
:1. Introduction
2. Use of Green Walnut at a Global Perspective
2.1. Asia
2.2. Europe
2.3. America
2.4. Africa and Middle East
3. Literature Review Methodology
4. Nutritional Composition of Green Walnut
5. Pharmacological Properties and Bioactive Compounds
6. Bioactive Components from Different Plant Parts of Juglans regia
6.1. Husk
6.2. Kernel
6.3. Shell
6.4. Leaves
6.5. Shoot
6.6. Bark
6.7. Root
| Parts of Green Walnut | Name of Bioactive Compounds | Medicinal Uses | Experiment-Conducted Country, Doses | References |
|---|---|---|---|---|
| Leaves | Juglone, galactosidase, arabinoside, xyloside, rhamnoside, 1,4-naphthoquinone, juglone phenolic acids, tannins, essential fatty acids, ascorbic acid, flavonoids, caffeic acid, para-coumaric acid, hydrojuglone-β-D-glucopyranoside, hydrojuglone rutinoside, hydrojuglone rhamnoside, hydrojuglone pentoside, hydrojuglone derivative 1 and 2, hydrojuglone derivative pentoside 1 and 2, hydrojuglone hexoside derivative, hydrojuglone dihexoside derivative, 5-hydroxy-2,3-dihydro-1,4-naphthalenedione, dihydroxytetralone hexoside | Anti-diabetic effect, anticancer effect, hepato-protective activity and anti-ageing activity, antioxidant activity, lipid-lowering effect, antihypertensive effect, antimicrobial effects, gastroprotective activity, hypercholesteraemic activity | In the regions of Europe, Asia, Iran and United states, studies were conducted on the identification of various bioactive compounds from the walnut tree leaves and its application in the food and medical fields through several clinical trials. 400 PPM leaf extracts had antioxidant activities. | [59,60,61,62,63,64] |
| Root | Polyphenolic compounds alkaloids, 1,4-naphthoquinone, juglone, α-hydrojuglone, flavonoids, hydrojuglone derivative 1, hydrojuglone derivative 5, hydrojuglone derivative rhamnoside, dihydroxytetralone hexoside | Hair fall, dandruff, skin disorders | In China and India, research was carried out on the extraction of bioactive compounds from the roots of the walnut tree and identified its various applications in the medical field. Addition of tender roots in mustard oil for 2–3 months and its application on hair to stop hair fall and dandruff. | [57,58,63,64,65,66] |
| Husk | Flavonoid, phenolic acids, vitamin C, 1,4-naphthoquinone, juglone, tannins, citric acid, malic acid, juglone, polyphenols, α-hydrojuglone, hydrojuglone-β-D-glucopyranoside, hydrojuglone rutinoside, hydrojuglone rhamnoside, hydrojuglone pentoside, hydrojuglone derivative 5, hydrojuglone derivative pentoside 1,2 and 3, hydrojuglone derivative rhamnoside, hydrojuglone hexoside derivative, bisjuglone, juglanin b, p-hydroxymetoxybenzobijuglone, regiolone, 5-hydroxy-2,3-dihydro-1,4-naphthalenedione, 4,5,8-trihydroxynaphthalene-5-D-glucopyranoside, 1,4,8-trihydroxynaphthalene-1-D-glucopyranoside, dihydroxytetralone hexoside, dihydroxytetralone galloyl hexoside, trihydroxytetralone derivative | Liver- and kidney-protective, antioxidant, anti-inflammatory, and anticancer properties and improves immune function | Experiments on the determination of bioactive compounds from green walnut husks were carried out in Hungary and the United States. Clinical trials were conducted to evaluate the application of husks in various medical conditions. 1.25 to 5 mg/mL of husk extract presented antimicrobial activity. | [43,63,64,67,68] |
| Shell | Pyroligneous acids, juglone, tannins, phenolic compounds, dihydroxytetralone hexoside | Antioxidant, anticancer, and anti-inflammatory properties | In Serbia, an experiment was carried out on the determination of bioactive compounds and production of green walnut liquor. | [63,64,69,70,71,72] |
| Bark | Flavonoids, polyphenols, 1,4-naphthoquinone, juglone, α-hydrojuglone, hydrojuglone-β-D-glucopyranoside, hydrojuglone rutinoside, hydrojuglone rhamnoside, hydrojuglone pentoside, hydrojuglone derivative 1, 2, 3, 4, 5, and 6, hydrojuglone derivative pentoside 1, 2, and 3, hydrojuglone derivative rhamnoside, dihydroxytetralone hexoside, procyanidin dimer 2 (+)catechin, ellagic acid derivative | Prevents tooth ache and tooth decay, has antimicrobial activity, antioxidant activity, antifungal activity, platelet aggregation, and is antiseptic | Study regarding extraction of bioactive compounds from the walnut tree bark and its assessment conducted in Pakistan. Dried bark twigs used as toothbrush. | [55,63,64,65,73,74,75] |
| Kernel | Ellagitannin, pedunculagin, hydrojuglone-β-D-glucopyranoside | Hypolipidemic effect, antioxidant activity, antibacterial properties, antiproliferative, chemo-preventive properties | In Hungary and parts of Africa, experiments were conducted on the exploration of the therapeutic potential and molecular mechanisms of the bioactive compounds present in green walnut kernels. With several dosages, clinical studies were carried out for medicinal and food purposes. 5 g of fruit kernel and its consumption in 500 mL of boiled milk was found to be a remedy for constipation and as a memory booster. | [24,44,46,63,64,76] |


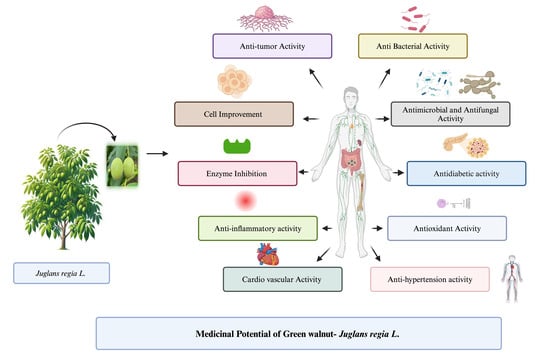
7. Health Benefits
8. Memory Cell Improvement
9. Antimicrobial, Antifungal Activity, and Antibacterial Activity
10. Antitumor Activity
11. Cell Improvement
12. Anti-Diabetic Activity
13. Enzyme Inhibition
14. Antioxidant Activity
15. Anti-Inflammatory Activity
16. Cardiovascular Activity
17. Antihypertension Activity
18. Traditional vs. Modern Medicine
19. Walnut Application in the Pharmaceutical Industry
20. Walnut Application in Food Industry as a Nutraceutical
21. Contemporary Research
22. Regulatory Approvals and Adverse Health Effects
23. Challenges and Future Directions
24. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WGH | Walnut green husk |
| WSP | Walnut septum |
| WMBP | Walnut meal bioactive peptide |
| WM | Walnut meal |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
References
- Sharma, M.; Sharma, M.; Sharma, M. A comprehensive review on ethnobotanical, medicinal and nutritional potential of walnut (Juglans regia L.). Proc. Indian Natl. Sci. Acad. Part A Phys. Sci. 2022, 88, 601–616. [Google Scholar] [CrossRef]
- Verma, G.; Sharma, V. A Scientific Update on Juglans Regia Linn. Asian J. Pharm. Res. Dev. 2020, 8, 166–175. [Google Scholar] [CrossRef]
- Ghasemi, M.; Arzani, K.; Hassani, D. Evaluation and identification of walnut (Juglans regia L.) genotypes in Markazi province of Iran. Crop Breed. J. 2012, 2, 119–124. [Google Scholar]
- Wu, S.; Ni, Z.; Wang, R.; Zhao, B.; Han, Y.; Zheng, Y.; Liu, F.; Gong, Y.; Tang, F.; Liu, Y. The effects of cultivar and climate zone on phytochemical components of walnut (Juglans regia L.). Food Energy Secur. 2020, 9, e196. [Google Scholar] [CrossRef]
- de Rigo, D.; Enescu, C.M.; Durrant, T.H.; Tinner, W.; Caudullo, G. Juglans regia in Europe: Distribution, Habitat, Usage and Threats; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Habibi, A.; Hamedpour-Darabi, M.; Vahdati, K. Local cultural values of Persian walnut in Iran. In The Cultural Value of Trees; Routledge: London, UK, 2022; pp. 162–173. [Google Scholar]
- Hajipuor, S.; Alinia-Ahandani, E.; Selamoglu, Z. A Closer Look at Some Medical Use of Green Persian Walnut Shell. Eurasian J. Med. Biol. Sci. 2022, 17. [Google Scholar]
- Zhang, R.; Liu, B.; Xin, G.; Zhang, X.; Li, J.; Wang, Y. Evaluation of Cold Tolerance of Seven Walnut Varieties. Cryoletters 2022, 43, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Aryapak, S.; Ziarati, P. Nutritive value of Persian walnut (Juglans regia L.) Orchards. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 1228–1235. [Google Scholar]
- Gandev, S. Budding and grafting of the walnut (Juglans regia L.) and their effectiveness in Bulgaria. Bulg. J. Agric. Sci. 2007, 13, 683. [Google Scholar]
- Milind, P.; Deepa, K. Walnut: Not a hard nut to crack. Int. Res. J. Pharm. 2011, 2, 8–17. [Google Scholar]
- Şen, S.M.; Karadeniz, T. The nutritional value of walnut. J. Hyg. Eng. Des. 2015, 11, 68–71. [Google Scholar]
- Pan, Z.; Zhang, R.; Zicari, S. Integrated Processing Technologies for Food and Agricultural by-Products; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Berkey, C.S.; Tamimi, R.M.; Willett, W.C.; Rosner, B.; Hickey, M.; Toriola, A.T.; Frazier, A.L.; Colditz, G.A. Adolescent alcohol, nuts, and fiber: Combined effects on benign breast disease risk in young women. NPJ Breast Cancer 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Jang, D.S.; Oh, M.S. Juglans mandshurica leaf extract protects skin fibroblasts from damage by regulating the oxidative defense system. Biochem. Biophys. Res. Commun. 2012, 421, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Saling, S.C.; Comar, J.F.; Mito, M.S.; Peralta, R.M.; Bracht, A. Actions of juglone on energy metabolism in the rat liver. Toxicol. Appl. Pharmacol. 2011, 257, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Stampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M. Traditional walnut liqueur—Cocktail of phenolics. Food Chem. 2006, 95, 627–631. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G.; Jankowska, A. Antioxidant properties, profile of polyphenolic compounds and tocopherol content in various walnut (Juglans regia L.) varieties. Eur. Food Res. Technol. 2019, 245, 607–616. [Google Scholar] [CrossRef]
- Chudhary, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Hamrouni, L. Walnut. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 671–684. [Google Scholar]
- Zhao, P.; Zhou, H.-J.; Potter, D.; Hu, Y.-H.; Feng, X.-J.; Dang, M.; Feng, L.; Zulfiqar, S.; Liu, W.-Z.; Zhao, G.-F.; et al. Population genetics, phylogenomics and hybrid speciation of Juglans in China determined from whole chloroplast genomes, transcriptomes, and genotyping-by-sequencing (GBS). Mol. Phylogenetics Evol. 2018, 126, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Kithi, L.; Lengyel-Kónya, É.; Berki, M.; Bujdosó, G. Role of the green husks of persian walnut (Juglans regia L.)—A Review. Horticulturae 2023, 9, 782. [Google Scholar] [CrossRef]
- Birkle, C.; Pendlebury, D.A.; Schnell, J.; Adams, J. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020, 1, 363–376. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Sabharanjak, S.M.; Zengin, G.; Mollica, A.; Szostak, A.; Simirgiotis, M.; Huminiecki, Ł.; Horbanczuk, O.K.; Nabavi, S.M.; Mocan, A. Pecan nuts: A review of reported bioactivities and health effects. Trends Food Sci. Technol. 2018, 71, 246–257. [Google Scholar] [CrossRef]
- Feldman, E.B. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J. Nutr. 2002, 132, 1062S–1101S. [Google Scholar] [CrossRef] [PubMed]
- Savage, G.P. Chemical composition of walnuts (Juglans regia L.) grown in New Zealand. Plant Foods Hum. Nutr. 2001, 56, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, S.; Cappelloni, M.; Gambelli, L.; Nicoli, S.; Carnovale, E. Chemical composition and nutritive value of nuts grown in Italy. Ital. J. Food Sci. 1998, 10, 243–252. [Google Scholar]
- Lavedrine, F.; Zmirou, D.; Ravel, A.; Balducci, F.; Alary, J. Blood cholesterol and walnut consumption: A cross-sectional survey in France. Prev. Med. 1999, 28, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P.P. Determination of sterol and fatty acid compo- sitions, oxidative stability, and nutritional value of six wal- nut (Juglans regia L.) cultivars grown in Portugal. J. Agric. Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Sabate, J.; Fraser, G.E.; Burke, K.; Knutsen, S.F.; Bennett, H.; Lindsted, K.D. Effects of walnuts on serum lipid levels and blood pressure in normal men. N. Engl. J. Med. 1993, 328, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Chen, C.-Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Qadir, R.; Abbas, A. Cold Pressed Walnut (Juglans regia L.) Oil, in Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 491–495. [Google Scholar]
- Ozcan, M.M. Some nutritional characteristics of fruit and oil of walnut (Juglans regia L.) growing in Turkey. Iranian J. Chem. Chem. Eng. Int. Engl. Ed. 2009, 28, 57–62. [Google Scholar]
- Siahnouri, Z.; Sadeghian, M.; Salehisormghi, M.; Qomi, M. Determination of Iranian walnut and pistachio mineral contents. J. Basic Appl. Sci. Res. 2013, 3, 217–220. [Google Scholar]
- Alaviani, S.; Mahmoudyar, F.; Miraftabi, S.F.; Salehisormghi, M.H.; Qomi, M. Determination of Iranian Almond, Peanut and Hazelnut Mineral Contents. J. Basic Appl. Chem. 2012, 2, 50–54. [Google Scholar]
- Özkutlu, F.; Doğru, Y.Z.; Özenç, N.; Yazici, G.; Turan, M.; Akçay, F. The importance of Turkish hazelnut trace and heavy metal contents for human nutrition. J. Soil Sci. Environ. Manag. 2011, 2, 25–33. [Google Scholar]
- Li, W.; Li, D.-Y.; Wang, H.-D.; Zheng, Z.-J.; Hu, J.; Li, Z.-Z. Juglans regia hexane extract exerts antitumor effect, apoptosis induction and cell circle arrest in prostate cancer cells in vitro. Trop. J. Pharm. Res. 2015, 14, 399–405. [Google Scholar] [CrossRef]
- Noshirvani, N.; Fasihi, H.; Moradipayam, A. Study on the Antioxidant Effects of Extract and Powder of Green Walnut Hulls on the Oxidation of Sunflower Oil. Iran. J. Nutr. Sci. Food Technol. 2015, 10, 79–90. [Google Scholar]
- Salejda, A.M.; Janiewicz, U.; Korzeniowska, M.; Kolniak-Ostek, J.; Krasnowska, G. Effect of walnut green husk addition on some quality properties of cooked sausages. LWT-Food Sci. Technol. 2016, 65, 751–757. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, A.; Tahmasbi, M. Anti-platelet aggregation activity of walnut hull extract via suppression of ROS generation and caspase activation. J. Acupunct. Meridian Stud. 2017, 10, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentao, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Greve, L.C.; McGranahan, G.; Hasey, J.; Snyder, R.; Kelly, K.; Goldhamer, D.; Labavitch, J.M. Variation in polyunsaturated fatty acids composition of Persian walnut. J. Am. Soc. Hortic. Sci. 1992, 117, 518–522. [Google Scholar] [CrossRef]
- Elmadfa, I.; Kornsteiner, M. Dietary fat intake–A global perspective. Ann. Nutr. Metab. 2009, 54 (Suppl. S1), 8–14. [Google Scholar] [CrossRef]
- Moghaddam, P.Z.; Mohammadi, A.; Feyzi, P.; Alesheikh, P. In vitro antioxidant and antibacterial activity of various extracts from exocarps and endocarps of walnut. Pak. J. Pharm. Sci. 2017, 30, 1725–1731. [Google Scholar] [PubMed]
- Jahanban-Esfahlan, A.; Amarowicz, R. Walnut (Juglans regia L.) shell pyroligneous acid: Chemical constituents and functional applications. RSC Adv. 2018, 8, 22376–22391. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Shi, G.; Wang, Y.; Mao, G.; Wang, D.; Wang, Z. Chemical compositions and biological activities of pyroligneous acids from walnut shell. BioResources 2015, 10, 1715–1729. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Optimization of ultrasound-assisted hydroalcoholic extraction of phenolic compounds from walnut leaves using response surface methodology. Pharm. Biol. 2016, 54, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Qa’Dan, F.; Thewaini, A.-J.; Ali, D.A.; Afifi, R.; Elkhawad, A.; Matalka, K.Z. The antimicrobial activities of Psidium guajava and Juglans regia leaf extracts to acne-developing organisms. Am. J. Chin. Med. 2005, 33, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Solar, A.; Colarič, M.; Usenik, V.; Stampar, F. Seasonal variations of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.). Plant Sci. 2006, 170, 453–461. [Google Scholar] [CrossRef]
- Cheniany, M.; Ebrahimzadeh, H.; Vahdati, K.; Preece, J.E.; Masoudinejad, A.; Mirmasoumi, M. Content of different groups of phenolic compounds in microshoots of Juglans regia cultivars and studies on antioxidant activity. Acta Physiol. Plant. 2013, 35, 443–450. [Google Scholar] [CrossRef]
- Taha, N.A.; Al-wadaan, M.A. Utility and importance of walnut, Juglans regia Linn: A. Afr. J. Microbiol. Res. 2011, 5, 5796–5805. [Google Scholar]
- Thakur, A. Juglone: A therapeutic phytochemical from Juglans regia L. J. Med. Plants Res. 2011, 5, 5324–5330. [Google Scholar]
- Bhatia, K.; Rahman, S.; Ali, M.; Raisuddin, S. In vitro antioxidant activity of Juglans regia L. bark extract and its protective effect on cyclophosphamide-induced urotoxicity in mice. Redox Rep. 2006, 11, 273–279. [Google Scholar] [CrossRef]
- NirmlaDevi, T.; Apraj, V.; Bhagwat, A.; Mallya, R.; Sawant, L.; Pandita, N. Pharmacognostic and phytochemical investigation of Juglans regia Linn. bark. Pharmacogn. J. 2011, 3, 39–43. [Google Scholar] [CrossRef]
- Raja, V.; Ahmad, S.I.; Irshad, M.; Wani, W.A.; Siddiqi, W.A.; Shreaz, S. Anticandidal activity of ethanolic root extract of Juglans regia (L.): Effect on growth, cell morphology, and key virulence factors. J. De Mycol. Médicale 2017, 27, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Allaie, A.H.; Singh, R.; Ganaie, A.A.; Mishra, R.P.; Ali, S. Phytochemically analyzed Juglans regia root extracts and their bactericidal facets against some urinary tract infection causing bacteria. Int. J. Biol. Med. Res. 2018, 9, 6304–6308. [Google Scholar]
- Rahimipanah, M.; Hamedi, M.; Mirzapour, M. Antioxidant activity and phenolic contents of Persian walnut (Juglans regia L.) green husk extract. Afr. J. Food Sci. Technol 2010, 1, 105–111. [Google Scholar]
- Al-Snai, A. Iraqi medicinal plants with antifungal effect—A review. IOSR J. Pharm. 2019, 9, 16–56. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Thul, S.T. Phytochemical analysis of the leaf volatile oil of walnut tree (Juglans regia L.) from western Himalaya. Ind. Crops Prod. 2013, 42, 195–201. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Trabelsi, N.; Hajlaoui, H.; Ksouri, R.; Valentin, E.; Bakhrouf, A. Antibacterial, anticandidal and antioxidant activities of Salvadora persica and Juglans regia L. extracts. J. Med. Plants Res. 2011, 5, 4138–4146. [Google Scholar]
- Medic, A.; Hudina, M.; Jakopic, J.; Veberic, R. Is juglone really the only allelochemical in J. regia affecting plant growth and seed germination? In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Quality Seeds and Transplants for Horticultural 1365, Angers, France, 14–20 August 2022. [Google Scholar]
- Medic, A.; Jakopic, J.; Solar, A.; Hudina, M.; Veberic, R. Walnut (J. regia) agro-residues as a rich source of phenolic compounds. Biology 2021, 10, 535. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.N.; Grace, B.L.; Shafi, G.; Al-Hazzani, A.A.; Alshatwi, A.A. Anti-proliferative effects of organic extracts from root bark of Juglans regia L. (RBJR) on MDA-MB-231 human breast cancer cells: Role of Bcl-2/Bax, caspases and Tp53. Asian Pac. J. Cancer Prev. 2011, 12, 525–530. [Google Scholar]
- Gupta, A.; Behl, T.; Panichayupakaranan, P. Review of phytochem- istry and pharmacology profile of Juglans regia. Obes. Med. 2019, 16, 100142. [Google Scholar] [CrossRef]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut Polyphenolics Inhibit In Vitro Human Plasma and LDL Oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P.; Kabiri, N.; Asgary, S.; Setorki, M. Anti-diabetic effects of walnut oil on alloxan-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2655–2661. [Google Scholar]
- Kazemipour, M.; Ansari, M.; Tajrobehkar, S.; Majdzadeh, M.; Kermani, H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008, 150, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sial, T.A.; Shaheen, S.M.; Lan, Z.; Korai, P.K.; Ghani, M.I.; Khan, M.N.; Syed, A.-u.-A.; Ali, M.N.A.; Rajpar, I.; Memon, M.; et al. Addition of walnut shells biochar to alkaline arable soil caused contradictory effects on CO2 and N2O emissions, nutrients availability, and enzymes activity. Chemosphere 2022, 293, 133476. [Google Scholar] [CrossRef] [PubMed]
- Owens, N.; Lee, D. The use of micro bubble flotation technology in secondary and tertiary produced water treatment—A technical comparison with other separation technologies. In Proceedings of the TUV NEL, 5th Produced Water Workshop, Aberdeen, Scotland, 30–31 May 2007. [Google Scholar]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, J.; Saadipour, K.; Delaviz, H.; Mohammadi, B. Anti-diabetic effects of an alcoholic extract of Juglans regia in an animal model. Turk. J. Med. Sci. 2011, 41, 685–691. [Google Scholar] [CrossRef]
- Ibrar, M.; Hussain, F.; Sultan, A. Ethnobotanical studies on plant resources of Ranyal Hills, District Shangla, Pakistan. Pak. J. Bot. 2007, 39, 329–337. [Google Scholar]
- Zakavi, F.; Hagh, L.G.; Sheikh, A.F.; Daraeighadikolaei, A.; Shooshtari, Z.L. Antibacterial effect of Juglans regia bark against oral pathologic bacteria. Int. J. Dent. 2013, 2013, 854765. [Google Scholar] [CrossRef] [PubMed]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Antioxidant activity and phenolic content of fresh and dry nuts with or without the seed coat. J. Food Compos. Anal. 2009, 22, 184–188. [Google Scholar] [CrossRef]
- Blomhoff, R.; Carlsen, M.H.; Andersen, L.F.; Jacobs, D.R. Health benefits of nuts: Potential role of antioxidants. Br. J. Nutr. 2006, 96, S52–S60. [Google Scholar] [CrossRef] [PubMed]
- Tsasi, G.; Milošević-Ifantis, T.; Skaltsa, H. Phytochemical study of Juglans regia L. pericarps from Greece with a chemotaxonomic approach. Chem. Biodivers. 2016, 13, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, D.; Farías, D.; Oyanedel, E.; Castro, M.; Lampinen, B.; Tixier, A.; Saa, S. The morphology of a Walnut (Juglans regia L.) shoot is affected by its position in the canopy and correlated to the number and size of its fruits. Sci. Hortic. 2017, 220, 303–309. [Google Scholar] [CrossRef]
- Tapsell, L.; Batterham, M.; Tan, S.-Y.; Warensjö, E. The effect of a calorie controlled diet containing walnuts on substrate oxidation during 8-hours in a room calorimeter. J. Am. Coll. Nutr. 2009, 28, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-G.; Zhang, Q.-L.; Liu, X.-L.; Wu, H.; Zheng, J.-L.; Xiang, Y.-B. Dietary protein intake and risk of type 2 diabetes: A dose–response meta-analysis of prospective studies. Eur. J. Nutr. 2019, 58, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Manson, J.E.; Stampfer, M.J.; Liu, S.; Willett, W.C.; Hu, F.B. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002, 288, 2554–2560. [Google Scholar] [CrossRef]
- Prokisch, J.; El-Ramady, H.; Daróczi, L.; Nagy, É.; Badgar, K.; Kiss, A.; Shaikh, A.M.; Gilányi, I.; Oláh, C. Functional Yogurt Fortified with Honey Produced by Feeding Bees Natural Plant Extracts for Controlling Human Blood Sugar Level. Plants 2022, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; An, J.M.; Han, Y.M.; Surh, Y.J.; Hwang, S.J.; Kim, S.J.; Hahm, K.B. Walnut polyphenol extracts inhibit Helicobacter pylori-induced STAT3Tyr705 phosphorylation through activation of PPAR-γ and SOCS1 induction. J. Clin. Biochem. Nutr. 2020, 67, 248–256. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Korkmaz, A.; Fuentes-Broto, L.; Hardman, W.E.; Rosales-Corral, S.A.; Qi, W. A walnut-enriched diet reduces the growth of LNCaP human prostate cancer xenografts in nude mice. Cancer Investig. 2013, 31, 365–373. [Google Scholar] [CrossRef]
- Figueroa, F.; Marhuenda, J.; Zafrilla, P.; Villaño, D.; Martínez-Cachá, A.; Tejada, L.; Cerdá, B.; Mulero, J. High-performance liquid chromatography-diode array detector determination and availability of phenolic compounds in 10 genotypes of walnuts. Int. J. Food Prop. 2017, 20, 1074–1084. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, F.; Zhang, Y.S.; Thakur, K.; Zhang, J.-G.; Liu, Y.; Kan, H.; Wei, Z.-J. Mechanism of juglone-induced cell cycle arrest and apoptosis in Ishikawa human endometrial cancer cells. J. Agric. Food Chem. 2019, 67, 7378–7389. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Zhang, Y.; Seeram, N.P.; Heber, D.; Chen, S. Pomegranate ellagitannin–derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev. Res. 2010, 3, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-G.; Kan, H.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Comparison of phenolic compounds extracted from Diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020, 321, 126672. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Khan, M.R.; Shabbir, M.A. In vitro antioxidant activity and punicalagin content quantification of pomegranate peel obtained as agro-waste after juice extraction. Pak. J. Agric. Sci. 2018, 55, 197–201. [Google Scholar]
- Pandareesh, M.D.; Chauhan, V.; Chauhan, A. Walnut supplementation in the diet reduces oxidative damage and improves antioxidant status in transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 64, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Bober, J.R.; Beisel, C.L.; Nair, N.U. Synthetic biology approaches to engineer probiotics and members of the human microbiota for biomedical applications. Annu. Rev. Biomed. Eng. 2018, 20, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology 2018, 155, 33–37.e6. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Chagas, P.; Chiva-Blanch, G. Diet and cardiovascular disease: Effects of foods and nutrients in classical and emerging cardiovascular risk factors. Curr. Med. Chem. 2019, 26, 3639–3651. [Google Scholar] [CrossRef]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef] [PubMed]
- Nergiz-Ünal, R.; Kuijpers, M.J.; de Witt, S.M.; Heeneman, S.; Feijge, M.A.; Caraballo, S.C.G.; Biessen, E.A.; Haenen, G.R.; Cosemans, J.M.; Heemskerk, J.W. Atheroprotective effect of dietary walnut intake in ApoE-deficient mice: Involvement of lipids and coagulation factors. Thromb. Res. 2013, 131, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Comstock, S.S.; Gershwin, L.J.; Teuber, S.S. Effect of walnut (Juglans regia) polyphenolic compounds on ovalbumin-specific IgE induction in female BALB/c mice. Ann. N. Y. Acad. Sci. 2010, 1190, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, A.; Okamura, T.; Sugiyama, D.; Higashiyama, A.; Watanabe, M.; Okuda, N.; Kadota, A.; Miyagawa, N.; Fujiyoshi, A.; Yoshita, K.; et al. Vegetable protein intake was inversely associated with cardiovascular mortality in a 15-year follow-up study of the general Japanese population. J. Atheroscler. Thromb. 2019, 26, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Wang, Y.; Zhang, X.; Sun, X. Recent studies on protective effects of walnuts against neuroinflammation. Nutrients 2022, 14, 4360. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhong, D.; Liu, H.; Yang, T.; Liang, Q.; Wang, J.; Zhang, R.; Zhang, Y. Water soluble dietary fiber from walnut meal as a prebiotic in preventing metabolic syndrome. J. Funct. Foods 2021, 78, 104358. [Google Scholar] [CrossRef]
- Wang, J.; Du, K.; Fang, L.; Liu, C.; Min, W.; Liu, J. Evaluation of the antidiabetic activity of hydrolyzed peptides derived fromJuglans mandshuricaMaxim. fruits in insulin-resistant HepG2 cells and type 2 diabetic mice. J. Food Biochem. 2018, 42, e12518. [Google Scholar] [CrossRef]
- Feng, L.; Wang, X.; Peng, F.; Liao, J.; Nai, Y.; Lei, H.; Li, M.; Xu, H. Walnut protein hydrolysates play a protective role on neurotoxicity induced by d-galactose and aluminum chloride in mice. Molecules 2018, 23, 2308. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, A.; Ficai, D.; Craciun, L.; Ficai, A.; Andronescu, E. Evaluation and exploitation of bioactive compounds of walnut, Juglans Regia. Curr. Pharm. Des. 2019, 25, 119–131. [Google Scholar] [CrossRef]
- Chaleshtori, R.S.; Chaleshtori, F.S.; Rafieian, M. Biological characterization of Iranian walnut (Juglans regia) leaves. Turk. J. Biol. 2011, 35, 635–639. [Google Scholar] [CrossRef]
- Moori Bakhtiari, N.; Khalafi, E. Antibacterial Activity of the Hydro-Alcoholic Extract of Juglans regia L. Stem Bark on Human Bacterial Infection. Int. Arch. Health Sci. 2015, 2, 139–143. [Google Scholar]
- Barekat, S.; Nasirpour, A.; Keramat, J.; Dinari, M.; Meziane-Kaci, M.; Paris, C.; Desobry, S. Phytochemical composition, antimicrobial, anticancer properties, and antioxidant potential of green husk from several walnut varieties (Juglans regia L.). Antioxidants 2022, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Soto-Madrid, D.; Gutiérrez-Cutiño, M.; Pozo-Martínez, J.; Zúñiga-López, M.C.; Olea-Azar, C.; Matiacevich, S. Dependence of the ripeness stage on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts from industrial by-products. Molecules 2021, 26, 2878. [Google Scholar] [CrossRef] [PubMed]
- Abedi, P.; Yaralizadeh, M.; Fatahinia, M.; Namjoyan, F.; Nezamivand-Chegini, S.; Yaralizadeh, M. Comparison of the effects of Juglans nigra green husk and clotrimazole on Candida albicans in rats. Jundishapur J. Microbiol. 2018, 11, e58151. [Google Scholar] [CrossRef]
- Yabalak, E.; Eliuz, E.A.E. Green synthesis of walnut shell hydrochar, its antimicrobial activity and mechanism on some pathogens as a natural sanitizer. Food Chem. 2022, 366, 130608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Zou, C.L.; Duan, Y.X.; Wu, F.; Li, G. Activity guided isolation and modification of juglone from Juglans regia as potent cytotoxic agent against lung cancer cell lines. BMC Complement. Altern. Med. 2015, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Hasan, T.N.; Shafi, G.; Syed, N.A.; Al-Assaf, A.H.; Alamri, M.S.; Al-Khalifa, A.S. Validation of the antiproliferative effects of organic extracts from the green husk of Juglans regia L. on PC-3 human prostate cancer cells by assessment of apoptosis-related genes. Evid. Based Complement. Altern. Med. 2012, 2012, 103026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhao, C.; Sui, H.; Li, C.F.; Zhong, L.; Zhou, Q.; Bai, Y.; An, S.; Du, X.; et al. Green walnut husk extracts Proliferation and Migration in Gastric Cancer. J. Cancer 2022, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, Z.; Shameli, K.; Hara, H.; Taib, S.H.M. Cytotoxicity assay of biosynthesis gold nanoparticles mediated by walnut (Juglans regia) green husk extract. J. Mol. Struct. 2018, 1151, 97–105. [Google Scholar] [CrossRef]
- Dzidziguri, D.; Rukhadze, M.; Modebadze, I.; Bakuradze, E.; Kurtanidze, M.; Giqoshvili, V. The study of the immune corrective properties of greek walnut (Juglans regia L.) septa on the experimental model of leukopenia. Georgian Med. News 2016, 252, 84–89. [Google Scholar]
- Raja, G.; Shaker, I.A.; Sailaja, I.; Swaminathan, R.; Babu, K.S.; Basha, S.S. Nutritional analysis of nuts extract of Juglans regia L. Int. J. Bioassays 2012, 1, 68–73. [Google Scholar] [CrossRef]
- Ramishvili, L.; Gordeziani, M.; Tavdishvili, E.; Bedineishvili, N.; Dzidziguri, D.; Kotrikadze, N. The effect of extract of Greek walnut (Juglans regia L.) septa on some functional characteristics of erythrocytes. Georgian Med. News 2016, 261, 51–57. [Google Scholar]
- Moravej, H.; Salehi, A.; Razavi, Z.; Moein, M.R.; Etemadfard, H.; Karami, F.; Ghahremani, F. Chemical composition and the effect of walnut hydrosol on glycemic control of patients with type 1 diabetes. Int. J. Endocrinol. Metab. 2016, 14, e34726. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oyeleye, S.I.; Ogunsuyi, O.B.; Oboh, G. Phenolic analysis and erectogenic function of African Walnut (Tetracarpidium conophorum) seeds: The impact of the seed shell on biological activity. J. Food Biochem. 2019, 43, e12815. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Fernie, A.R.; Alseekh, S.; Ezzat, S.M. Potential valorization of edible nuts by-products: Exploring the immune-modulatory and antioxidants effects of selected nut shells extracts in relation to their metabolic profiles. Antioxidants 2022, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.; Trandafir, I. Anti-oxidant activities and total phenolics contents of leaf extracts from 14 cultivars of walnut (Juglans regia L.). J. Hortic. Sci. Biotechnol. 2012, 87, 504–508. [Google Scholar] [CrossRef]
- Carey, A.N.; Fisher, D.R.; Joseph, J.A.; Shukitt-Hale, B. The ability of walnut extract and fatty acids to protect against the deleterious effects of oxidative stress and inflammation in hippocampal cells. Nutr. Neurosci. 2013, 16, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Zarei, H.; Taghiabadi, E. Antinociceptive, anti-inflammatory and acute toxicity effects of Juglans regia L. leaves in mice. Iran. Red Crescent Med. J. 2011, 13, 27. [Google Scholar] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Shukla, P.; Chopada, K.; Sakure, A.; Hati, S. Current Trends and Applications of Food-derived Antihypertensive Peptides for the Management of Cardiovascular Disease. Protein Pept. Lett. 2022, 29, 408–428. [Google Scholar] [CrossRef]
- Wang, F.J.; Yin, X.Y.; Regenstein, J.M.; Wang, J.Z. Separation and purification of angiotensin-I-converting enzyme (ACE) inhibitory peptides from walnuts (Juglans regia L.) meal. Eur. Food Res. Technol. 2016, 242, 911–918. [Google Scholar] [CrossRef]
- Mahwasane, S.; Middleton, L.; Boaduo, N. An ethnobotanical survey of indigenous knowledge on medicinal plants used by the traditional healers of the Lwamondo area, Limpopo province, South Africa. S. Afr. J. Bot. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Bakkalbașı, E.; Yılmaz, Ö.; Artık, N. Physical properties and chemical composition of some walnut cultivars grown in Turkey. Akad. Gida 2010, 8, 6–12. [Google Scholar]
- Wu, L.; Piotrowski, K.; Rau, T.; Waldmann, E.; Broedl, U.C.; Demmelmair, H.; Koletzko, B.; Stark, R.G.; Nagel, J.M.; Mantzoros, C.S.; et al. Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: A randomized controlled cross-over clinical trial. Metabolism 2014, 63, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Zibaeenezhad, M.J.; Shamsnia, S.J.; Khorasani, M. Walnut Consum. Hyperlipidemic Patients. Angiology 2005, 56, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M. Chemical, mineral, and fatty acid compositions of various types of walnut (Juglans regia L.) in Turkey. Bulg. Chem. Commun. 2016, 48, 66–70. [Google Scholar]
- Serrano, A.; Cofrades, S.; Ruiz-Capillas, C.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Jiménez-Colmenero, F. Nutritional Profile of Restructured Beef Steak With Added Walnuts. Meat Sci. 2005, 70, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Nouri, M.; Baghi, M. The effect of adding walnut green husk extract on antioxidant and antimicrobial properties of ketchup. J. Food Bioprocess Eng. 2019, 2, 93–100. [Google Scholar]
- Fulin, Y.A.N.; Tiantian, Y.I.N. Chemical constituents and antitumor activities of walnut green husk. Med. Plant 2019, 10, 17. [Google Scholar]
- Ros, E.; Izquierdo-Pulido, M.; Sala-Vila, A. Beneficial effects of walnut consumption on human health: Role of micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Tehrani-Bagha, A.R.; Gharanjig, K.; Hashemi, E. Use of pomegranate peels and walnut green husks as the green antimicrobial agents to reduce the consumption of inorganic nanoparticles on wool yarns. J. Clean. Prod. 2019, 231, 1463–1473. [Google Scholar] [CrossRef]
- Food and Drug Administration. Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption: What You Need to Know about the FDA Regulation: Guidance for Industry Small Entity Com-Pliance Guide; Food and Drug Administration, Ed.; FDA: Silver Spring, MA, USA, 2017. [Google Scholar]




| Nutritional Information | Content (per 100 g) | Reference |
|---|---|---|
| Protein (g) | 14.60 | [24,25] |
| Carbohydrates (g) | 10.30 | |
| Fat (g) | 64.5 g/100 g | |
| Total MUFA (g) * | 15.28 g/100 g lipid | |
| Total PUFA (g) * | 72.96 g/100 g lipid | |
| Linoleic acid (C18:2n6) (g) | 59.79/100 g lipid | |
| Alpha-linolenic acid (C18:3n3) (g) | 13.17/100 g lipid | |
| Vitamins A | 0.036 | |
| Vitamins B1 | 0.26 | |
| Vitamins B2 | 0.15 | |
| Niacin | 1.13 | |
| Vitamins C | 1.3 | |
| Vitamin B6 | 0.54 | |
| Vitamins E | 43.21 | |
| K (mg) | 441.00 | |
| P (mg) | 346.00 | |
| Mn (mg) | 3.80 | |
| Zn (mg) | 3.09 | |
| Cu (mg) | 1.50 | |
| Mg (mg) | 158.00 | |
| Ca (mg) | 98.00 | |
| Se (mg) | 4.62 | |
| Ai (mg) | 0.58 | |
| Na (mg) | 2.00 | |
| Fe (mg) | 2.90 | |
| Dietary fibre content | 9.79 g/100 g | [26] |
| Bioactivities on Human Health | Bioactive Compounds | Description | References |
|---|---|---|---|
| Blood sugar level control | Dietary fats Walnut kernels with polyphenolic extracts | Helps to improve the health level of patients with type 2 diabetes and reduces insulin levels. Also helps to decrease blood glucose levels. Reduces the total cholesterol and triglycerides. | [82,83,84,85] |
| Anticancer | Juglone Walnut polyphenol extracts | Triggers cell cycle arrest and programmed cell death (apoptosis) in human endometrial cancer cells. Suppresses the growth of Caco-2, MCF-7, and other cell types. Decreases the rate of prostate tumour growth by 30% to 40%. Decreases the likelihood of developing breast cancer; slows down the rate at which tumours grow. | [86,87,88,89] |
| Antioxidation | Ellagitannin-derived Walnut polyphenol extracts | Controls the activity of the enzyme. Contains the antioxidant activity of the scavenging ability for DPPH and ABTS+. Contains chemical substances that decrease the number of electrons in a reaction, substances that remove unstable molecules with unpaired electrons, and substances that supply hydrogen atoms. Enhances blood lipid and endothelial function. | [90,91,92,93] |
| Gut health | Walnut polyphenol extracts containing omega-3 and omega-6 fatty acids | Controls the gut microbiome. Immunomodulation refers to the process of modifying or regulating the immune system. Enhances the composition of gut bacteria and promotes the production of secondary bile acids; decreases the concentration of LDL cholesterol. | [94,95] |
| Cardiovascular activity | Ellagic acid Ellagitannin Walnut kernels | Facilitates the initiation of neurodegenerative disorders. Reduces the likelihood of developing cardiovascular diseases and improves blood lipid levels. The enhancement of the defense mechanism of HDL cholesterol and antioxidants, together with the reduction in total cholesterol and LDL, effectively averts the occurrence of cardiovascular illnesses and alleviates the irritation. | [96,97,98,99,100,101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukarram, S.A.; Wandhekar, S.S.; Ahmed, A.E.M.; Várallyay, S.; Pandey, V.K.; József, P.; Bela, K. Global Perspectives on the Medicinal Implications of Green Walnut and Its Benefits: A Comprehensive Review. Horticulturae 2024, 10, 433. https://doi.org/10.3390/horticulturae10050433
Mukarram SA, Wandhekar SS, Ahmed AEM, Várallyay S, Pandey VK, József P, Bela K. Global Perspectives on the Medicinal Implications of Green Walnut and Its Benefits: A Comprehensive Review. Horticulturae. 2024; 10(5):433. https://doi.org/10.3390/horticulturae10050433
Chicago/Turabian StyleMukarram, Shaikh Ayaz, Sangram S. Wandhekar, Abdelhakam Esmaeil Mohamed Ahmed, Szilvia Várallyay, Vinay Kumar Pandey, Prokisch József, and Kovács Bela. 2024. "Global Perspectives on the Medicinal Implications of Green Walnut and Its Benefits: A Comprehensive Review" Horticulturae 10, no. 5: 433. https://doi.org/10.3390/horticulturae10050433