Aerated Irrigation and Pruning Residue Biochar on N2O Emission, Yield and Ion Uptake of Komatsuna
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Soil Properties and Soil Temperature Changes
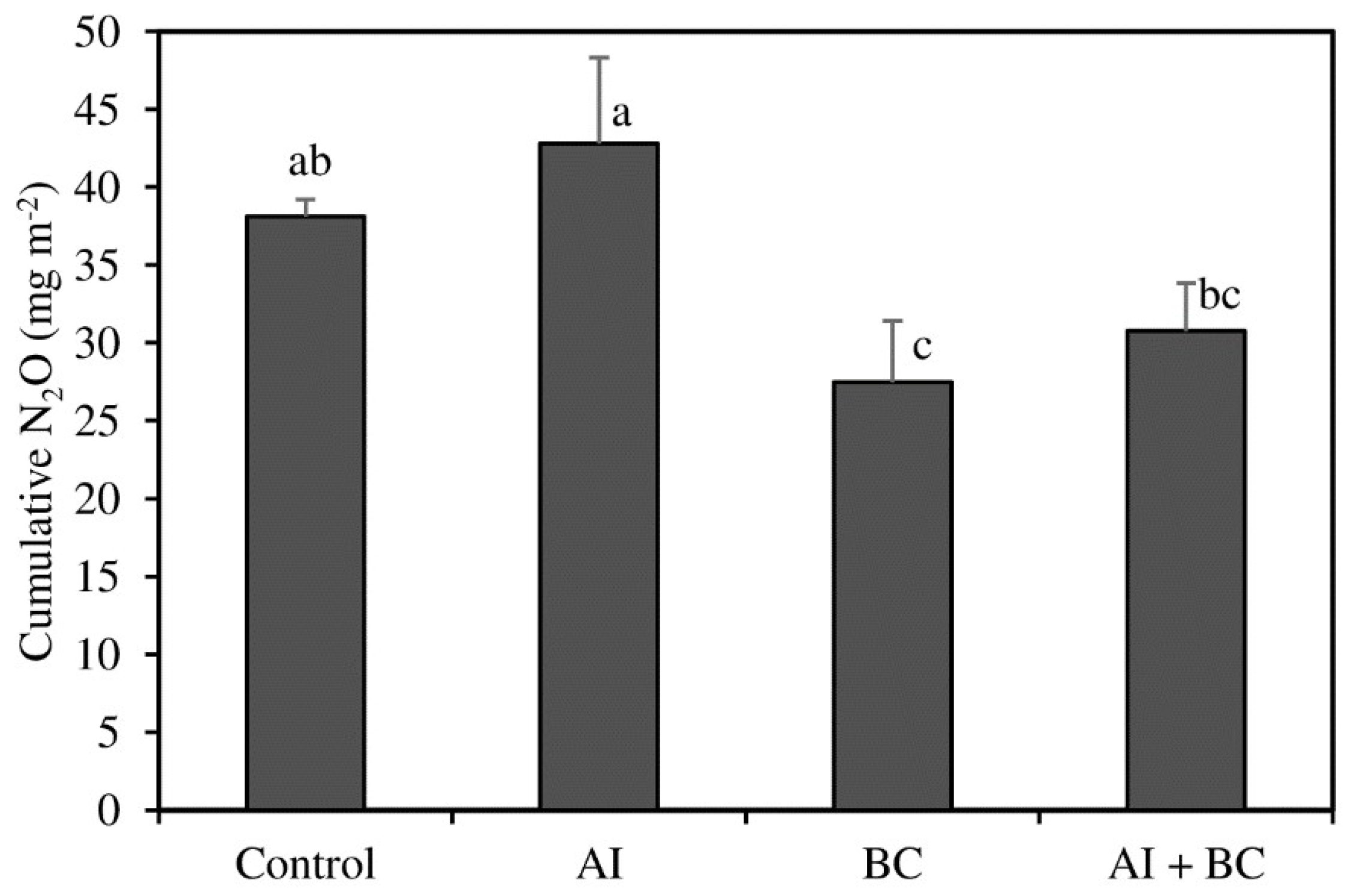
3.2. N2O Emissions
3.3. Growth and Yield of Komatsuna
3.4. Ion Concentration and Uptake of Komatsuna
4. Discussion
4.1. Aerated Irrigation and Biochar on Yield and Ion Uptake of Komatsuna
4.2. Aerated Irrigation and Biochar on Soil N2O Emissions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Smith, K.A.; Crutzen, P.J.; Mosier, A.; Winiwarter, W. The global N2O budget: A reassessment. In Nitrous Oxide and Climate Change; Smith, K., Ed.; Earthscan: London, UK, 2010; ISBN 9781844077571. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD). Environmental indicators for agriculture. In Methods and Results; Publications Service, OECD: Paris, France, 2001; Volume 3, pp. 281–283. [Google Scholar]
- Hou, H.; Chen, H.; Cai, Hi.; Yang, F.; Li, D.; Wang, F. CO2 and N2O emissions from Lou soils of greenhouse tomato fields under aerated irrigation. Atmos. Environ. 2016, 132, 69–76. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, C.H.; Li, Q.L.; Li, B.; Zhu, Y.Y.; Xiong, Z.Q. A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agric. Ecosyst. Environ. 2015, 201, 43–50. [Google Scholar] [CrossRef]
- Huang, S.; Pant, H.K.; Lu, J. Effects of water regimes on nitrous oxide emission from soils. Ecol. Eng. 2007, 131, 9–15. [Google Scholar] [CrossRef]
- Scheer, C.; Grace, P.R.; Rowlings, D.W.; Payero, J. Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management. Nutr. Cycl. Agroecosyst. 2013, 95, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Abuara, M.; Mostafa, E.; Ibrahim, M. Effect of air injection under subsurface drip irrigation on yield and water use efficiency of corn in a sandy clay loam soil. J. Adv. Res. 2013, 4, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, H.; Livet, J.; Schnitzler, S.W. Effect of soil aeration on nitrogen availability and growth of selected vegetables-preliminary results. Acta Hortic. 2001, 563, 147–154. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Huber, S.; Midmore, D.J. Aerated subsurface irrigation water gives growth and yield benefits to Zucchini, vegetable soybean and cotton in heavy clay soils. Ann. Appl. Biol. 2004, 144, 285–298. [Google Scholar] [CrossRef]
- Chen, X.; Dhungel, J.; Bhattarai, S.P.; Torabi, M.; Pendergast, L.; Midmore, D.J. Impact of oxygation on soil respiration, yield and water use efficiency of three crop species. J. Plant Ecol. 2011, 4, 236–248. [Google Scholar] [CrossRef]
- Zhu, L.F.; Yu, S.M.; Jin, Q.Y. Effects of aerated irrigation on leaf senescence at late growth stage and grain yield of rice. Rice Sci. 2012, 19, 44–48. [Google Scholar] [CrossRef]
- Machefert, S.E.; Dise, N.B.; Goulding, K.W.T.; Whitehead, P.G. Nitrous oxide emission from a range of land uses across Europe. Hydrol. Earth Syst. Sci. 2002, 6, 325–337. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.; van Groenigen, J.W.; Hungate, B.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Chen, H.; Li, B.; Xiong, Z. Biochar reduces yield-scaled emissions of reactive nitrogen gases from vegetable soils across China. Biogeosciences 2017, 14, 2851–2863. [Google Scholar] [CrossRef] [Green Version]
- Oo, A.Z.; Sudo, S.; Akiyama, H.; Win, K.T.; Shibata, A.; Yamamoto, A.; Hirono, Y.; Sano, T. Effect of dolomite and biochar addition on N2O and CO2 emissions from acidic tea field soil. PLoS ONE 2018, 13, E0192235. [Google Scholar] [CrossRef] [PubMed]
- Oo, A.Z.; Sudo, S.; Win, K.T.; Shibata, A.; Gonai, T. Influence of pruning waste biochar and oyster shell on N2O and CO2 emissions from Japanese pear orchard soil. Heliyon 2018, 4, e00568. [Google Scholar] [CrossRef] [PubMed]
- Oo, A.Z.; Sudo, S.; Win, K.T.; Shibata, A.; Sano, T.; Hirono, Y. Returning Tea Pruning Residue and Its Biochar Had a Contrasting Effect on Soil N2O and CO2 Emissions from Tea Plantation Soil. Atmosphere 2018, 9, 109. [Google Scholar] [CrossRef]
- Clough, T.; Bertram, J.; Ray, J.; Condron, L.; O’Callaghan, M.; Sherlock, R.; Wells, N. Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil. Soil Sci. Soc. Am. J. 2010, 74, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Saarnio, S.; Heimonen, K.; Kettunen, R. Biochar addition indirectly affects N2O emissions via soil moisture and plant N uptake. Soil Biol. Biochem. 2013, 58, 99–106. [Google Scholar] [CrossRef]
- Field, J.L.; Keske, C.M.H.; Birch, G.L.; Defoort, M.W.; Cotrufo, M.F. Distributed biochar and bioenergy coproduction: A regionally specific case study of environmental benefits and economic impacts. Glob. Chang. Biol. Bioenergy 2013, 5, 177–191. [Google Scholar] [CrossRef]
- Cayuela, M.; Van Zwieten, L.; Singh, B.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Ebid, A.; Ueno, H.; Ghoneim, A.; Asagi, N. Uptake of carbon and nitrogen derived from carbon-13 and nitrogen-15 dual-labeled maize residue compost applied to radish, komatsuna, and chingensai for three consecutive croppings. Plant Soil 2008, 304, 241–248. [Google Scholar] [CrossRef]
- Amkha, S.; Sakamoto, A.; Tachibana, M.; Inubushi, K. Controlled mineralizing acetaldehyde condensation urea (CM-CDU) fertilizer can reduce nitrate leaching and N2O emission from an Andisol with continuous cropped komatsuna (Brassica napa L.). Soil Sci. Plant Nutr. 2009, 55, 772–777. [Google Scholar] [CrossRef]
- Minamikawa, K.; Takahashi, M.; Makino, T.; Tago, K.; Hayatsu, M. Irrigation with oxygen-nanobubble water can reduce methane emission and arsenic dissolution in a flooded rice paddy. Environ. Res. Lett. 2015, 10, 084012. [Google Scholar] [CrossRef] [Green Version]
- Parkin, T.B.; Venterea, R.T. Chamber-Based Trace Gas Flux Measurements. In Sampling Protocols; Follet, R.F., Ed.; 2010; Chapter 3; pp. 3-1–3-39. Available online: http://www.ars.usda.gov/research/GRACEnet (accessed on 29 September 2018).
- Rochette, P.; Chadwick, D.R.; de Klein, C. Deployment Protocol. In Nitrous Oxide Chamber Methodolog Guidelines; De Klein, C., Harvey, M., Eds.; Ministry for Primary Industries: Wellington, UK, 2013; Chapter 3. [Google Scholar]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, W.Q.; Wang, J.W.; Liu, L.; Zhang, M.Z.; Xu, J. Effects of artificial soil aeration volume and frequency on soil enzyme activity and microbial abundance when cultivating greenhouse tomato. Soil Sci. Soc. Am. J. 2016, 80, 1208–1221. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enaria, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A: Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Du, Y.; Niu, W.; Zhang, Q.; Cui, B.; Gu, X.; Guo, L.; Liang, B. Effects of Nitrogen on Soil Microbial Abundance, Enzyme Activity, and Nitrogen Use Efficiency in Greenhouse Celery under Aerated Irrigation. Soil Sci. Soc. Am. J. 2018, 82, 606–613. [Google Scholar] [CrossRef]
- Park, J.; Ohashi, K.; Kurata, K.; Lee, J. Promotion of lettuce growth by application of microbubbles in nutrient solution using different rates of electrical conductivity and under periodic intermittent generation in a deep flow technique culture system. Eur. J. Hortic. Sci. 2010, 75, 198–203. [Google Scholar]
- Ikeura, H.; Takahashi, H.; Kobayashi, F.; Sato, M.; Tamaki, M. Effects of microbubble generation methods and dissolved oxygen concentrations on growth of Japanese mustard spinach in hydroponic culture. J. Hortic. Sci. Biotechnol. 2017, 92, 483–490. [Google Scholar] [CrossRef]
- Carter, D.C.; Harris, D.; Youngquist, J.B.; Persaud, N. Soil properties, crop water use and cereal yields in Botswana after additions of mulch and manure. Field Crops Res. 1992, 30, 97–109. [Google Scholar] [CrossRef]
- Czyz, E.A. Effect of traffic on soil aeration, bulk density and growth of barley. Soil Tillage Res. 2004, 79, 153–166. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosys. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Pan, G.; Hussain, Q.; Li, L.; Zheng, J.; Zhang, X. Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China plain. Plant Soil 2012, 351, 263–275. [Google Scholar] [CrossRef]
- Chan, K.Y.; Xu, Z. Biochar: Nutrient properties and their enhancement, Biochar for environmental management. Sci. Technol. 2009, 1, 67–84. [Google Scholar]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soilapplied black carbon: Downward migration, leaching and soil respiration. Glob. Chang. Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of biochar amendment on fertility of a Southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effect of biochar from slow pyrolysis of paper mill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; Salazar, P.; Barron, V.; Torrent, J.; del Carmen del Campillo, M.; Gallardo, A.; Villar, R. Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron. Sustain. Dev. 2013, 33, 475–484. [Google Scholar] [CrossRef]
- Morard, P.; Lacoste, L.; Silvestre, J. Effect of oxygen deficiency on uptake of water and mineral nutrients by tomato plants in soilless culture. J. Plant Nutr. 2000, 23, 1063–1078. [Google Scholar] [CrossRef]
- Yang, F.; Lee, X.; Theng, B.K.G.; Wang, B.; Cheng, J.; Wang, Q. Effect of biochar addition on short-term N2O and CO2 emissions during repeated drying and wetting of an anthropogenic alluvial soil. Environ. Geochem. Health 2016, 39, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Müller, C.; Weiske, A.; Benckiser, G.; Ottow, J. Nitrification and denitrification as sources of atmospheric nitrous oxide—Role of oxidizable carbon and applied nitrogen. Biol. Fertil. Soils 2002, 35, 54–61. [Google Scholar]
- Chen, H.; Hou, H.; Wang, X.; Zhu, Y.; Saddique, Q.; Wang, Y.; Cai, H. The effects of aeration and irrigation regimes on soil CO2 and N2O emissions in a greenhouse tomato production system. J. Integr. Agric. 2018, 17, 449–460. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Pendergast, L.; Midmore, D.J. Root aeration improves yield and water use efficiency of tomato in heavy clay and saline soils. Sci. Hortic. 2006, 108, 278–288. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Z.; Niu, W.; Wang, J.; Zhang, M. Effect of Post-Infiltration Soil Aeration at Different Growth Stages on Growth and Fruit Quality of Drip-Irrigated Potted Tomato Plants (Solanum lycopersicum). PLoS ONE 2015, 10, e0143322. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, W.; Wang, J.; Zhang, M. Effects of aeration on rhizosphere soil enzyme activities and soil microbes for muskmelon in plastic greenhouse. Trans. Chin. Soc. Agric. Mach. 2015, 46, 121–129. [Google Scholar]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 13–32. [Google Scholar]
- Rogovska, N.; Laird, D.; Cruse, R.; Fleming, P.; Parkin, T.; Meek, D. Impact of biochar on manure carbon stabilization and greenhouse gas emissions. Soil Sci. Soc. Am. J. 2011, 75, 871–879. [Google Scholar] [CrossRef]
- Heincke, M.; Kaupenjohann, M. Effects of soil solution on the dynamics of N2O emissions: A review. Nutr. Cycl. Agroecosyst. 1999, 55, 133–157. [Google Scholar] [CrossRef]
- Liu, B.; Morkved, P.T.; Frostegard, A.; Bakken, L.R. Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh-Toosi, A.; Clough, T.J.; Condron, L.M.; Sherlock, R.R.; Anderson, C.R.; Craigie, R.A. Biochar incorporation into pasture soil suppresses in situ nitrous oxide emissions from ruminant urine patches. J. Environ. Qual. 2011, 40, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Y.; Wang, S.; Ma, B.; Ge, S.; Wang, Z.; Huang, H.; Zhang, J.; Zhang, L. Pathways and organisms involved in ammonia oxidation and nitrous oxide emission. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2213–2296. [Google Scholar] [CrossRef]
- Feng, Z.; Zhu, L. Impact of biochar on soil N2O emissions under different biochar-carbon/fertilizer-nitrogen ratios at a constant moisture condition on a silt loam soil. Sci. Total Environ. 2017, 15, 584–585. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value |
|---|---|
| pH | 5.3 |
| EC (mS cm−1) | 0.1 |
| P2O5 (mg 100 g−1) | 4.1 |
| K2O (mg 100 g−1) | 45.9 |
| NO3-N (mg 100 g−1) | 2.4 |
| NO2-N (mg 100 g−1) | 0.2 |
| Total N (g kg−1) | 3.7 |
| Total C (g kg−1) | 45 |
| Bulk density (g cm−3) | 0.76 |
| Coarse sand (%) | 3.1 |
| Fine sand (%) | 23.6 |
| Silt (%) | 50.4 |
| Clay (%) | 22.9 |
| Classification | Silk loam |
| pH (H2O) | Total N (g kg−1 Soil) | Total C (g kg−1 Soil) | C: N Ratio | Bulk Density (g cm−3) | Soil Porosity (%) | |
|---|---|---|---|---|---|---|
| Control | 6.54 ± 0.05 d | 4.66 ± 0.27 a | 52.1 ± 2.0 b | 11.19 ± 0.21 b | 0.70 ± 0.07 | 53.0 ± 3.6 |
| AI | 6.76 ± 0.07 c | 4.82 ± 0.16 a | 53.5 ± 0.4 b | 11.12 ± 0.29 b | - | - |
| BC | 7.12 ± 0.11 b | 5.10 ± 0.24 a | 90.1 ± 22.7 a | 17.56 ± 3.82 a | 0.63 ± 0.03 | 59.4 ± 1.9 |
| AI + BC | 7.25 ± 0.05 a | 5.34 ± 0.39 a | 93.9 ± 14.5 a | 17.53 ± 1.49 a | - | - |
| Analysis of variance P value | ||||||
| Treatment | <0.01 | >0.05 | <0.05 | <0.05 | - | - |
| Shoot Length (cm) | Shoot Fresh wt. (g pot−1) | Shoot Dry wt. (g pot−1) | |
|---|---|---|---|
| Control | 23.6 ± 2.2 ab | 59.5 ± 3.2 ab | 7.20 ± 0.08 b |
| AI | 21.5 ± 0.9 b | 55.5 ± 3.3 b | 6.73 ± 0.39 b |
| BC | 25.3 ± 1.2 a | 65.6 ± 0.8 a | 7.80 ± 0.08 a |
| AI + BC | 24.5 ± 1.9 ab | 60.3 ± 5.6 ab | 7.54 ± 0.36 ab |
| Analysis of variance P value | |||
| Treatment | ≤0.05 | ≤0.05 | ≤0.05 |
| Concentration (mg g−1) | |||||
|---|---|---|---|---|---|
| N | P | K | Mg | Ca | |
| Control | 16.72 ± 2.7 a | 0.64 ± 0.06 a | 13.9 ± 2.7 b | 2.16 ± 0.05 a | 20.9 ± 0.2 a |
| AI | 20.26 ± 0.9 a | 0.63 ± 0.17 a | 15.6 ± 1.9 ab | 2.16 ± 0.21 a | 21.0 ± 3.1 a |
| BC | 19.63 ± 3.1 a | 0.73 ± 0.03 a | 18.5 ± 2.4 a | 1.97 ± 0.24 a | 16.9 ± 0.6 b |
| AI + BC | 18.45 ± 1.0 a | 0.76 ± 0.09 a | 16.8 ± 2.8 ab | 2.04 ± 0.21 a | 15.7 ± 1.2 b |
| Analysis of variance P value | |||||
| Treatment | >0.05 | >0.05 | ≤0.05 | >0.05 | ≤0.05 |
| Uptake (mg pot−1) | |||||
|---|---|---|---|---|---|
| N | P | K | Mg | Ca | |
| Control | 120.7 ± 11.8 b | 99.4 ± 9.9 ab | 100.5 ± 20.9 b | 15.6 ± 0.7 a | 150.5 ± 3.0 a |
| AI | 136.3 ± 14.5 ab | 89.9 ± 18.0 b | 104.2 ± 11.6 ab | 14.5 ± 0.4 a | 139.8 ± 6.5 b |
| BC | 152.9 ± 12.9 a | 121.9 ± 6.1 ab | 143.9 ± 17.9 a | 15.4 ± 1.8 a | 131.6 ± 5.7 b |
| AI + BC | 139.2 ± 10.6 ab | 122.8 ± 10.1 a | 127.4 ± 26.9 ab | 15.5 ± 2.3 a | 118.6 ± 9.8 c |
| Analysis of variance P value | |||||
| Treatment | ≤0.05 | ≤0.05 | ≤0.05 | >0.05 | ≤0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oo, A.Z.; Sudo, S.; Matsuura, S.; Win, K.T.; Gonai, T. Aerated Irrigation and Pruning Residue Biochar on N2O Emission, Yield and Ion Uptake of Komatsuna. Horticulturae 2018, 4, 33. https://doi.org/10.3390/horticulturae4040033
Oo AZ, Sudo S, Matsuura S, Win KT, Gonai T. Aerated Irrigation and Pruning Residue Biochar on N2O Emission, Yield and Ion Uptake of Komatsuna. Horticulturae. 2018; 4(4):33. https://doi.org/10.3390/horticulturae4040033
Chicago/Turabian StyleOo, Aung Zaw, Shigeto Sudo, Shoji Matsuura, Khin Thuzar Win, and Takeru Gonai. 2018. "Aerated Irrigation and Pruning Residue Biochar on N2O Emission, Yield and Ion Uptake of Komatsuna" Horticulturae 4, no. 4: 33. https://doi.org/10.3390/horticulturae4040033





