Colored Shading Nets Differentially Affect the Phytochemical Profile, Antioxidant Capacity, and Fruit Quality of Piquin Peppers (Capsicum annuum L. var. glabriusculum)
Abstract
:1. Introduction
2. Materials and Methods
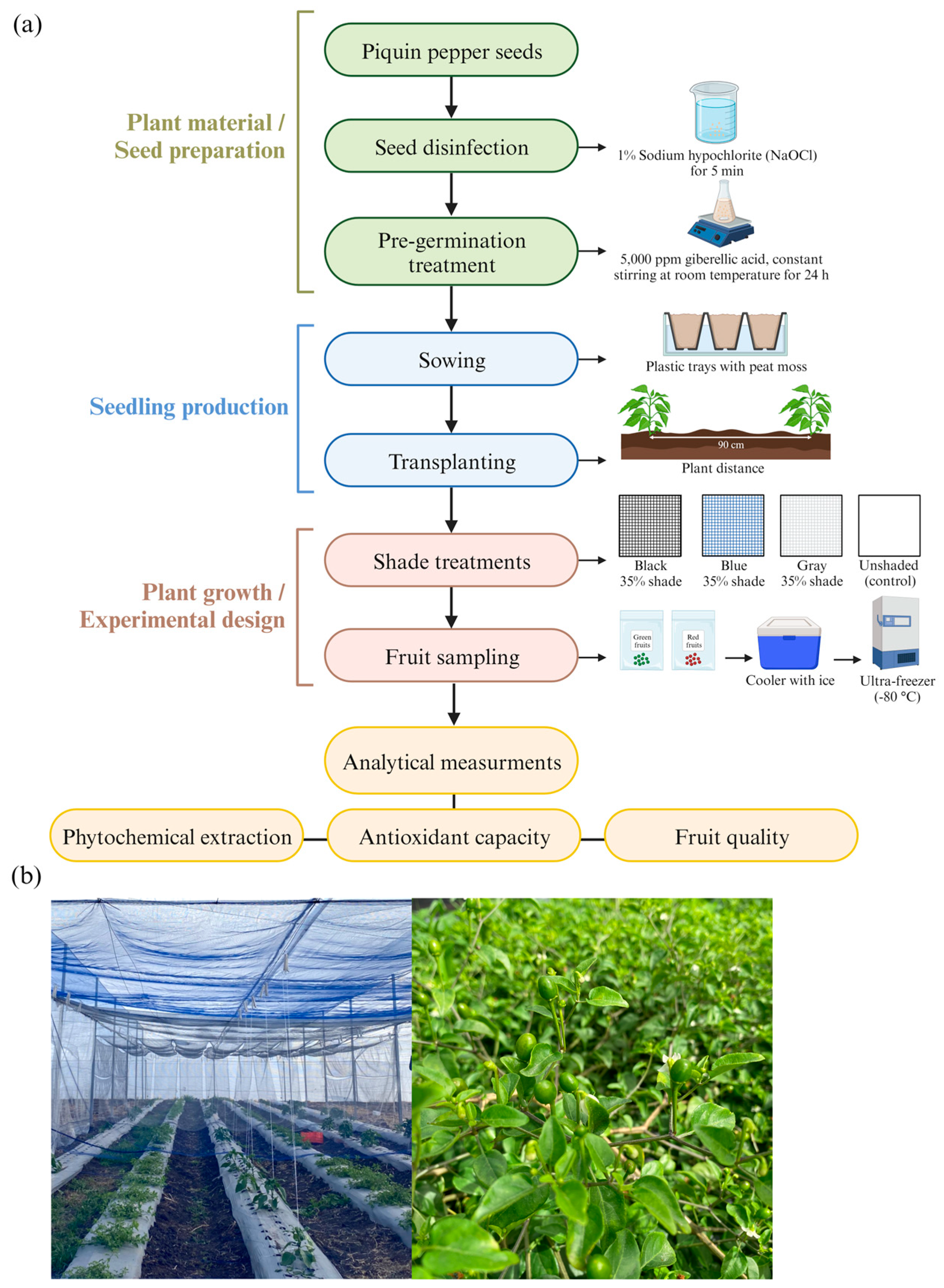
2.1. Plant Material and Experimental Site
2.2. Experimental Design
2.3. Sample Preparation
2.4. HPLC Analysis for Capsaicinoid Content
2.5. HPLC Analysis for Carotenoid Content
2.6. Total Phenolic Compounds
2.7. Antioxidant Capacity
2.7.1. ABTS Method
2.7.2. DPPH Method
2.8. Fruit Quality
2.8.1. Analysis of Color
2.8.2. Morphological Analysis
2.9. Statistical Analysis
3. Results and Discussion
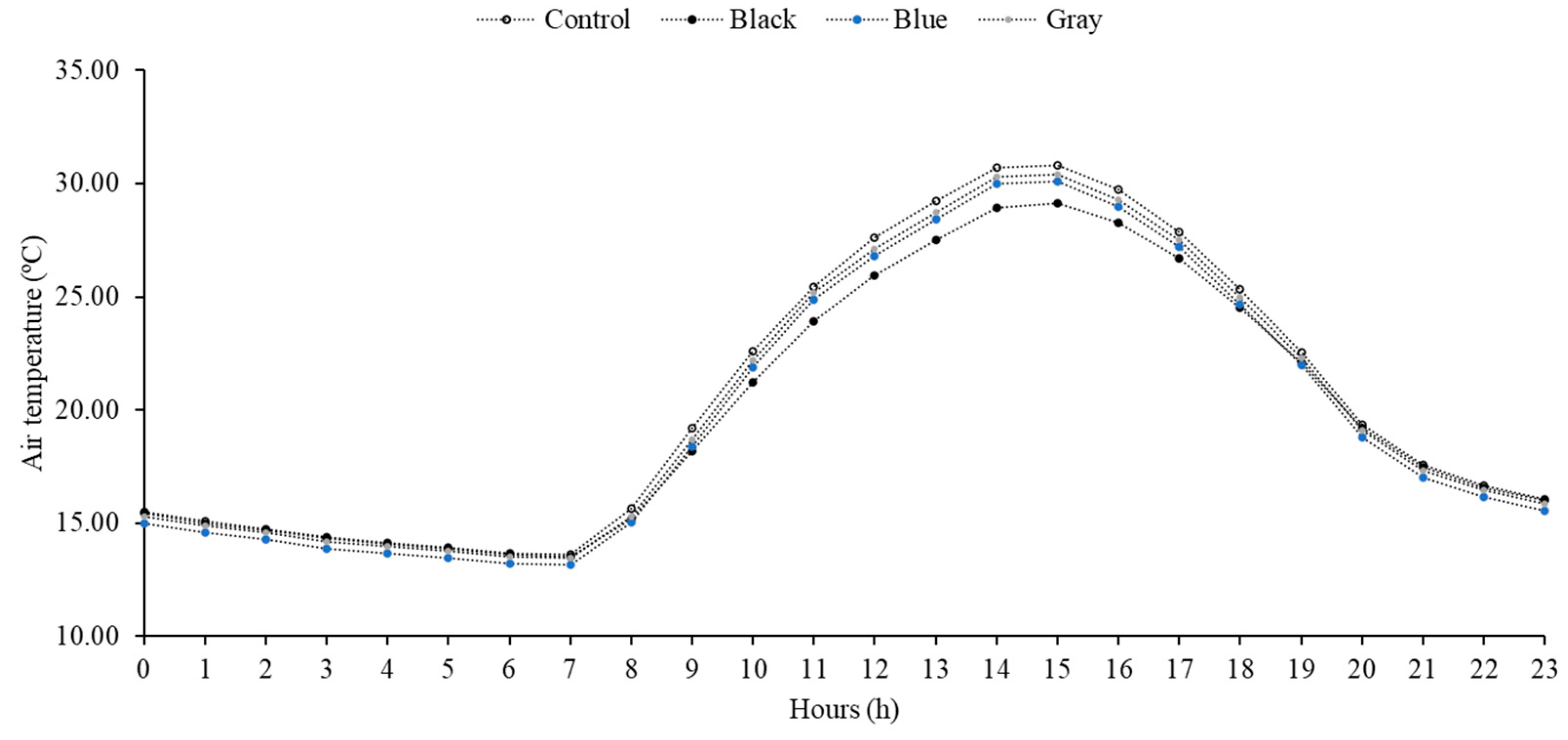
3.1. Temperature
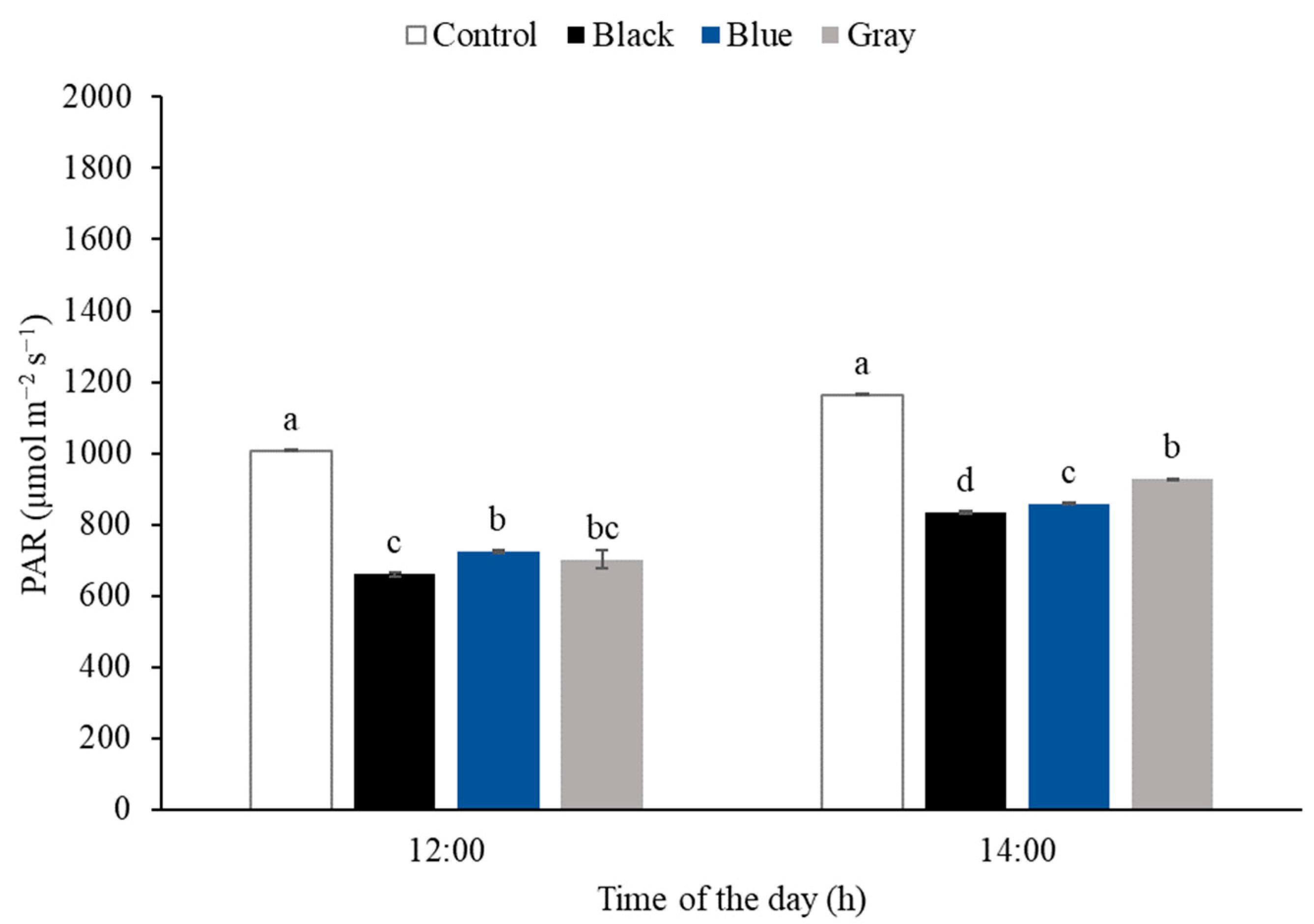
3.2. Photosynthetically Active Radiation (PAR)
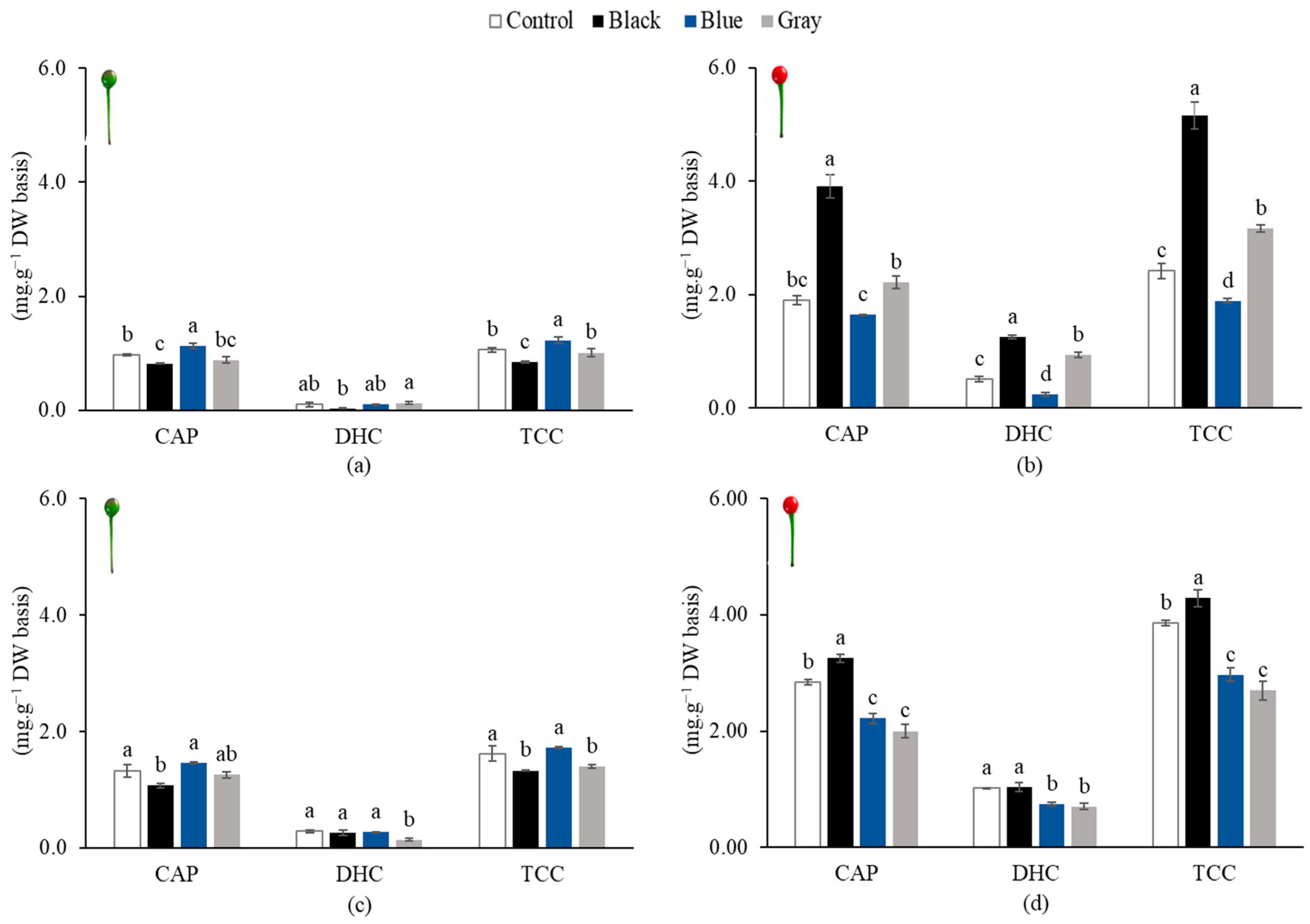
3.3. Capsaicinoid Content of Piquin Peppers
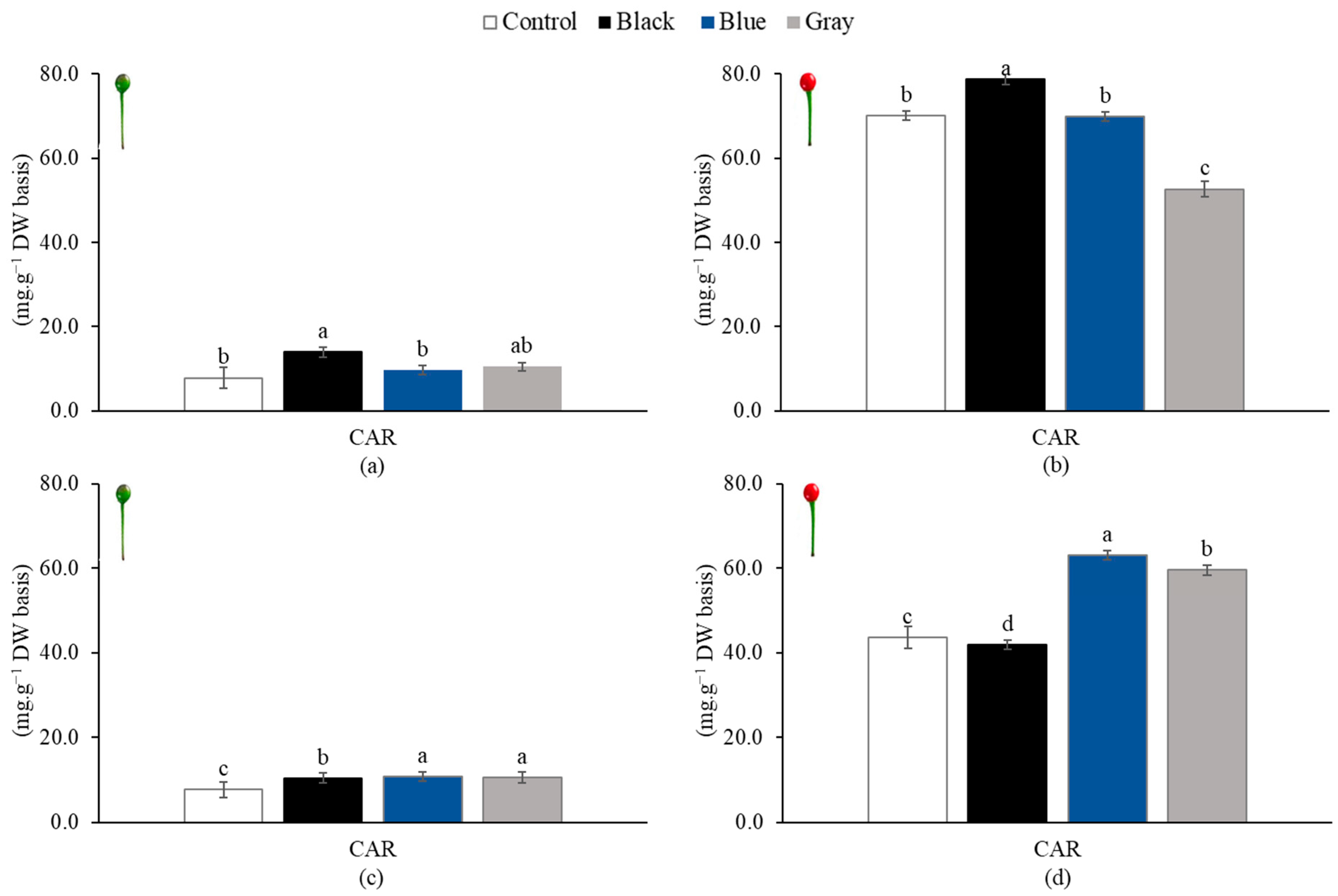
3.4. Carotenoid Content of Piquin Peppers
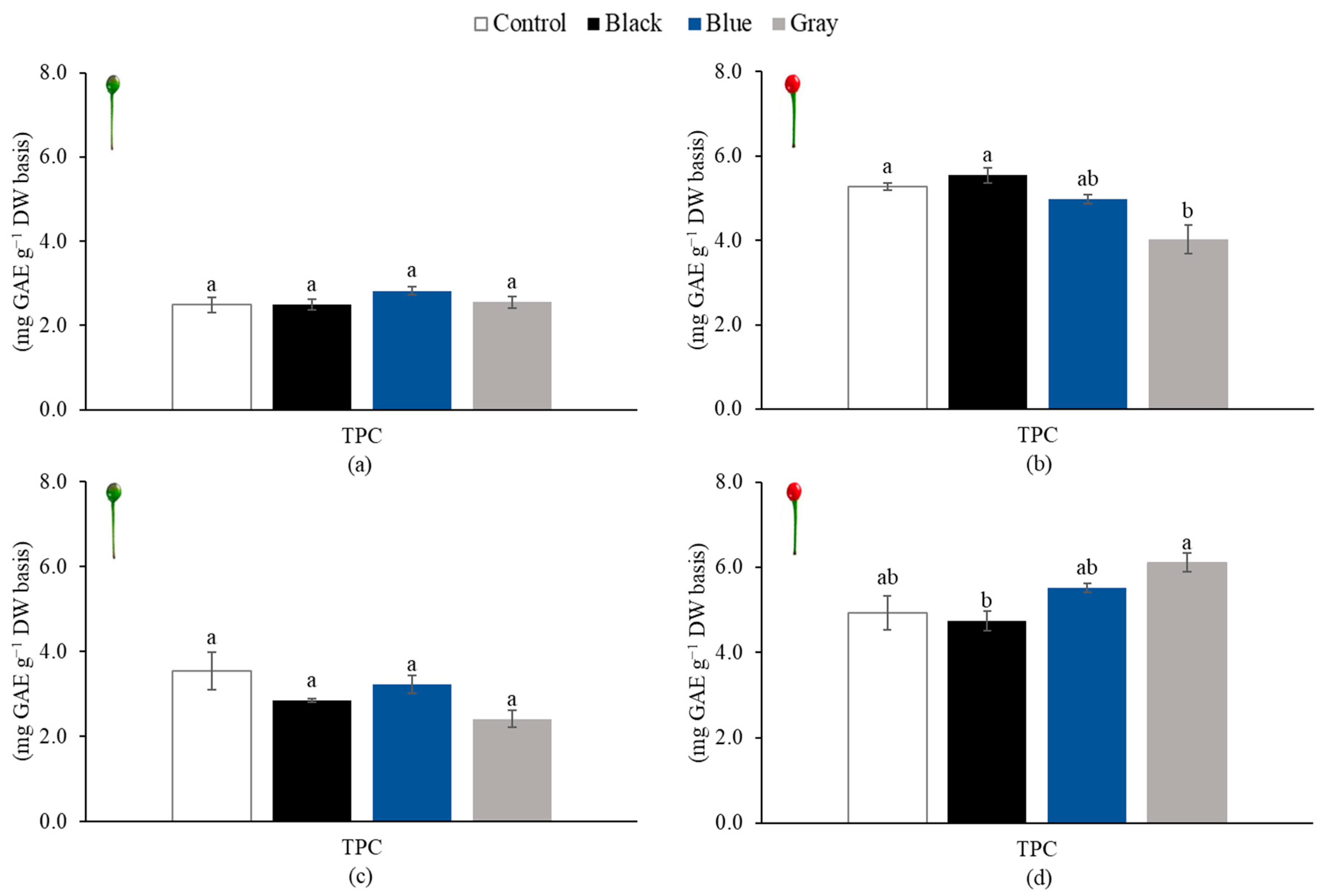
3.5. Total Phenolic Compounds of Piquin Peppers
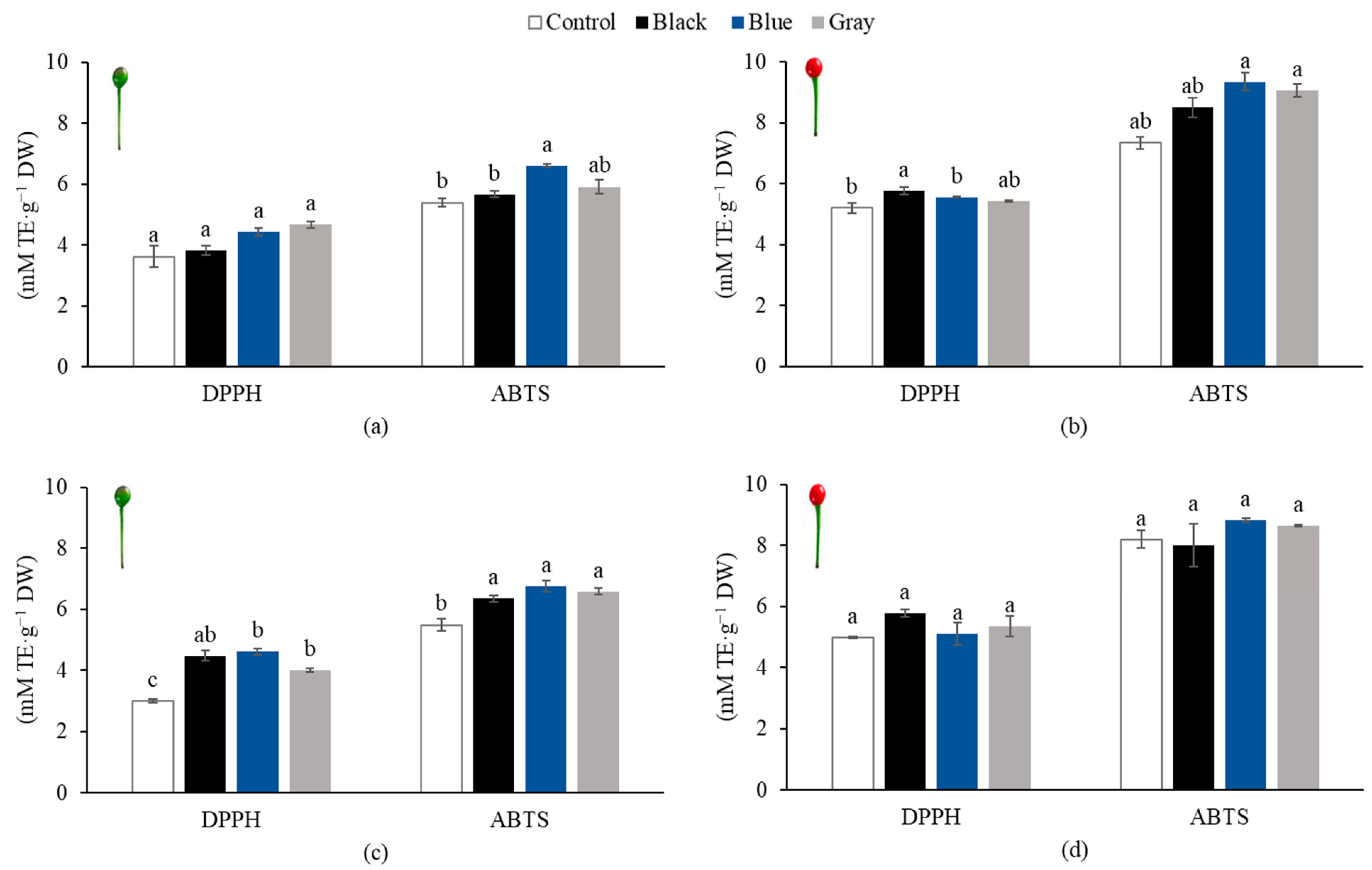
3.6. Antioxidant Capacity
3.7. Fruit Quality
3.7.1. Effects on Pepper Color
3.7.2. Size and Weight
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mares-Quiñones, M.D.; Valiente-Banuet, J.I. Horticultural aspects for the cultivated production of piquin peppers (Capsicum annuum L. var. Glabriusculum) a review. HortScience 2019, 54, 70–75. [Google Scholar] [CrossRef]
- Rodríguez-Del Bosque, L.A. Preferencia del consumidor por el chile piquín en comparación con otros chiles en el noreste de México. Rev. Chapingo Ser. Hortic. 2005, 11, 279–281. Available online: https://www.redalyc.org/articulo.oa?id=60911214 (accessed on 5 October 2023).
- Coronado-García, M.A.; Córdova-Yánez, A.; García-Porchas, M.; Santiago-Hernández, V.G.; Vásquez Navarro, R.A. Estrategias de mercado para productos elaborados a base de chiltepín en la sierra de Sonora. Rev. Mex. Agroneg. 2013, 32, 359–370. [Google Scholar]
- Rodríguez-del Bosque, L.A. Producción intensiva de chile piquín en el norte de Tamaulipas. Ficha tecnológica por sistema producto. INIFAP 2008. Available online: http://www.inifapcirne.gob.mx/Biblioteca/Publicaciones/536.pdf (accessed on 6 October 2023).
- Meckelmann, S.W.; Riegel, D.W.; van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Capsaicinoids, flavonoids, tocopherols, antioxidant capacity and color attributes in 23 native peruvian chili peppers (Capsicum spp.) grown in three different locations. Eur. Food Res. Technol. 2014, 240, 273–283. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Anju, T.; Ramchiary, N. Genetic, epigenetic, and hormonal regulation of fruit development and ripening in Capsicum L. species. Annu. Plant Rev. Online 2021, 4, 295–356. [Google Scholar] [CrossRef]
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706. [Google Scholar]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. An R2R3-MYB transcription factor regulates capsaicinoid biosynthesis. Plant Physiol. 2017, 174, 1359–1370. [Google Scholar] [CrossRef]
- Naves, E.R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Peres, L.E.P.; Zsögön, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef]
- Llorente, B.; D’Andrea, L.; Ruiz-Sola, M.A.; Botterweg, E.; Pulido, P.; Andilla, J.; Loza-Alvarez, P.; Rodriguez-Concepcion, M. Tomato fruit carotenoid biosynthesis is adjusted to actual ripening progression by a light-dependent mechanism. Plant J. 2016, 85, 107–119. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar]
- Lemos, V.C.; Reimer, J.J.; Wormit, A. Color for life: Biosynthesis and distribution of phenolic compounds in pepper (Capsicum annuum). Agriculture 2019, 9, 81. [Google Scholar] [CrossRef]
- Ncise, W.; Daniels, C.W.; Nchu, F. Effects of light intensities and varying watering intervals on growth, tissue nutrient content and antifungal activity of hydroponic cultivated Tulbaghia violacea L. under greenhouse conditions. Heliyon 2020, 6, e03906. [Google Scholar] [CrossRef]
- Jiménez-Viveros, Y.; Núñez-Palenius, H.G.; Fierros-Romero, G.; Valiente-Banuet, J.I. Modification of light characteristics affect the phytochemical profile of peppers. Horticulturae 2023, 9, 72. [Google Scholar] [CrossRef]
- Mashabela, M.N.; Selahle, K.M.; Soundy, P.; Crosby, K.M.; Sivakumar, D. Bioactive compounds and fruit quality of green sweet pepper grown under different colored shade netting during postharvest storage. J. Food Sci. 2015, 80, H2612–H2618. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Barać, S.; Mastilović, J.; Kevrešan, Ž.; Fallik, E. Effect of shading by coloured nets on yield and fruit quality of sweet pepper. Zemdirbyste 2017, 104, 53–62. [Google Scholar] [CrossRef]
- Valiente-Banuet, J.I.; Gutierrez-Ochoa, A. Effect of irrigation frequency and shade levels on vegetative growth, yield, and fruit quality of piquin pepper (Capsicum annuum L. Var. glabriusculum). HortScience 2016, 51, 573–579. [Google Scholar] [CrossRef]
- Mohawesh, O.; Albalasmeh, A.; Deb, S.; Singh, S.; Simpson, C.; Alkafaween, N.; Mahadeen, A. Effect of colored shading nets on the growth and water use efficiency of sweet pepper grown under semi-arid conditions. Horttechnology 2022, 32, 21–27. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jifon, J.; Soundy, P. Spectral quality of photo-selective shade nettings improves antioxidants and overall quality in selected fresh produce after postharvest storage. Food Rev. Int. 2018, 34, 290–307. [Google Scholar] [CrossRef]
- Santana, J.Q.; Balbino, M.A.; Tavares, T.R.; Bezerra, R.S.; Farias, J.G.; Ferreira, R.C. Effect of photoselective screens in the development and productivity of red and yellow sweet pepper. Acta Hortic. 2012, 956, 493–500. [Google Scholar] [CrossRef]
- Stamps, R.H. Use of colored shade netting in horticulture. HortScience 2009, 44, 239–241. [Google Scholar] [CrossRef]
- Castellano, S.; Mugnozza, G.S.; Russo, G.; Briassoulis, D.; Mistriotis, A.; Hemming, S.; Waaijenberg, D. Plastic nets in agriculture: A general review of types and applications. Appl. Eng. Agric. 2008, 24, 799–808. [Google Scholar] [CrossRef]
- Britannica. Tropical and Subtropical Steppe Climate, Deserts, arid Regions, Semi-Arid. Available online: https://www.britannica.com/science/tropical-and-subtropical-steppe-climate (accessed on 11 July 2023).
- INEGI. Available online: https://www.inegi.org.mx/app/biblioteca/ficha.html?upc=702825293147 (accessed on 11 July 2023).
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Hornero-Méndez, D. Separation and quantification of the carotenoid pigments in red peppers (Capsicum annuum L.), paprika, and oleoresin by reversed-phase HPLC. J. Agric. Food Chem. 1993, 41, 1616–1620. [Google Scholar] [CrossRef]
- Blanco-Ríos, A.K.; Medina-Juárez, L.Á.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of the phenolic and oily fractions of different sweet bell peppers. J. Mex. Chem. Soc. 2013, 57, 137–143. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Rajurkar, N.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds†. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I. Effect of Water Activity and Temperature on the Color Change of Red Pepper (Capsicum annuum L.) Powder. Food Sci. Biotechnol. 2011, 20, 215–222. [Google Scholar] [CrossRef]
- Ibraheem, N.A.; Hasan, M.M.; Khan, R.Z.; Mishra, P.K. Understanding color models: A review. ARPN J. Sci. Technol. 2012, 2, 265–275. [Google Scholar] [CrossRef]
- Ahemd, H.A.; Al-Faraj, A.A.; Abdel-Ghany, A.M. Shading greenhouses to improve the microclimate, energy and water saving in hot regions: A review. Sci. Hortic. 2016, 201, 36–45. [Google Scholar] [CrossRef]
- Olivares-Soto, H.; Bastías, R.M. Photosynthetic efficiency of apples under protected shade nets. Chil. J. Agric. Res. 2018, 78, 126–138. [Google Scholar] [CrossRef]
- Bastías, R.M.; Boini, A. Apple Production under Protective Netting Systems. In Apple Cultivation—Recent Advances, 1st ed.; Küden, A., Ed.; IntechOpen: London, UK, 2022; pp. 91–102. [Google Scholar]
- Fayos, O.; De Aguiar, A.C.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Garcés-Claver, A.; Martínez, J.; Mallor, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G.; et al. Ontogenetic variation of individual and total capsaicinoids in malagueta peppers (Capsicum Frutescens) during fruit maturation. Molecules 2017, 22, 736. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Daood, H.; Neményi, A.; Ambrózy, Z.; Pék, Z.; Helyes, L. Impact of shading net color on phytochemical contents in two chili pepper hybrids cultivated under greenhouse conditions. Korean J. Hortic. Sci. Technol. 2017, 35, 418–430. [Google Scholar] [CrossRef]
- Agyemang, D.S.; Nagy, Z.; e Souza, C.S.; Pék, Z.; Neményi, A.; Helyes, L. Effect of net shading technology on the yield quality and quantity of chilli pepper under greenhouse cultivation. Acta Agrar. Debr. 2021, 1, 5–9. [Google Scholar] [CrossRef]
- Pacheco, F.V.; Alvarenga, I.C.A.; Junior, P.M.R.; Pinto, J.E.B.P.; Avelar, R.d.P.; Alvarenga, A.A. Growth and production of secondary compounds in monkey-pepper (Piper Aduncum L.) leaves cultivated under altered ambient light. Aust. J. Crop Sci. 2014, 8, 1510–1516. [Google Scholar] [CrossRef]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum Chinense Jacq.) cultivars. Hortic. Environ. Biotechnol. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Hassan, N.M.; Yusof, N.A.; Yahaya, A.F.; Rozali, N.N.M.; Othman, R. Carotenoids of Capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD. Food Chem. X 2020, 6, 100092. [Google Scholar] [CrossRef]
- Salinas Hernández, M.; Ma, R.; Liévano, L.; Andrés, E.; Jiménez, P. Caracterización morfológica y cambios durante la vida postcosecha de cuatro tipos de chile amashito (Capsicum annuum L.) variedad glabriusculum (Dunal) Heiser & Pickersgill. Rev. Iberoam. Tecnol. Postcosecha 2010, 11, 92–100. Available online: https://www.redalyc.org/articulo.oa?id=81315093012 (accessed on 17 August 2023).
- Ambrózy, Z.; Daood, H.; Nagy, Z.; Darázsi Ledó, H.; Helyes, L. Effect of net shading technology and harvest times on yield and fruit quality of sweet pepper. Appl. Ecol. Environ. Res. 2016, 14, 99–109. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Artés-Hernández, F. Postharvest UV radiation enhanced biosynthesis of flavonoids and carotenes in bell peppers. Postharvest Biol. Technol. 2022, 184, 111774. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Ombódi, A.O.; Pék, Z.; Szuvandzsiev, P.; Lugasi, A.; Ledóné Darázsi, H.; Helyes, L. Effect of coloured shade nets on some nutritional characteristics of a kapia type pepper grown in plastic tunnel. Columella J. Agric. Environ. Sci. 2016, 3, 25–33. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; St. John, K.; Kabir, M.Y.; Alvarado-Chávez, J.A.; Cutiño-Jiménez, A.M.; Bautista, J.; Gunawan, G.; Nambeesan, S.U. Bell Pepper (Capsicum Annum L.) under colored shade nets: Fruit yield, postharvest transpiration, color, and chemical composition. HortScience 2020, 55, 181–187. [Google Scholar] [CrossRef]
- Horváth, G.; Kissimon, J.; Faludi-Dániel, Á. effect of light intensity on the formation of carotenoids in normal and mutant maize leaves. Phytochemistry 1972, 11, 183–187. [Google Scholar] [CrossRef]
- Sandmann, G.; Kuhn, M.; Böger, P. Carotenoids in photosynthesis: Protection of D1 degradation in the light. Photosynth. Res. 1993, 35, 185–190. [Google Scholar] [CrossRef]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef]
- Ionică, M.E.; Nour, V. Bioactive compounds and antioxidant activity of hot pepper fruits at different stages of growth and ripening. J. Appl. Bot. Food Qual. 2017, 90, 232–237. [Google Scholar] [CrossRef]
- Angmo, P.; Dolma, T.; Phuntsog, N.; Chaurasia, O.P.; Stobdan, T. Effect of shading and high temperature amplitude on yield and phenolic contents of greenhouse capsicum (Capsicum annuum L.). J. Biol. Pharm. 2021, 4, 30–39. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Nagarajan, J.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Krishnamurthy, N.P. Carotenoids. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Academic Press: Cambridge, MA, USA, 2017; pp. 259–296. [Google Scholar] [CrossRef]
- Mercy, E.R.; David, U. Potential health benefits of conventional nutrients and phytochemicals of Capsicum peppers. Pharm. Pharmacol. Int. J. 2018, 6, 62–69. [Google Scholar] [CrossRef]
- Del Rocio Moreno-Ramírez, Y.; Martínez-Ávila, G.C.G.; González-Hernández, V.A.; Castro-López, C.; Torres-Castillo, J.A. Free radical-scavenging capacities, phenolics and capsaicinoids in wild piquin chili (Capsicum annuum var. glabriusculum). Molecules 2018, 23, 2655. [Google Scholar] [CrossRef]
- Castro-Concha, L.A.; Tuyub-Che, J.; Moo-Mukul, A.; Vazquez-Flota, F.A.; Miranda-Ham, M.L.; Bekatorou, A.; Tariq, A.; Tripathi, N.K. Antioxidant capacity and total phenolic content in fruit tissues from accessions of Capsicum Chinense Jacq. (Habanero pepper) at different stages of ripening. Sci. World J. 2014, 2014, 809073. [Google Scholar] [CrossRef]
- Pola, W.; Sugaya, S.; Photchanachai, S. Color development and phytochemical changes in mature green chili (Capsicum annuum L.) exposed to red and blue light-emitting diodes. J. Agric. Food Chem. 2020, 68, 59–66. [Google Scholar] [CrossRef]
- Chaki, M.; Álvarez De Morales, P.; Ruiz, C.; Begara-Morales, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M. Ripening of Pepper (Capsicum annuum) Fruit is characterized by an enhancement of protein tyrosine nitration. Ann. Bot. 2015, 116, 637. [Google Scholar] [CrossRef] [PubMed]
- Ombódi, A.; Pék, Z.; Szuvandzsiev, P.; Taskovics, Z.T.; Koházi-Kis, A.; Kovács, A.; Darázsi, H.L.; Helyes, L. Effects of external coloured shade nets on sweet peppers cultivated in walk-in plastic tunnels. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 398–403. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C. Bell pepper (Capsicum annum L.) crop as affected by shade level: Fruit yield, quality, and postharvest attributes, and incidence of phytophthora blight (caused by Phytophthora capsici Leon.). HortScience 2014, 49, 891–900. [Google Scholar] [CrossRef]

| Treatment | Green Fruits | Red Fruits | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| Control | 30.88 ± 2.32 a | −8.67 ± 5.09 a | 31.20 ± 2.28 a | 28.51 ± 0.88 a | 43.69 ± 4.92 a | 38.24 ± 1.72 b |
| Black | 30.26 ± 2.69 a | −15.45 ± 21.48 a | 28.04 ± 3.07 b | 27.64 ± 2.42 a | 43.39 ± 1.40 a | 38.60 ± 3.07 b |
| Blue | 29.33 ± 1.57 a | −9.98 ± 0.38 a | 28.46 ± 1.83 b | 28.83 ± 1.60 a | 45.00 ± 1.07 a | 40.50 ± 2.12 ab |
| Gray | 30.16 ± 1.58 a | −10.24 ± 0.31 a | 31.73 ± 1.85 a | 28.88 ± 1.58 a | 44.55 ± 1.07 a | 42.06 ± 2.92 a |
| Treatment | Green Fruits | Red Fruits | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| Control | 28.64 ± 1.78 a | −7.43 ± 1.60 a | 40.47 ± 2.15 a | 36.77 ± 2.29 a | 35.02 ± 2.23 a | 20.84 ± 1.97 a |
| Black | 24.81 ± 1.59 b | −9.03 ± 0.94 b | 35.57 ± 2.70 bc | 38.06 ± 0.60 a | 34.00 ± 1.40 a | 19.22 ± 1.63 a |
| Blue | 23.73 ± 2.20 b | −10.29 ± 0.78 c | 36.90 ± 1.61 b | 35.30 ± 8.86 a | 21.49 ± 19.67 b | 22.36 ± 7.29 a |
| Gray | 24.69 ± 2.56 b | −9.82 ± 0.38 bc | 34.61 ± 2.28 c | 38.19 ± 1.16 a | 34.17 ± 0.77 a | 20.25 ± 1.10 a |
| Treatment | Green Fruits | Red Fruits | ||||
|---|---|---|---|---|---|---|
| Fruit Width | Fruit Length | Weight | Fruit Width | Fruit Length | Weight | |
| Control | 8.73 ± 0.32 ab | 10.22 ± 0.40 ab | 2683.83 ± 344.82 a | 8.84 ± 0.33 a | 9.98 ± 0.40 a | 2553.73 ± 180.66 a |
| Black | 8.91 ± 0.24 a | 10.81 ± 0.49 a | 2883.33 ± 336.51 a | 8.33 ± 0.42 bc | 9.57 ± 0.58 a | 2242.59 ± 423.48 a |
| Blue | 8.23 ± 0.35 c | 10.42 ± 0.80 ab | 2711.35 ± 319.59 a | 8.62 ± 0.33 ab | 10.01 ± 0.57 a | 2348.14 ± 428.44 a |
| Gray | 8.39 ± 0.26 bc | 10.01 ± 0.38 b | 2802.46 ± 306.35 a | 8.14 ± 0.41 c | 9.99 ± 0.57 a | 2731.55 ± 411.68 a |
| Treatment | Green Fruits | Red Fruits | ||||
|---|---|---|---|---|---|---|
| Fruit Width | Fruit Length | Weight | Fruit Width | Fruit Length | Weight | |
| Control | 8.56 ± 0.28 a | 10.32 ± 0.43 a | 2977.50 ± 283.44 a | 7.93 ± 0.19 b | 9.54 ± 0.20 b | 2924.50 ± 162.81 b |
| Black | 8.47 ± 0.27 a | 10.02 ± 0.34 a | 2852.78 ± 187.14 ab | 8.54 ± 0.17 a | 9.84 ± 0.41 ab | 3046.45 ± 232.93 b |
| Blue | 8.02 ± 0.33 b | 8.91 ± 0.31 b | 2484.24 ± 107.96 b | 8.40 ± 0.15 a | 10.21 ± 0.33 a | 3375.51 ± 46.85 a |
| Gray | 8.24 ± 0.38 ab | 9.26 ± 0.33 b | 2586.40 ± 154.60 ab | 8.37 ± 0.26 a | 10.27 ± 0.50 a | 2555.88 ± 99.13 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Viveros, Y.; Valiente-Banuet, J.I. Colored Shading Nets Differentially Affect the Phytochemical Profile, Antioxidant Capacity, and Fruit Quality of Piquin Peppers (Capsicum annuum L. var. glabriusculum). Horticulturae 2023, 9, 1240. https://doi.org/10.3390/horticulturae9111240
Jiménez-Viveros Y, Valiente-Banuet JI. Colored Shading Nets Differentially Affect the Phytochemical Profile, Antioxidant Capacity, and Fruit Quality of Piquin Peppers (Capsicum annuum L. var. glabriusculum). Horticulturae. 2023; 9(11):1240. https://doi.org/10.3390/horticulturae9111240
Chicago/Turabian StyleJiménez-Viveros, Yamir, and Juan Ignacio Valiente-Banuet. 2023. "Colored Shading Nets Differentially Affect the Phytochemical Profile, Antioxidant Capacity, and Fruit Quality of Piquin Peppers (Capsicum annuum L. var. glabriusculum)" Horticulturae 9, no. 11: 1240. https://doi.org/10.3390/horticulturae9111240





