Effects of 1–MCP Treatment on Postharvest Fruit of Five Pomegranate Varieties during Low-Temperature Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. 1–MCP Treatment
2.3. Exterior Quality Index
2.4. Interior Quality Index
2.5. Ethylene Production
2.6. Respiration Rate
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results
3.1. Effects of 1–MCP on Exterior Quality
3.1.1. Browning Index
3.1.2. Color
3.2. Effects of 1–MCP on Interior Quality
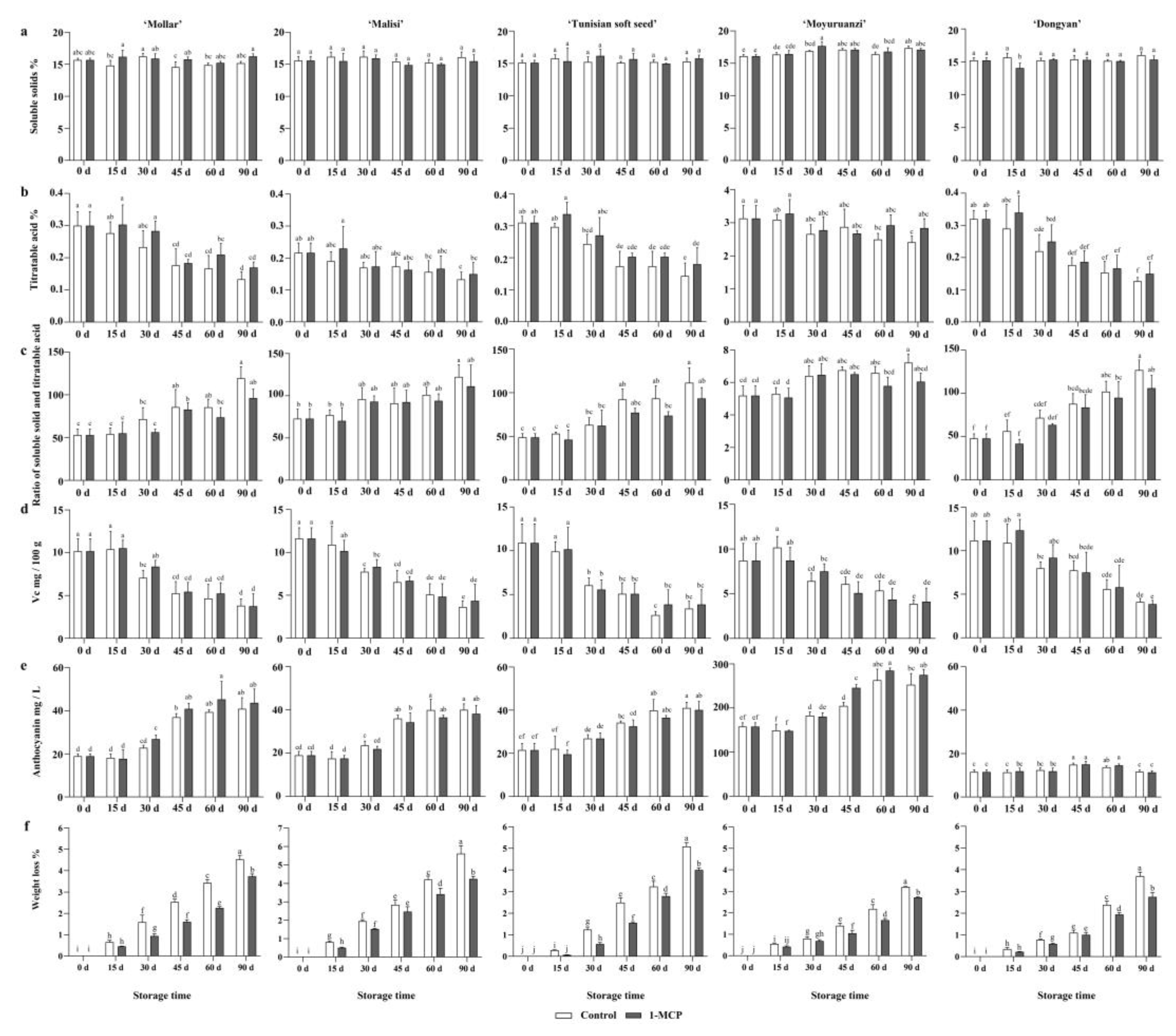
3.2.1. Total Soluble Solids and Titratable Acids
3.2.2. Vc and Anthocyanins
3.2.3. Weight Loss
3.3. Effects of 1–MCP on Respiration Rate and Ethylene Production
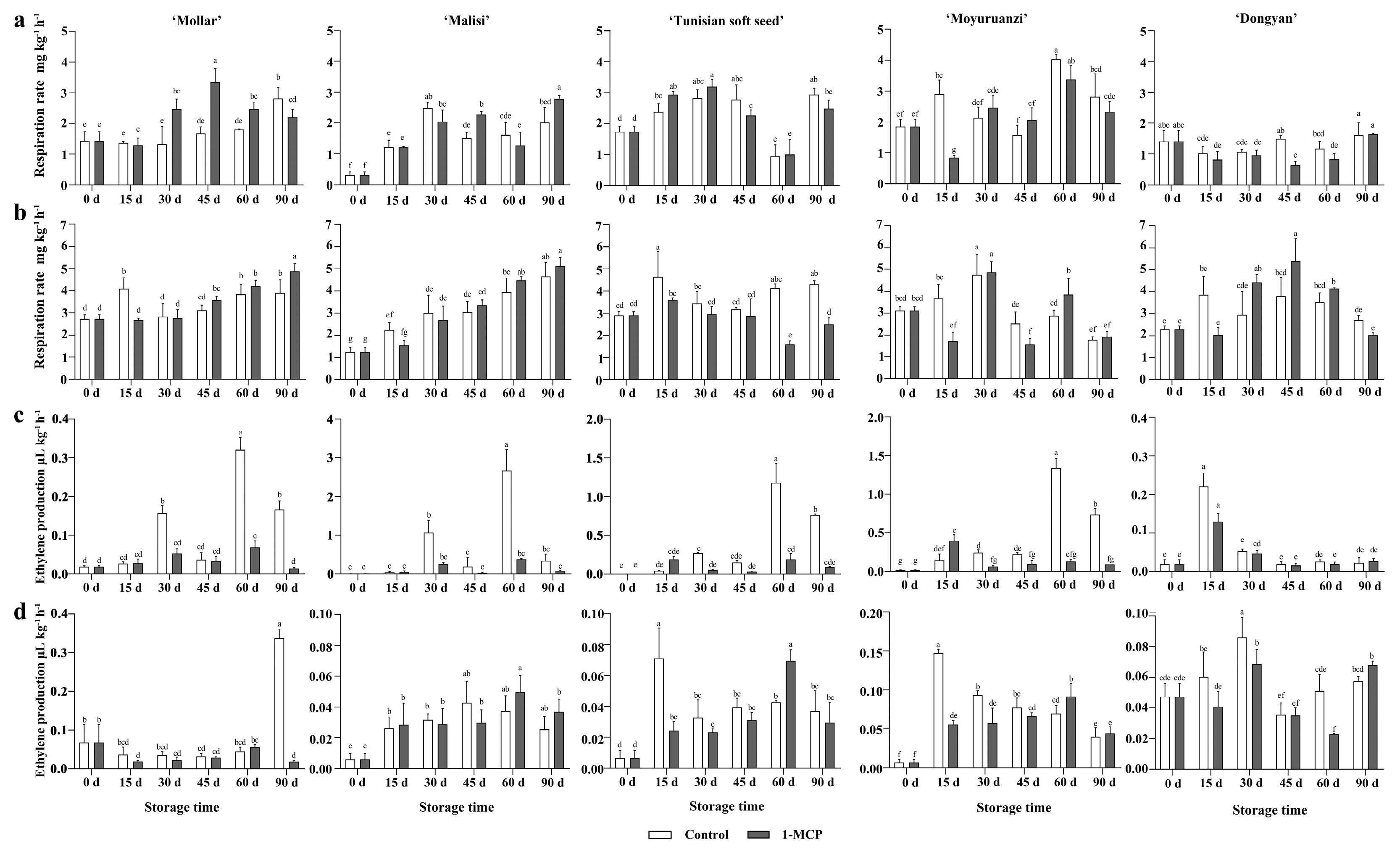
3.3.1. Respiration Rates of Whole Fruit and Arils
3.3.2. Ethylene Production of Whole Fruit and Arils
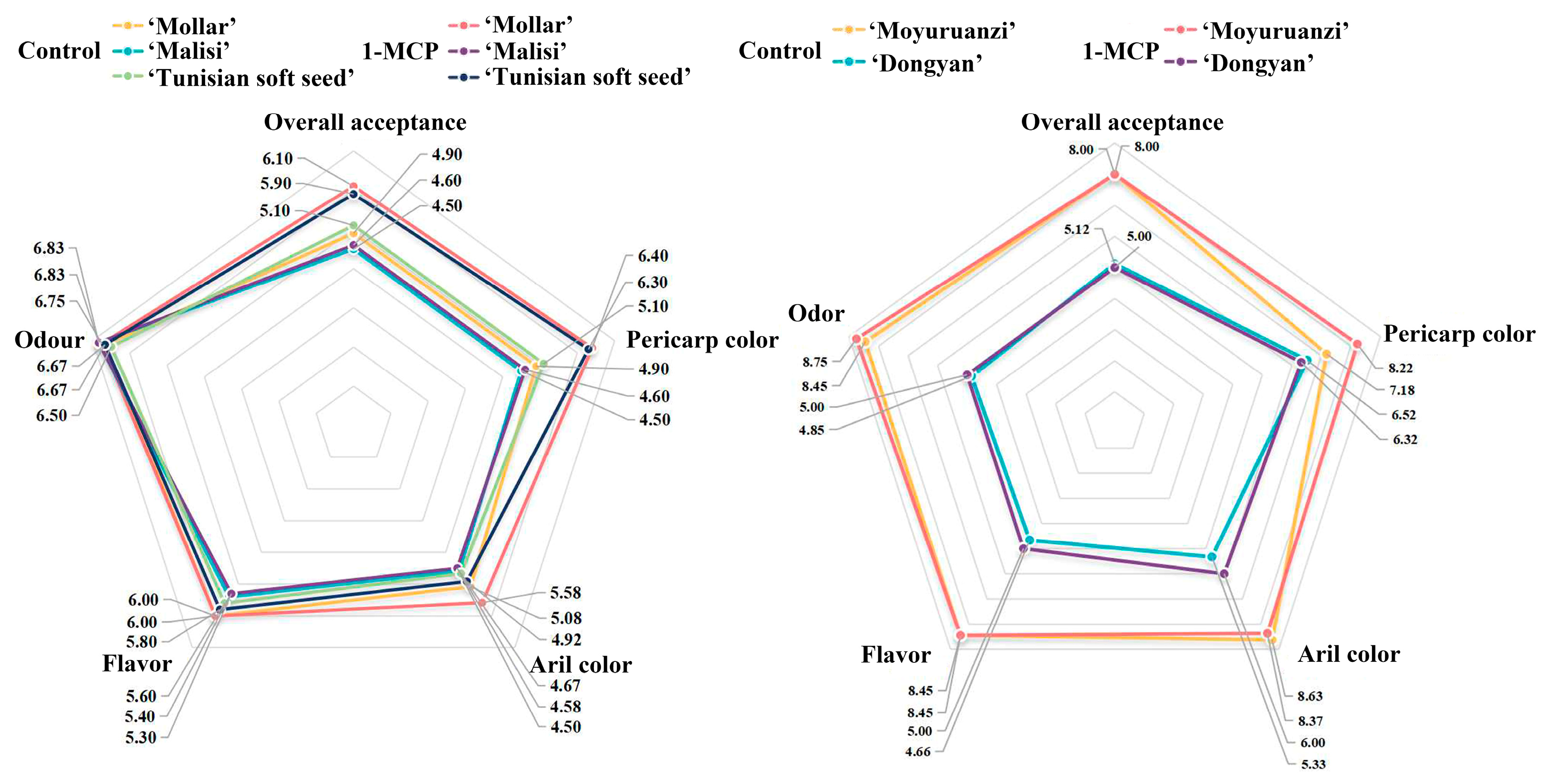
3.4. Effects of 1–MCP Treatment on Sensory Quality
4. Discussion
4.1. Peel Browning
4.2. Weight Loss
4.3. Fruit Quality
4.4. Respiration and Ethylene Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, Z.H.; Fang, Y.M.; Zhang, T.K.; Fei, Z.; Han, F.; Liu, C.; Liu, M.; Xiao, W.; Zhang, W.; Wu, S.; et al. The pomegranate (Punica granatum L.) genome provides insights into fruit quality and ovule developmental biology. Plant Biotechnol. J. 2018, 16, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Gbinigie, O.A.; Onakpoya, I.J.; Spencer, E.A. Evidence for the effectiveness of pomegranate supplementation for blood pressure management is weak: A systematic review of randomized clinical trials. Nutr. Res. 2017, 46, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo-Sánchez, P.; Núñez-Gómez, D.; Martínez-Nicolás, J.J.; Hernández, F.; Legua, P.; Melgarejo, P. Pomegranate variety and pomegranate plant part, relevance from bioactive point of view: A review. Bioresour. Bioprocess. 2021, 8, 2. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Opara, U.L.; Witthuhn, C.R. Modeling the effect of time and temperature on respiration rate of pomegranate arils (cv. ‘Acco’ and ‘Herskawitz’). J. Food Sci. 2012, 77, E80–E87. [Google Scholar] [CrossRef] [PubMed]
- Valdenegro, M.; Huidobro, C.; Monsalve, L.; Bernales, M.; Fuentes, L.; Simpson, R. Effects of ethrel, 1–MCP and modified atmosphere packaging on the quality of ‘Wonderful’ pomegranates during cold storage. J. Sci. Food Agric. 2018, 98, 4854–4865. [Google Scholar] [CrossRef]
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2379. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. The contribution of transpiration and respiration processes in the mass loss of pomegranate fruit (cv. Wonderful). Postharvest Biol. Technol. 2019, 157, 110982. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, X.; Du, J.; Guo, Y.; Guo, X.; Kou, L. Comparative analysis of husk microstructure, fruit quality and concentrations of bioactive compounds of different pomegranate cultivars during low temperature storage. Food Biosci. 2023, 52, 102400. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Mahajan, P.V.; Opara, U.L. Response of pomegranate arils (cv. ‘Wonderful’) to low oxygen stress under active modified atmosphere condition. J. Sci. Food Agric. 2019, 99, 1088–1097. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, S.; Wu, W.; Tong, R.; Wang, S.; Li, M.; Jian, Z.; Wan, R.; Hu, Q.; et al. Exogenous arginine treatment maintains the appearance and nutraceutical properties of hard- and soft-seed pomegranates in cold storage. Front. Nutr. 2022, 9, 828946. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Wan, R.; Jian, Z.; Hu, Q. Development status and suggestions of soft seed pomegranate industry in China. Deciduous Fruits 2020, 52, 1–4+79–80. [Google Scholar] [CrossRef]
- Guo, X.; Wu, X. Selection and breeding of ‘Shaanxi Dazi’, a new variety of pomegranate with large fruit and large grain. China Fruits 2010, 04, 14–15+22+79. [Google Scholar] [CrossRef]
- Xu, Z. Early high-yield cultivation technique of ‘Yudazi’ pomegranate. Mod. Agric. Res. 2011, 06, 25. [Google Scholar] [CrossRef]
- Yao, F.; Cao, S.; Wu, G.; Li, H.; Ma, G.; Zhao, Z. Breeding of a new soft-seeded pomegranate variety ‘Malisi’. North. Hortic. 2019, 13, 172+178–180. [Google Scholar]
- Dias, C.; Ribeiro, T.; Rodrigues, A.C.; Ferrante, A.; Vasconcelos, M.W.; Pintado, M. Improving the ripening process after 1–MCP application: Implications and strategies. Trends Food Sci. Technol. 2021, 113, 382–396. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. Recent advances in detecting and regulating ethylene concentrations for shelf-life extension and maturity control of fruit: A review. Trends Food Sci. Technol. 2019, 91, 66–82. [Google Scholar] [CrossRef]
- Botondi, R.; De Sanctis, F.; Bartoloni, S.; Mencarelli, F. Simultaneous application of ethylene and 1–MCP affects banana ripening features during storage. J. Sci. Food Agric. 2014, 94, 2170–2178. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.-G.; Kang, I.-K. 1–Methylcyclopropene (1–MCP) treatment delays modification of cell wall pectin and fruit softening in ‘Hwangok’ and ‘Picnic’ apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Li, L.; Kaplunov, T.; Zutahy, Y.; Daus, A.; Porat, R.; Lichter, A. The effects of 1-methylcyclopropane and ethylene on postharvest rachis browning in table grapes. Postharvest Biol. Technol. 2015, 107, 16–22. [Google Scholar] [CrossRef]
- Si, W.; Li, K.; Jiang, Y.; Zi, W.; Kai, M.; Sha, H.; Yi, G.; Xin, L. Effect of 1–MCP treatment on quality of winter jujube during low-temperature storage. Food Res. Dev. 2021, 42, 64–70. [Google Scholar] [CrossRef]
- De Reuck, K.; Sivakumar, D.; Korsten, L. Integrated application of 1–methylcyclopropene and modified atmosphere packaging to improve quality retention of litchi cultivars during storage. Postharvest Biol. Technol. 2009, 52, 71–77. [Google Scholar] [CrossRef]
- Aguayo, E.; Jansasithorn, R.; Kader, A.A. Combined effects of 1–methylcyclopropene, calcium chloride dip, and/or atmospheric modification on quality changes in fresh-cut strawberries. Postharvest Biol. Technol. 2006, 40, 269–278. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Chalupowicz, D.; Gamrasni, D.; Goldberg, T.; Nerya, O.; Ben-Arie, R.; Porat, R. Effects of the ethylene-action inhibitor 1–methylcyclopropene on postharvest quality of non-climacteric fruit crops. Postharvest Biol. Technol. 2016, 111, 322–329. [Google Scholar] [CrossRef]
- Gamrasni, D.; Gadban, H.; Tsvilling, A.; Goldberg, T.; Neria, O.; Ben-Arie, R.; Wolff, T.; Stern, Y.J. 1–MCP improves the quality of stored ‘Wonderful’ pomegranates. Acta Hortic. 2015, 1079, 229–234. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, R.; Liang, Q.; Guo, X.; Yao, G.; Li, Y.; Zhang, Y. Effects of low temperature combined with 1–MCP on postharvest quality of Tunisia soft-seed pomegranate. Food Ferment. Ind. 2021, 47, 147–155. [Google Scholar] [CrossRef]
- Wan, R.; Song, J.; Lv, Z.; Qi, X.; Han, X.; Guo, Q.; Wang, S.; Shi, J.; Jian, Z.; Hu, Q.; et al. Genome-wide identification and comprehensive analysis of the AP2/ERF gene family in pomegranate fruit development and postharvest preservation. Genes 2022, 13, 895. [Google Scholar] [CrossRef]
- Guo, Q. Effect of 1–MCP and Potassium Permanganate on Storage and Aging Mechanism of ‘Tunisian Soft-Seeded’ Pomegranate. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2019. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Li, L.; Li, Y. Effects of 1–MCP on peel browning of pomegranates. Acta Hortic. 2008, 774, 275–282. [Google Scholar] [CrossRef]
- Adams-Phillips, L.; Barry, C.; Giovannoni, J. Signal transduction systems regulating fruit ripening. Trends Plant Sci. 2004, 9, 331–338. [Google Scholar] [CrossRef]
- Matityahu, I.; Marciano, P.; Holland, D.; Ben-Arie, R.; Amir, R. Differential effects of regular and controlled atmosphere storage on the quality of three cultivars of pomegranate (Punica granatum L.). Postharvest Biol. Technol. 2016, 115, 132–141. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, H.; Wang, S.; Liu, X.; Hu, Q.; Jian, Z.; Wan, R.; Song, J.; Shi, J. Comprehensive evaluation of 20 pomegranate (Punica granatum L.) cultivars in China. J. Integr. Agric. 2022, 21, 434–445. [Google Scholar] [CrossRef]
- Shi, J.; Wang, S.; Tong, R.; Wang, S.; Chen, Y.; Wu, W.; He, F.; Wan, R.; Jian, Z.; Hu, Q.; et al. Widely targeted secondary metabolomics explored pomegranate aril browning during cold storage. Postharvest Biol. Technol. 2022, 186, 111839. [Google Scholar] [CrossRef]
- Osondu, H.A.A.; Akinola, S.A.; Shoko, T.; Pillai, S.K.; Sivakumar, D. Coating properties, resistance response, molecular mechanisms and anthracnose decay reduction in green skin avocado fruit (‘Fuerte’) coated with chitosan hydrochloride loaded with functional compounds. Postharvest Biol. Technol. 2022, 186, 111812. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, X. Pomegranate storage preservation technology. Northwest Hortic. 2017, 03, 14–15. [Google Scholar]
- Maghoumi, M.; Amodio, M.L.; Fatchurrahman, D.; Cisneros-Zevallos, L.; Colelli, G. Pomegranate husk scald browning during Storage: A review on factors involved, their modes of action, and its association to postharvest treatments. Foods 2022, 11, 3365. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Kaplan, Y.; Ginzberg, I. Mitigating chilling injury of pomegranate fruit skin. Sci. Hortic. 2022, 304, 111329. [Google Scholar] [CrossRef]
- Qi, X.; Zhao, J.; Jia, Z.; Cao, Z.; Liu, C.; Li, J.; Su, Y.; Pan, Y.; He, C.; Xu, Y.; et al. Potential metabolic pathways and related processes involved in pericarp browning for postharvest pomegranate fruits. Horticulturae 2022, 8, 924. [Google Scholar] [CrossRef]
- Valdenegro, M.; Fuentes, L.; Bernales, M.; Huidobro, C.; Monsalve, L.; Hernández, I.; Schelle, M.; Simpson, R. Antioxidant and fatty acid changes in pomegranate peel with induced chilling injury and browning by ethylene during long storage times. Front. Plant Sci. 2022, 13, 771094. [Google Scholar] [CrossRef]
- Kawhena, T.G.; Opara, U.L.; Fawole, O.A. Effects of gum arabic coatings enriched with lemongrass essential oil and pomegranate peel extract on quality maintenance of pomegranate whole fruit and arils. Foods 2022, 11, 593. [Google Scholar] [CrossRef]
- Lei, Y.; Niu, H.; Yun, Y.; Tian, J.; Lao, F.; Liao, X.; Gao, Z.; Ren, D.; Zhou, L. Analysis of coloration characteristics of Tunisian soft-seed pomegranate arils based on transcriptome and metabolome. Food Chem. 2022, 370, 131270. [Google Scholar] [CrossRef]
- Maghoumi, M.; Fatchurrahman, D.; Amodio, M.L.; Quinto, M.; Cisneros-Zevallos, L.; Colelli, G. Is pomegranate husk scald during storage induced by water loss and mediated by ABA signaling? J. Sci. Food Agric. 2023, 103, 2914–2925. [Google Scholar] [CrossRef]
- Ali, H.M.; Almagribi, W.; Al-Rashidi, M.N. Antiradical and reductant activities of anthocyanidins and anthocyanins, structure-activity relationship and synthesis. Food Chem. 2016, 194, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jiang, Y.; Duan, X.; Liu, H.; Li, Y.; Lin, W.; Zheng, Y. Effects of pure oxygen on the rate of skin browning and energy status in longan fruit. J. Food Technol. Biotechnol. 2005, 43, 359–365. [Google Scholar]
- Lin, Y.; Lin, Y.; Chen, Y.; Wang, H.; Shi, J.; Lin, H. Hydrogen peroxide induced changes in energy status and respiration metabolism of harvested longan fruit in relation to rericarp browning. J. Agric. Food Chem. 2016, 64, 4627–4632. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.; Zheng, Q.; En, Y.; Fu, W.; Hong, Q.; Yue, J. Respiratory activity and energy metabolism of harvested litchi fruit and their relationship to quality deterioration. J. Fruit Sci. 2010, 27, 946–951. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, R.; Song, J.; Lv, Z.; Qi, X.; Feng, Z.; Yang, Z.; Cao, X.; Shi, J.; Jian, Z.; Tong, R.; et al. Effects of 1–MCP Treatment on Postharvest Fruit of Five Pomegranate Varieties during Low-Temperature Storage. Horticulturae 2023, 9, 1031. https://doi.org/10.3390/horticulturae9091031
Wan R, Song J, Lv Z, Qi X, Feng Z, Yang Z, Cao X, Shi J, Jian Z, Tong R, et al. Effects of 1–MCP Treatment on Postharvest Fruit of Five Pomegranate Varieties during Low-Temperature Storage. Horticulturae. 2023; 9(9):1031. https://doi.org/10.3390/horticulturae9091031
Chicago/Turabian StyleWan, Ran, Jinhui Song, Zhenyang Lv, Xingcheng Qi, Zhiliang Feng, Zhenfeng Yang, Xinyue Cao, Jiangli Shi, Zaihai Jian, Ruiran Tong, and et al. 2023. "Effects of 1–MCP Treatment on Postharvest Fruit of Five Pomegranate Varieties during Low-Temperature Storage" Horticulturae 9, no. 9: 1031. https://doi.org/10.3390/horticulturae9091031




