Nuclear Magnetic Resonance, a Powerful Tool in Cultural Heritage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cleaning Process of Cultural Heritage Studied by Single-Sided NMR
- Natural and synthetic silicates, i.e., clays such as sepiolite and attapulgite.
- Cellulosic and wooden materials, i.e., cotton fibers, paper pulp, wooden pulp.
- Gelling materials, i.e., natural polysaccharide-based gels such as agarose and agaropectine, Gellan gum, Xanthan gum, etc.
2.2. Detection of Moisture in Historical Walls and Wall Paintings
2.3. Protective and Consolidating Treatment on Lapideous Material Studied by Single-Sided NMR
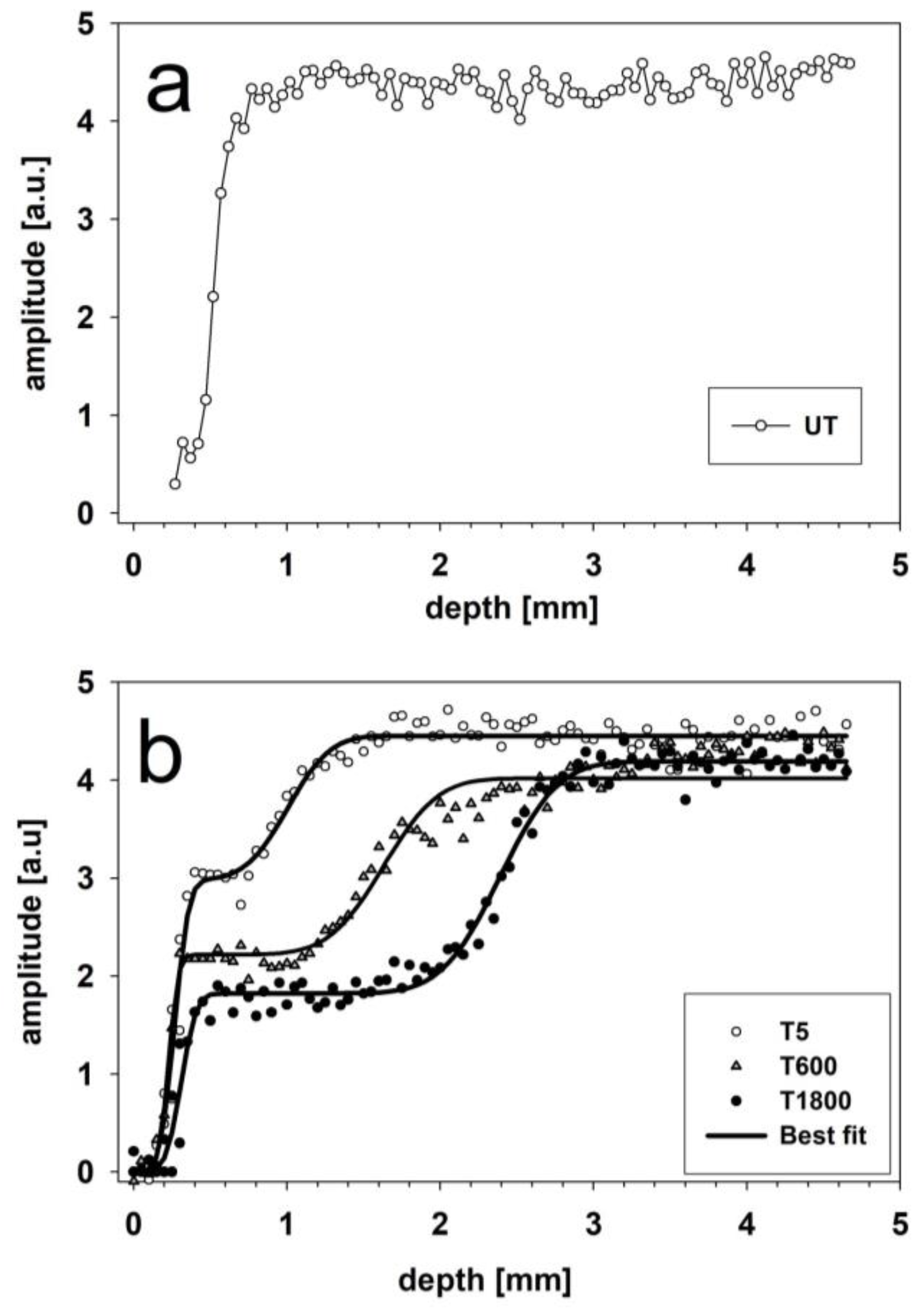
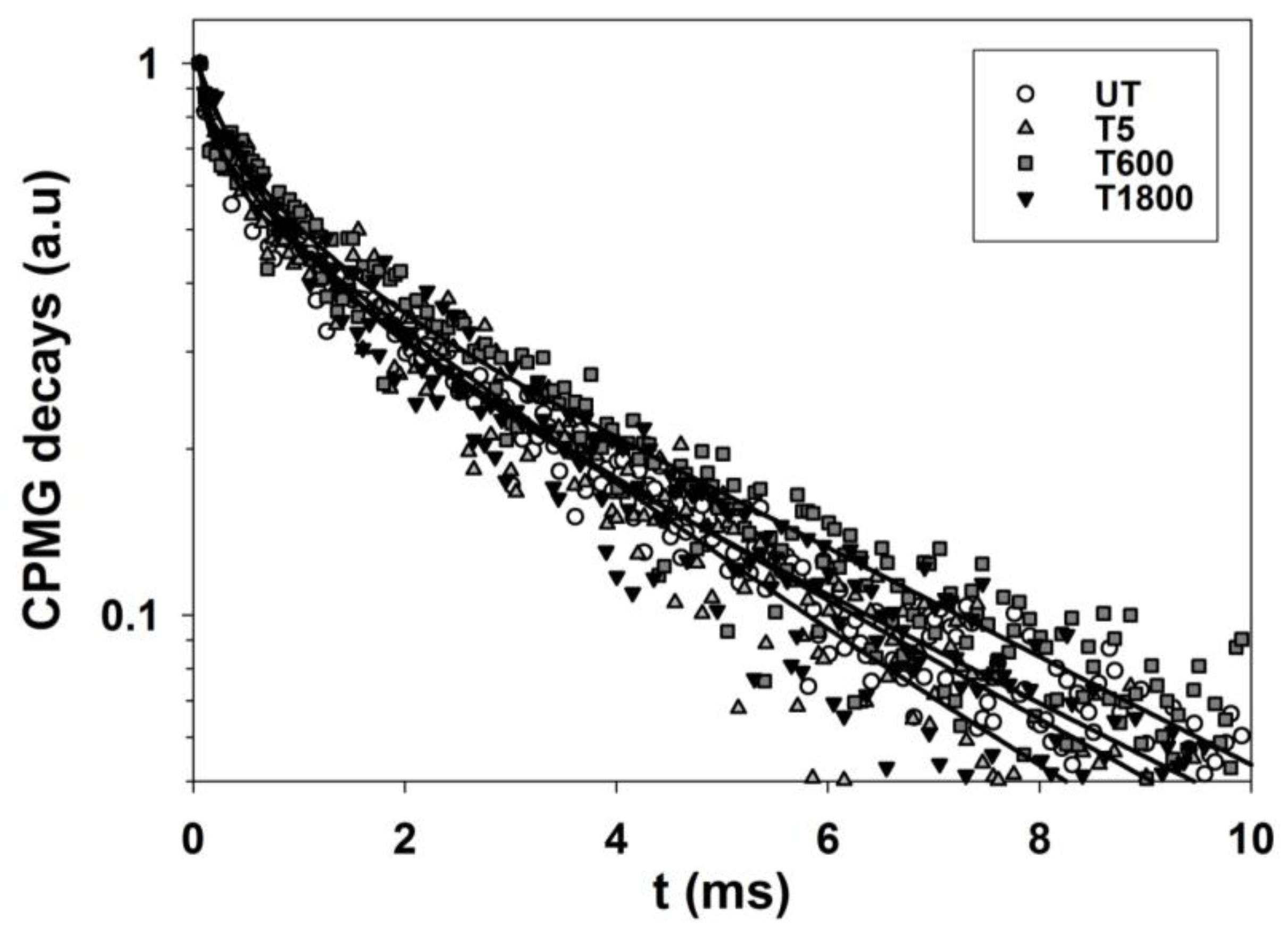
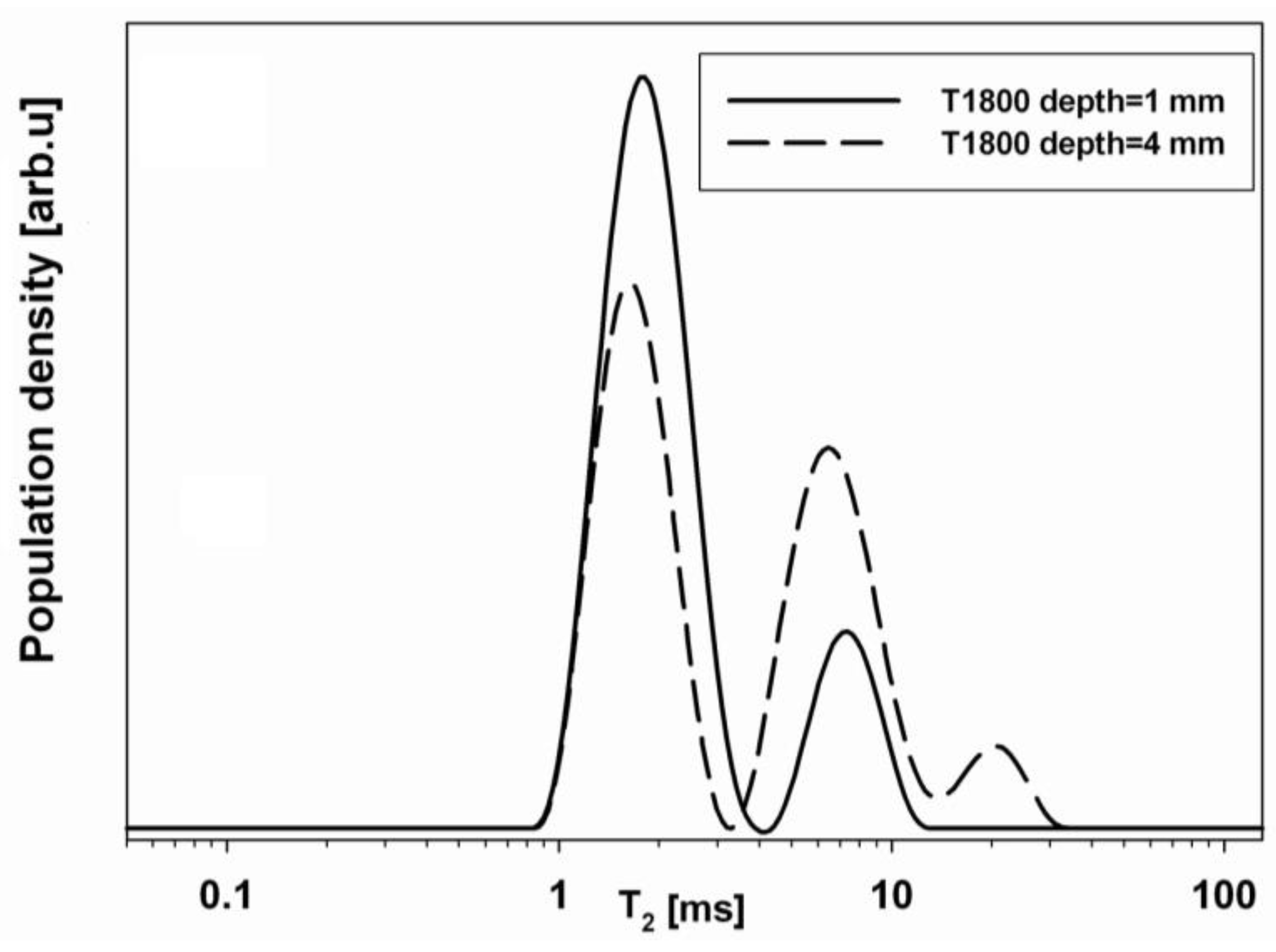
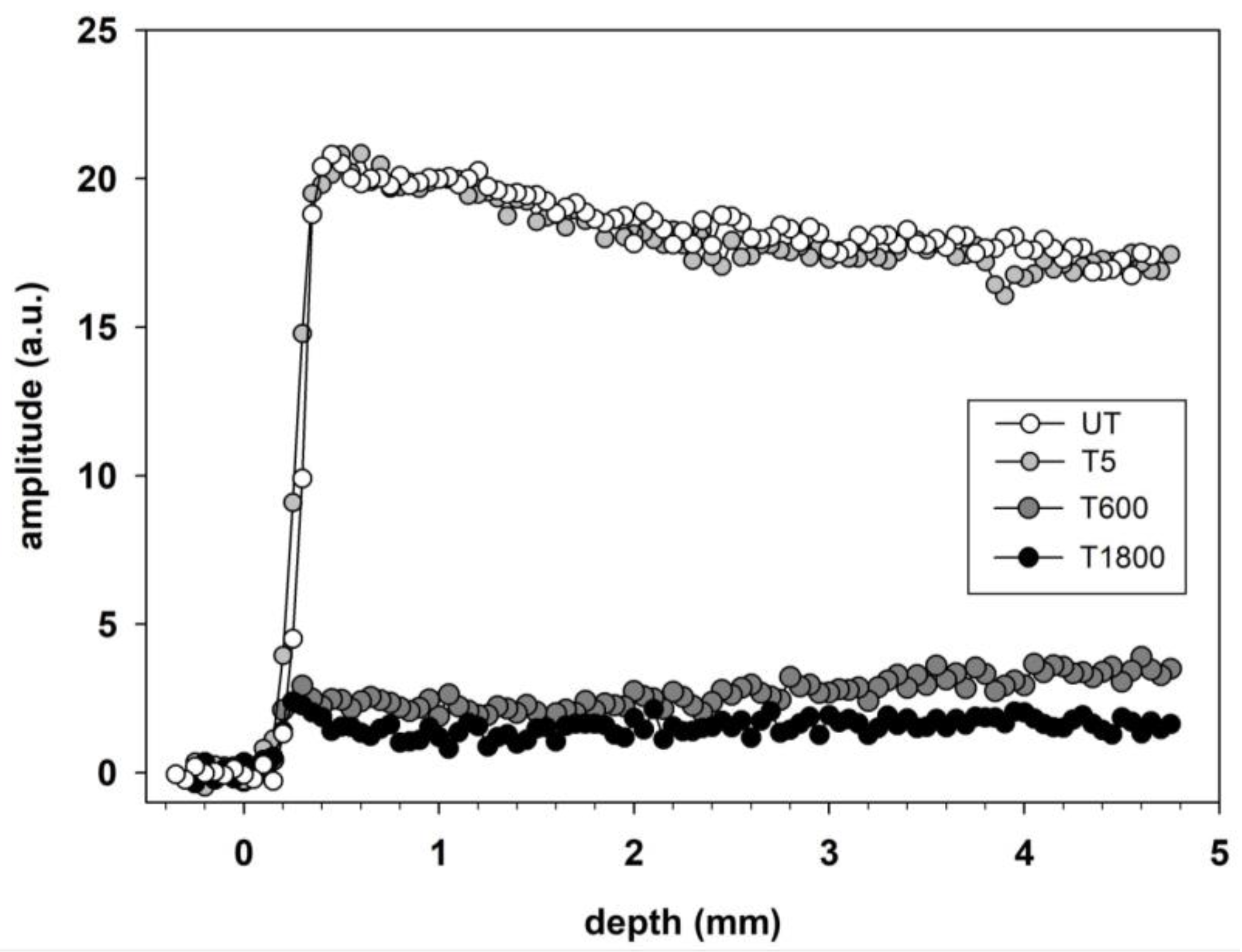
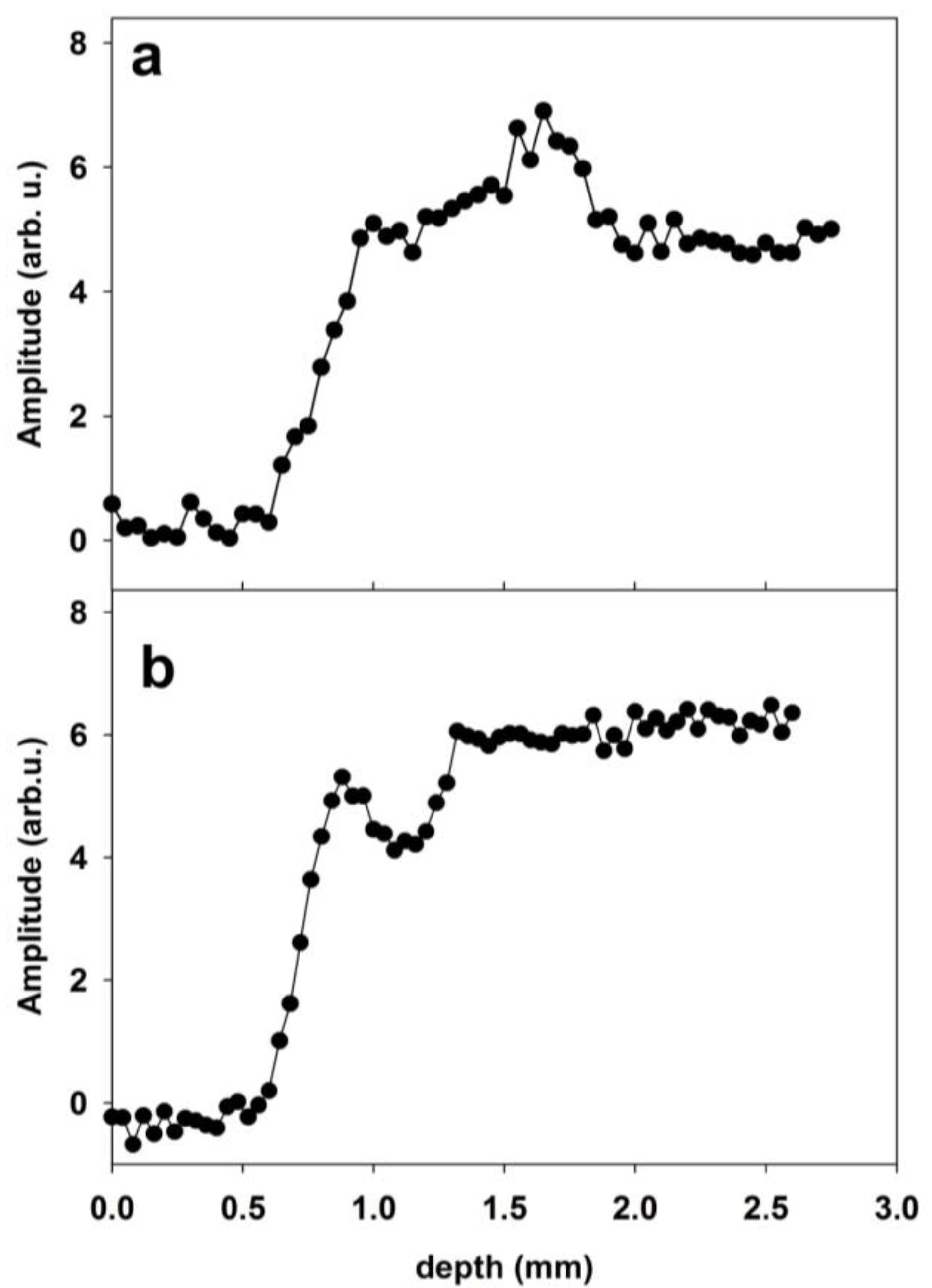
2.3.1. Sandstone Specimens Treated with Hydrophobic Treatments
2.3.2. An Unsuitable Consolidative Treatment on Calcarenite Studied by Single-Sided NMR
2.4. NMR Stratigraphy of Paintings
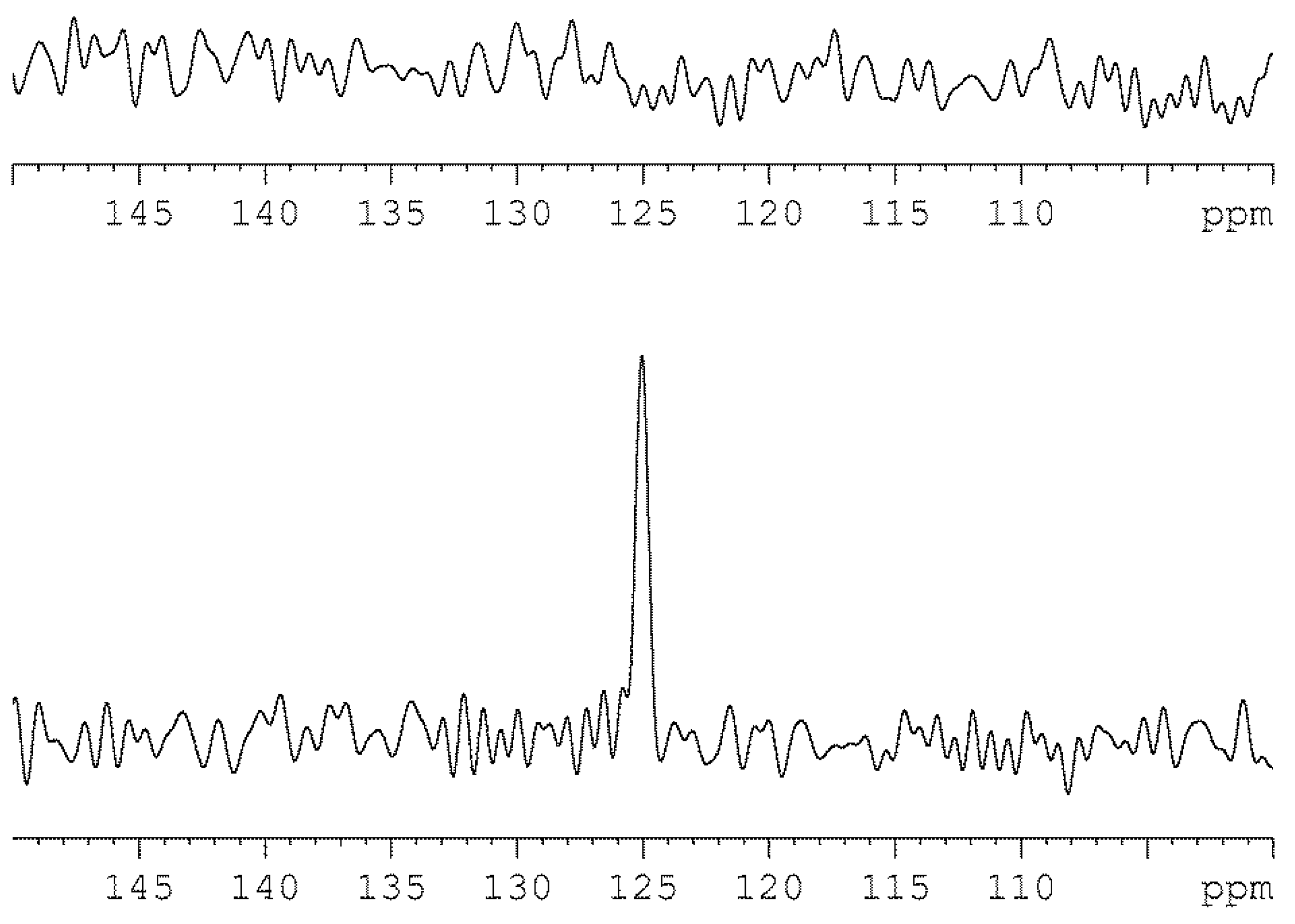
2.5. Detection of CO2 in Lapis Lazuli by 13C MAS NMR
3. Materials and Methods
3.1. Gel Preparation and Application to Wall Painting Specimens
3.2. Measurements on Hydrogel-Based Cleaning Systems
3.3. Moisture Measurements
3.4. Protective and Consolidating Treatment on Lapideous Material
3.4.1. Materials
3.4.2. Treatments Application
3.5. Depth Profiles and Transverse Relaxation Times Collected on Sandstone and Calcarenite
Processing of Depth Profiles
3.6. 1H NMR Stratigraphy of an Ancient Icon
3.7. Measurements on Lapis Lazuli
4. Conclusions
Conflicts of Interest
References
- Ernst, R.R.; Bodenhausen, G.; Wokaun, A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Schmidt-Rohr, K.; Spiess, H.W. Multidimensional Solid-State NMR and Polymers; Academic Press: London, UK, 1994. [Google Scholar]
- MacKenzie, K.J.D.; Smith, M.E. Multinuclear Solid-State NMR of Inorganic Materials; Pergamon Materials Series 6; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Spraul, M.; Feund, A.S.; Nast, R.E.; Withers, R.S.; Maas, W.E.; Corcoran, O. Advancing NMR sensitivity for LC-NMR-MS using a cryoflow probe: Application to the analysis of acetaminophen in urine. Anal. Chem. 2003, 75, 1546–1551. [Google Scholar] [CrossRef]
- Schlotterbeck, G.; Ross, A.; Hochstrasser, R.; Senn, H.; Kühn, T.; Marek, D.; Schett, O. High-resolution capillary tube NMR. A miniaturized 5-μL highsensitivity TXI Probe for mass-limited samples, off-line LC NMR, and HT NMR. Anal. Chem. 2002, 74, 4464–4471. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.M.; Jenkins, J.E. HR-MAS NMR Spectroscopy in Material Science. In Advanced Aspect of Spectroscopy; InTech: Rijeka, Croatia, 2012; pp. 279–306. [Google Scholar]
- Blümich, B.; Perlo, J.; Casanova, F. Mobile single-sided NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 52, 197–269. [Google Scholar] [CrossRef]
- Blümich, B.; Casanova, F.; Perlo, J.; Anferova, S.; Anferov, V.; Kremer, K.; Goga, N.; Kupferschläger, K.; Adams, M. Advances of unilateral mobile NMR in nondestructive materials testing. Magn. Reson. Imaging 2005, 23, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Blümich, B.; Anferova, S.; Kremer, K.; Sharma, S.; Herrmann, V.; Segre, A.L. Unilateral NMR for Quality Control: The NMR-MOUSE®. Spectroscopy 2003, 18, 18–32. [Google Scholar]
- Capitani, D.; Di Tullio, V.; Proietti, N. Nuclear Magnetic Resonance to characterize and monitor Cultural Heritage. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 64, 29–69. [Google Scholar] [CrossRef] [PubMed]
- Blümich, B.; Casanova, F.; Perlo, J.; Presciutti, F.; Anselmi, C.; Doherty, B. Noninvasive testing of art and cultural heritage by mobile NMR. Acc. Chem. Res. 2010, 43, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Baias, M. Mobile NMR: An essential tool for protecting our cultural heritage. Magn. Reson. Chem. 2017, 55, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Haber, A.; Blümich, B.; Souvorova, D.; Del Federico, E. Ancient Roman walls mapped non-destructively by portable NMR. Anal. Bioanal. Chem. 2011, 401, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Blümich, B.; Casanova, F.; Harber, A.; Del Federico, E.; Boardman, V.; Stiliano, A.; Wald, G.; Isolani, L. Non-Invasive Depth-Profiling of Walls by Portable Nuclear Magnetic Resonance. Anal. Bioanal. Chem. 2010, 397, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Fife, G.R.; Stabik, B.; Kelley, A.E.; King, J.N.; Blümich, B.; Hoppenbrouwers, R.; Meldrum, T. Characterization of aging and solvent treatments of painted surfaces using single-sided NMR. Magn. Reson. Chem. 2015, 53, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Presciutti, F.; Perlo, J.; Casanova, F.; Glöggler, S.; Miliani, C.; Blümich, B.; Brunetti, B.G.; Sgamellotti, A. Noninvasive nuclear magnetic resonance profiling of painting layers. Appl. Phys. Lett. 2008, 93, 033505-1–033505-3. [Google Scholar] [CrossRef]
- Proietti, N.; Di Tullio, V.; Presciutti, F.; Gentile, G.; Brunetti, B.G.; Capitani, D. A Multi-Analytical study of ancient nubian detached mural paintings. Microchem. J. 2016, 125, 719–725. [Google Scholar] [CrossRef]
- Di Tullio, V.; Capitani, D.; Atrei, A.; Benetti, F.; Perra, G.; Presciutti, F.; Proietti, N.; Marchettini, N. Advanced NMR methodologies and micro-analytical techniques to investigate the stratigraphy and materials of 14th century Sienese Wooden Paintings. Microchem. J. 2016, 125, 208–218. [Google Scholar] [CrossRef]
- Proietti, N.; Capitani, D.; Di Tullio, V. Applications of Nuclear Magnetic Resonance Sensors to Cultural Heritage. Sensor 2014, 14, 6977–6997. [Google Scholar] [CrossRef] [PubMed]
- Del Federico, E.; Centeno, S.; Kehlet, C.; Currier, P.; Jerschow, A. Unilateral NMR applied to the conservation of works of art. Anal. Bioanal. Chem. 2010, 396, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Lepore, A.; Baccaro, S.; Casieri, C.; Cemmi, A.; De Luca, F. Role of water in the ageing mechanism of paper. Chem. Phys. Lett. 2012, 531, 206–209. [Google Scholar] [CrossRef]
- Oligschläger, D.; Waldow, S.; Haber, A.; Zia, W.; Blümich, B. Moisture dynamics in wall paintings monitored by single-sided NMR. Magn. Reson. Chem. 2015, 53, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Di Tullio, V.; Proietti, N.; Gobbino, M.; Capitani, D.; Olmi, R.; Priori, S.; Riminesi, C.; Giani, E. Non-destructive mapping of dampness and salts in degraded wall paintings in hypogeous building: The case of St. Clement at mass fresco in St. Clement Basilica, Rome. Anal. Bioanal. Chem. 2010, 396, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Capitani, D.; Proietti, N.; Gobbino, M.; Soroldoni, L.; Casellato, U.; Valentini, M.; Rosina, E. An integrated study for mapping the moisture distribution in an ancient damaged wall painting. Anal. Bioanal. Chem. 2009, 395, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Catalano, J.; Yao, Y.; Murphy, A.; Zumbulyadis, N.; Centeno, S.A.; Dybowski, C. Nuclear magnetic resonance spectra and 207Pb chemical-shift tensors of lead carboxylates relevant to soap formation in oil paintings. Appl. Spectrosc. 2014, 68, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Catalano, J.; Murphy, A.; Yao, Y.; Alkan, F.; Zumbulyadis, N.; Centeno, S.A.; Dybowski, C. 207Pb and 119Sn Solid-State NMR and Relativistic Density Functional Theory Studies of the Historic Pigment Lead–Tin Yellow Type I and Its Reactivity in Oil Paintings. J. Phys. Chem. A 2014, 18, 7952–7958. [Google Scholar] [CrossRef] [PubMed]
- Del Federico, E.; Centeno, S.A.; Kehlet, C.; Ulrich, K.; Yamazaki-Kleps, A.; Jerschow, A. In Situ Unilateral 1H-NMR Studies of the Interaction Between Lead White Pigments and Collagen-Based Binders. Appl. Magn. Reson. 2012, 42, 363–376. [Google Scholar] [CrossRef]
- Kobayashi, T.; Perras, F.A.; Murphy, A.; Yao, Y.; Catalano, J.; Centeno, S.A.; Dybowski, C.; Zumbulyadis, N.; Pruski, M. DNP-enhanced ultrawideline 207Pb solid-state NMR spectroscopy: An application to cultural heritage science. Dalton Trans. 2017, 46, 3535–3540. [Google Scholar] [CrossRef] [PubMed]
- Del Federico, E.; Schoefberger, W.; Jerschow, A.; Tyne, L.; Schelvis, J.; Kapetanaki, S. Insight into Framework Destruction in Ultramarine Pigments. Inorg. Chem. 2006, 45, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.T.; Dybowski, C. Carbon-13 chemical-shift tensors in indigo: A two-dimensional NMR-ROCSA and DFT Study. Solid State Nucl. Magn. Reson. 2015, 7, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Spyros, A.; Anglos, D. Studies of organic paint binders by NMR spectroscopy. Appl. Phys. A 2006, 83, 705–708. [Google Scholar] [CrossRef]
- Sfakianaki, S.; Kouloumpi, E.; Anglos, D.; Spyros, A. Egg yolk identification and aging in mixed paint binding media by NMR spectroscopy. Magn. Reson. Chem. 2015, 53, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, G.; Knuutinen, U.; Laitinen, K.; Spyros, A. Analysis and aging of unsaturated polyester resins in contemporary art installations by NMR spectroscopy. Anal. Bioanal. Chem. 2010, 398, 3203–3214. [Google Scholar] [CrossRef] [PubMed]
- Proietti, N.; Di Tullio, V.; Capitani, D.; Tomassini, R.; Guiso, M. Nuclear Magnetic Resonance in contemporary art: The case of Moon Surface by Turcato. Appl. Phys. A Mater. Sci. Process. 2013, 113, 1009–1017. [Google Scholar] [CrossRef]
- Ulrich, K.; Centeno, S.A.; Arslanoglu, J.; Del Federico, E. Absorption and diffusion measurements of water in acrylic paint films by single-sided NMR. Prog. Organ. Coat. 2011, 71, 283–289. [Google Scholar] [CrossRef]
- Bardet, M.; Pournou, A. Fossil wood from the Miocene and Oligocene epoch: Chemistry and morphology. Magn. Reson. Chem. 2015, 53, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bardet, M.; Pournou, A. NMR Studies of Fossilized Wood. Ann. Rep. NMR Spectrosc. 2017, 90, 41–83. [Google Scholar]
- Bardet, M.; Gerbaud, G.; Doan, C.; Giffard, M.; Hediger, S.; De Paëpe, G.; Trân, Q.K. Dynamics property recovery of archaeological-wood fibers treated with polyethylene glycol demonstrated by high-resolution solid-sate NMR. Cellulose 2012, 19, 1537–1545. [Google Scholar] [CrossRef]
- Bardet, M.; Gerbaud, G.; Giffard, M.; Doan, C.; Hediger, S.; Le Pape, L. 13C high-resolution solid-sate NMR for structural elucidation of archaeological woods. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 55, 199–224. [Google Scholar] [CrossRef]
- Bardet, M.; Gerbaud, G.; Le Pape, L.; Hediger, S.; Trân, Q.K.; Boumlil, N. NMR and EPR as Analytical Tools to Investigate Structural Features of Archaeological Leathers. Anal. Chem. 2009, 81, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Badea, E.; Şendrea, C.; Carşote, C.; Adams, A.; Blümich, B.; Iovu, H. Unilateral NMR and thermal microscopy studies of vegetable tanned leather exposed to dehydrothermal treatment and light irradiation. Microchem. J. 2016, 129, 158–165. [Google Scholar] [CrossRef]
- Lambert, J.B.; Santiago-Blay, J.A.; Wu, Y.; Allison, J. Levy Examination of amber and related materials by NMR spectroscopy. Magn. Reson. Chem. 2015, 53, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.B.; Tsai, C.Y.H.; Shah, M.C.; Hurtley, A.E.; Santiago-Blay, J.A. Distinguishing Amber And Copal Classes By Proton Magnetic Resonance Spectroscopy. Archaeometry 2012, 54, 332–348. [Google Scholar] [CrossRef]
- Casieri, C.; De Luca, F.; Nodari, L.; Russo, U.; Terenzi, C.; Tudisca, V. Effects of time and temperature of firing on Fe-rich ceramics studied by Mossbauer spectroscopy and two-dimensional H-1-nuclear magnetic resonance relaxometry. J. Appl. Phys. 2012, 112. [Google Scholar] [CrossRef]
- Casieri, C.; Terenzi, C.; De Luca, F. Two-dimensional longitudinal and transverse relaxation time correlation as a low-resolution nuclear magnetic resonance characterization of ancient ceramics. J. Appl. Phys. 2009, 105, 034901. [Google Scholar] [CrossRef]
- Terenzi, C.; Casieri, C.; De Luca, F.; Quaresima, R.; Quarta, G.; Tudisca, V. Firing-Induced Microstructural Properties of Quasi-Diamagnetic Carbonate-Based Porous Ceramics: A 1H NMR Relaxation Correlation Study. Appl. Magn. Reson. 2015, 46, 1159–1178. [Google Scholar] [CrossRef]
- Camaiti, M.; Fantazzini, P.; Bortolotti, V. Stone Porosity, wettability changes and other features detected by MRI and NMR relaxometry: A more than 15-year study. Magn. Reson. Chem. 2015, 53, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Di Tullio, V.; Cocca, M.; Avolio, R.; Gentile, G.; Proietti, N.; Ragni, P.; Errico, M.E.; Capitani, D.; Avella, M. Unilateral NMR investigation of multifunctional treatments on stones based on colloidal inorganic and organic nanoparticles. Magn. Reson. Chem. 2015, 53, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Brai, M.; Casieri, C.; De Luca, F.; Fantazzini, P.; Gombia, M.; Terenzi, C. Validity of NMR pore-size analysis of cultural heritage ancient building materials containing magnetic impurities. Solid State Nucl. Magn. Reson. 2007, 25, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Di Tullio, V.; Proietti, N.; Capitani, D.; Nicolini, I.; Mecchi, A.M. NMR depth profiles as a non-invasive analytical tool to probe the penetration depth of hydrophobic treatments and inhomogeneities in treated porous stones. Anal. Bioanal. Chem. 2011, 400, 3151–3164. [Google Scholar] [CrossRef] [PubMed]
- Presciutti, F.; Doherty, B.; Anselmi, C.; Brunetti, B.G.; Sgamellotti, A.; Miliani, C. A non-invasive NMR relaxometric characterization of the cyclododecane–solvent system insideporous substrates. Magn. Reson. Chem. 2015, 53, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Canevali, C.; Fasoli, M.; Bertasa, M.; Botteon, A.; Colombo, A.; Di Tullio, V.; Capitani, D.; Proietti, N.; Scalarone, D.; Sansonetti, A. A multi-analytical approach for the study of copper stain removal by agar gels. Microchem. J. 2016, 129, 249–258. [Google Scholar] [CrossRef]
- Di Tullio, V.; Capitani, D.; Proietti, N. Unilateral NMR to study water diffusion and absorption in stone-hydrogel systems. Microporous Mesoporous Mater. 2017. [Google Scholar] [CrossRef]
- Angelova, L.; Ormsby, B.; Richardson, E. Diffusion of water from a range of conservation treatment gels into paint films studied by unilateral NMR. Part I. Acrylic emulsion paint. Microchem. J. 2016, 124, 311–320. [Google Scholar] [CrossRef]
- Capitani, D.; Di Tullio, V.; Proietti, N. Advanced Nuclear Magnetic Resonance Methodologies in Cultural Heritage. In Cultural Heritage Protection, Developments and International Perspectives; Frediani, P., Frediani, M., Rosi, L., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Troiano, F.; Gulotta, D.; Balloi, A.; Polo, A.; Toniolo, L.; Lombardi, E.; Daffonchio, D.; Sorlin, C.; Cappitelli, F. Successful combination of chemical and biological treatments for the cleaning of stone artworks. Int. Biodeterior. Biodegrad. 2013, 85, 294–304. [Google Scholar] [CrossRef]
- Burnstock, A.; White, R. Cleaning gels: Further studies. In Conservation Science in the UK; Tennent, N.H., Ed.; James & James: London, UK, 1993; pp. 36–39. [Google Scholar]
- Gioventù, E.; Lorenzi, P.F.; Villa, F.; Sorlini, C.; Rizzi, M.; Cagnini, A.; Griffo, A.; Cappitelli, F. Comparing the bioremoval of black crusts on colored artistic lithotypes of the Cathedral of Florence with chemical and laser treatment. Int. Biodeterior. Biodegrad. 2011, 65, 832–839. [Google Scholar] [CrossRef]
- Gauri, K.L.; Bandyopadhyay, J.K. Carbonate Stone: Chemical Behaviour, Durability and Conservation; John Wiley & Sons: New York, NY, UK, 1999. [Google Scholar]
- Baglioni, M.; Giorgi, R.; Berti, D.; Baglioni, P. Smart cleaning of cultural heritage: A new challenge for soft nanoscience. Nanoscale 2012, 4, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Challagan, P.T. Principles of Nuclear Magnetic Resonance Microscopy; Clarendon Press: Oxford, UK, 1991. [Google Scholar]
- Homans, S.W. A Dictionary of Concepts in NMR; Clarendon Press: Oxford, UK; New York, NY, USA, 1992. [Google Scholar]
- Kimmich, R. NMR: Tomography, Diffusiometry, Relaxometry; Springer: Berlin, Germany, 1979. [Google Scholar]
- Rata, D.G.; Casanova, F.; Perlo, J.; Demco, D.E.; Blümich, B. Self-diffusion measurements by a mobile single-sided NMR sensor with improved magnetic field gradient. J. Magn. Reson. 2006, 180, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, V.G.G.; Fassina, V. Stone Decay and Conservation; Elsevier: Lausanne, Switzerland, 1983. [Google Scholar]
- Camuffo, D. Physical weathering of stones. Sci. Total Environ. 1995, 167, 1–14. [Google Scholar] [CrossRef]
- Di Zenzo, S. A Note on the Gradient of a Multi-Image. Comput. Vis. Graph. Image Process. 1986, 33, 116–125. [Google Scholar] [CrossRef]
- Couran, R.; Herbert, R.; Ian, S. What is Mathematics? An Elementary Approach to Ideas and Methods; Oxford University Press: New York, NY, USA, 1996; p. 344. [Google Scholar]
- Watson, A.T.; Chang, C.T. Characterizing porous media with NMR methods. Prog. Nucl. Magn. Reson. Spectrosc. 1997, 31, 343–386. [Google Scholar] [CrossRef]
- Ferreira Pinto, A.P.; Delgado Rodrigues, J. Stone consolidation: The role of treatment procedures. J. Cult. Heritage 2008, 9, 38–53. [Google Scholar] [CrossRef]
- Miliani, C.; Daveri, A.; Brunetti, B.G.; Sgamellotti, A. CO2 entrapment in natural ultramarine blue. Chem. Phys. Lett. 2008, 466, 148–151. [Google Scholar] [CrossRef]
- Omi, H.; Ueda, T.; Miyakubo, K.; Eguchi, T. Dynamics of CO2 molecules confined in the micropores of solids as studied by 13C NMR. Appl. Surf. Sci. 2005, 252, 660–667. [Google Scholar] [CrossRef]
- SefciK, M.D.; Schaefer, J.; Stejskal, E.O. Molecular Sieves II; Katzer, J.R., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1977; Volume 40. [Google Scholar]
- Perlo, J.; Casanova, F.; Blümich, B. Profiles with microscopic resolution by single-sided NMR. J. Magn. Reson. 2005, 176, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Perlo, J.; Casanova, F.; Blümich, B. Single-Sided NMR; Springer-Verlag: Berlin, Germany, 2011. [Google Scholar]
- Goelman, G.; Prammer, M.G. The CPMG pulse sequence in strong magnetic field gradients with applications to oil-well logging. J. Magn. Reson. 1995, 113, 11–18. [Google Scholar] [CrossRef]
- Hurlimann, M.D.; Griffin, D.D. Spin dynamics of Carr-Purcell-Meiboom-Gill-like sequences in grossly inhomogeneous B(0) and B(1) fields and application to NMR well logging. J. Magn. Reson. 2000, 143, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Belton, P.S. NMR and the mobility of water in polysaccharide gels. Int. J. Biol. Macromol. 1997, 21, 81–88. [Google Scholar] [CrossRef]
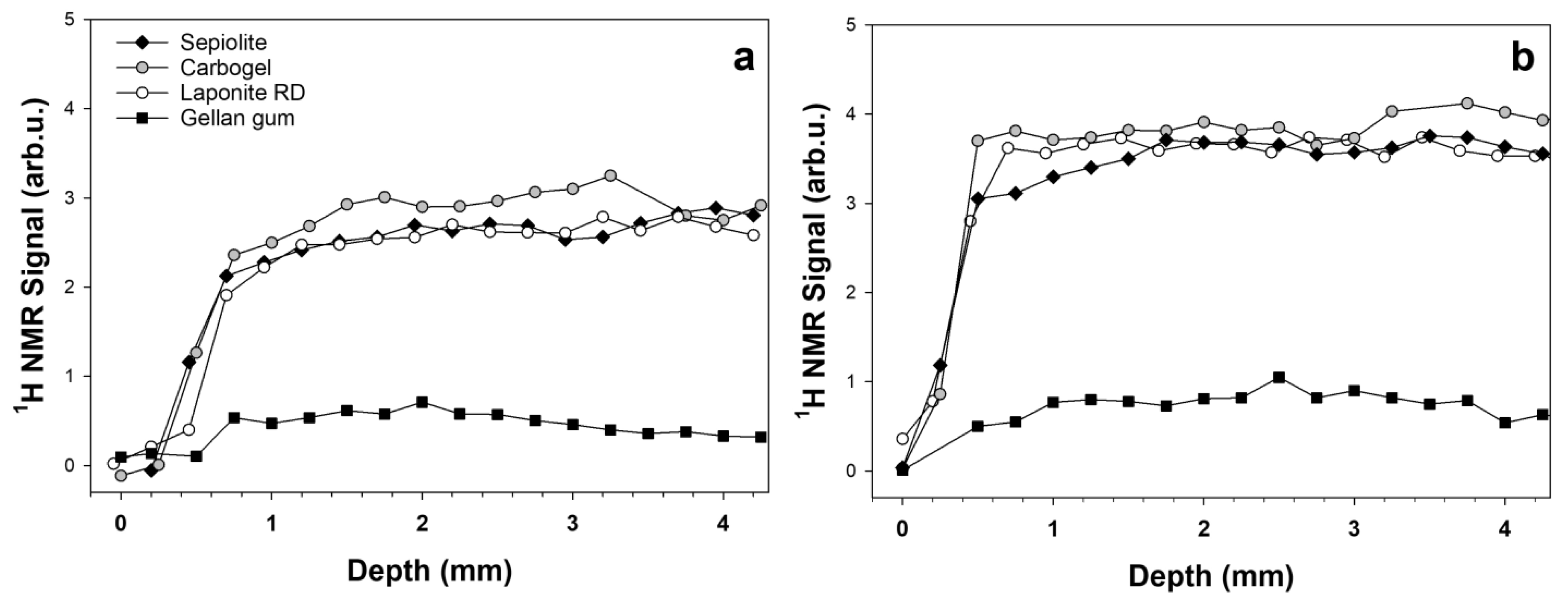
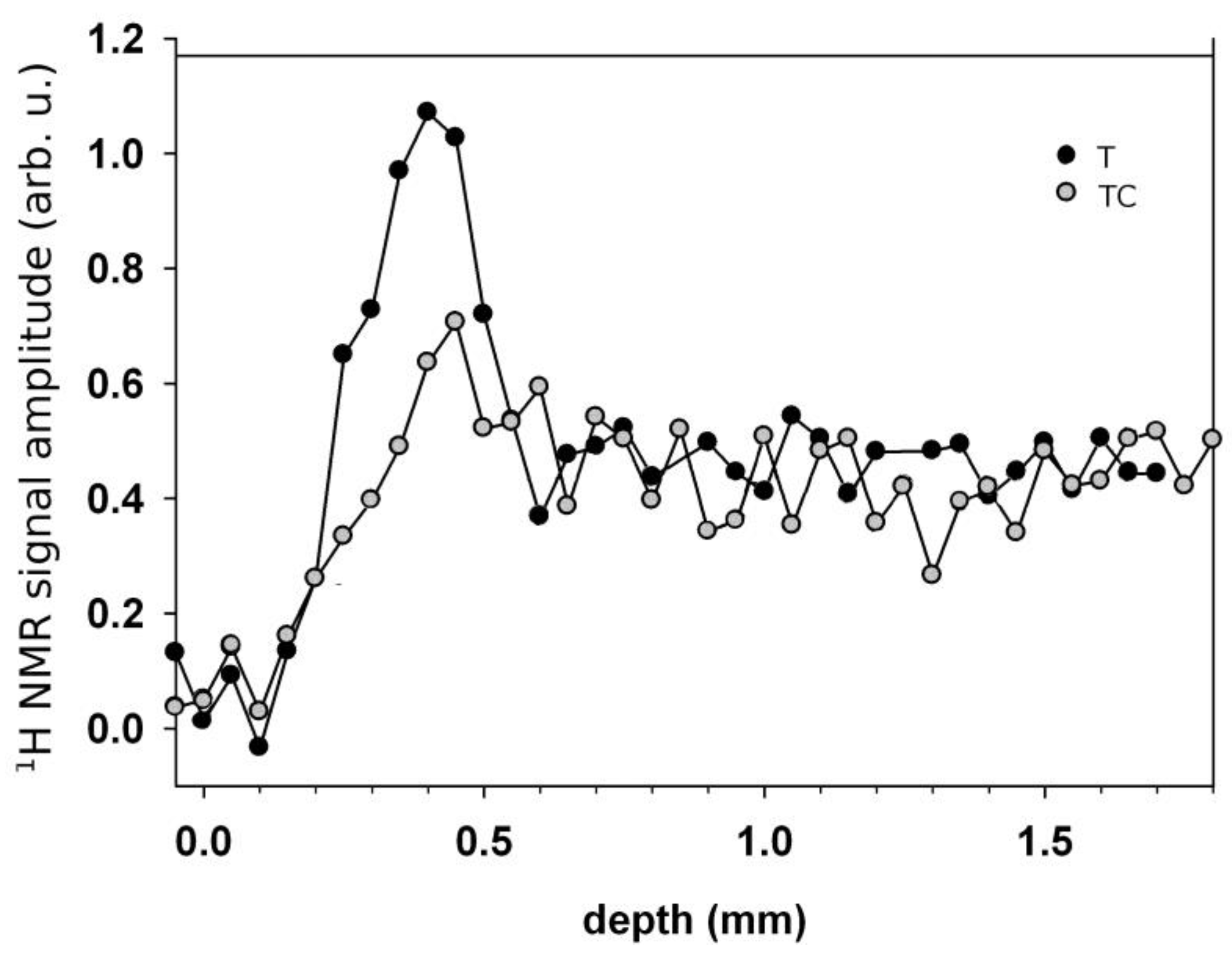

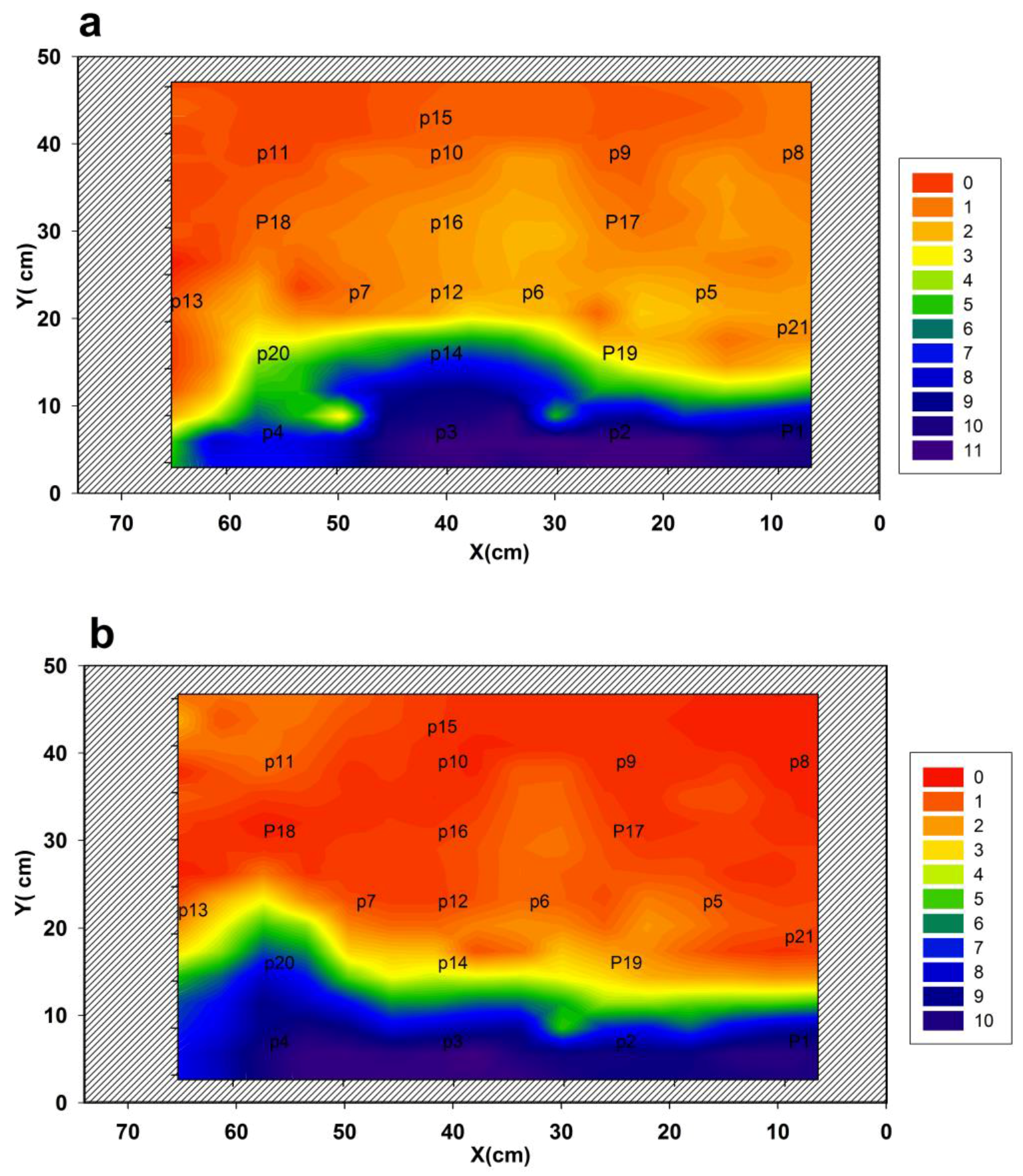
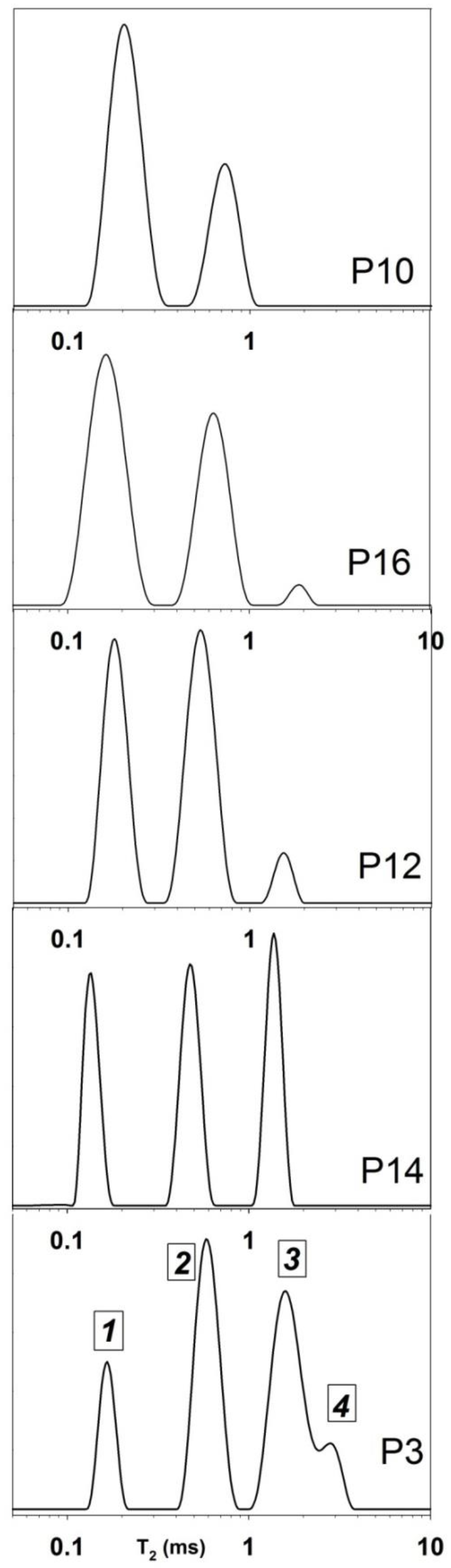

| Sample | T1 (s) | WA (%) | T2A (ms) | WB (%) | T2B (ms) | D × 10–9 m2/s |
|---|---|---|---|---|---|---|
| Carbogel 0.5% | 1.7 ± 0.1 | 10 | 6.4 ± 0.8 | 90 | 52.2 ± 0.4 | 2.05 ± 0.05 |
| Laponite RD 5% | 1.2 ± 0.1 | 9 | 4.7 ± 0.4 | 91 | 41.9 ± 0.1 | 1.89 ± 0.02 |
| Sepiolite 6% | 0.03 ± 0.01 | 29 | 3.4 ± 0.4 | 71 | 11.2 ± 0.1 | 1.75 ± 0.03 |
| Gellan 2% | 0.7 ± 0.1 | 5 | 5.6 ± 0.5 | 95 | 39.2 ± 0.1 | 1.97 ± 0.02 |
| (x,y) (cm) | Wa (%) | T2a (ms) | Wb (%) | T2b (ms) | Wc (%) | T2c (ms) | Wd (%) | T2d (ms) |
|---|---|---|---|---|---|---|---|---|
| P3 (40,7) | 14 | 0.17 | 38 | 0.60 | 40 | 1.63 | 8 | 2.85 |
| P14 (40,16) | 29 | 0.15 | 36 | 0.40 | 34 | 1.57 | -- | - |
| P12 (40,23) | 43 | 0.18 | 52 | 0.54 | 5 | 1.58 | -- | - |
| P16 (40,31) | 58 | 0.17 | 40 | 0.65 | 2 | 1.7 | -- | - |
| P10 (40,39) | 69 | 0.20 | 21 | 0.80 | -- | -- | -- | - |
| Specimen | x1 (mm) | Δ1 (mm) | w1 (arb.u.) | x2 (mm) | Δ2 (mm) | w2 (arb.u.) | R2 | b1 | b2 |
|---|---|---|---|---|---|---|---|---|---|
| T5 | 0.27 ± 0.01 | 0.073 ± 0.008 | 2.97 ± 0.09 | 1.00 ± 0.03 | 0.22 ± 0.04 | 1.48 ± 0.09 | 0.99 | 14.29 | 2.325 |
| T600 | 0.23 ± 0.01 | 0.05 ± 0.01 | 2.22 ± 0.06 | 1.63 ± 0.03 | 0.28 ± 0.04 | 1.80 ± 0.06 | 0.96 | 14.29 | 2.174 |
| T1800 | 0.31 ± 0.01 | 0.08 ± 0.01 | 1.82 ± 0.03 | 2.39 ± 0.02 | 0.30 ± 0.02 | 2.37 ± 0.04 | 0.99 | 8.33 | 2.703 |
| Specimen | Wa (%) | T2a (ms) | Wb (%) | T2b (ms) | Wc (%) | T2c (ms) |
|---|---|---|---|---|---|---|
| UT | 40 | 0.18 | 40 | 0.9 | 20 | 4.4 |
| T5 | 42 | 0.17 | 42 | 1.1 | 16 | 4.7 |
| T600 | 38 | 0.20 | 44 | 0.71 | 16 | 4.3 |
| T1800 | 31 | 0.20 | 44 | 0.93 | 25 | 4.1 |
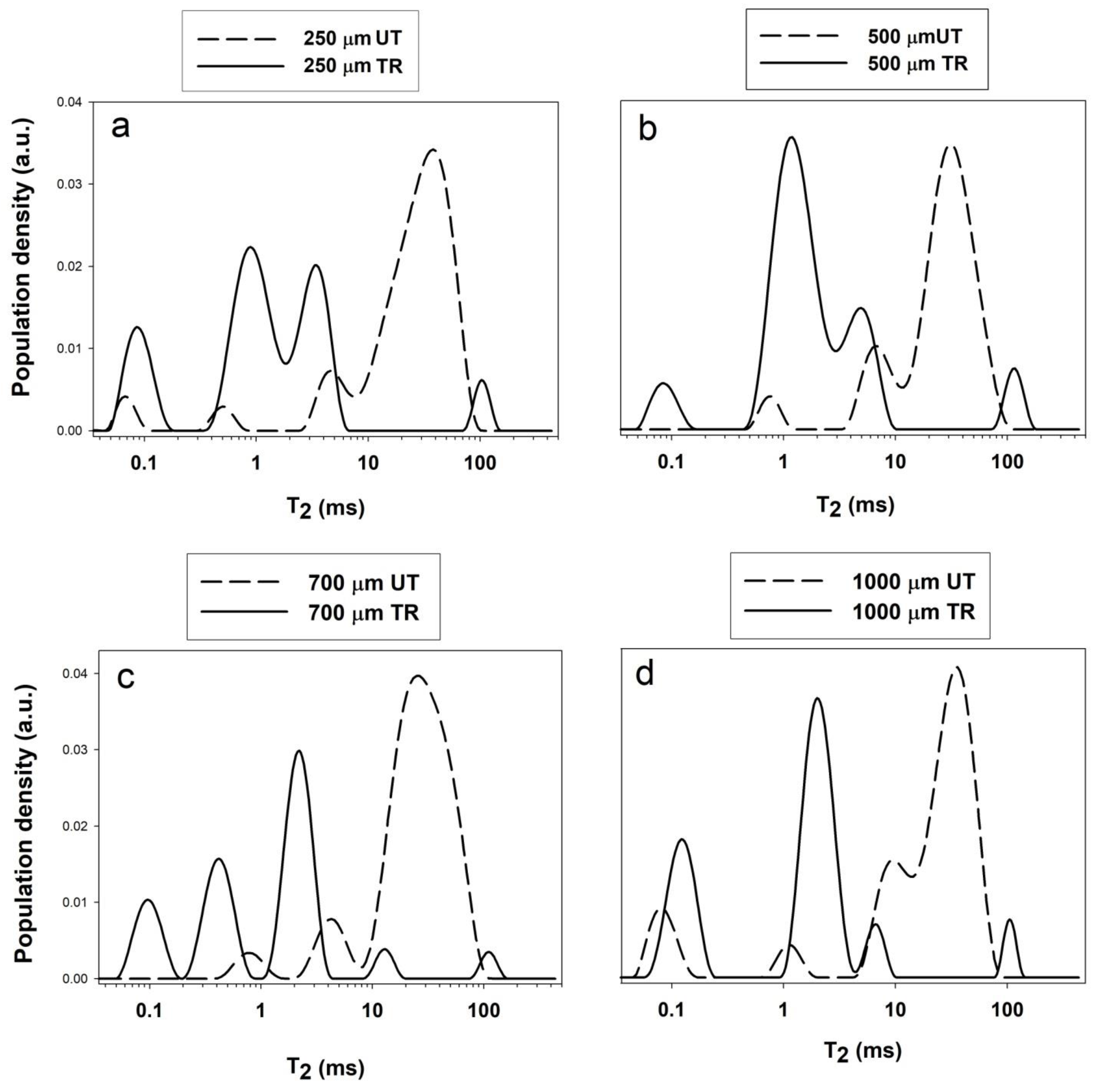
| Depth (μm) | Sample | Wa | T2a (ms) | Wb | T2b (ms) | Wc | T2c (ms) | Wd | T2d (ms) | We | T2e (ms) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 | UT | 3% | 0.070 | 2% | 0.52 | - | - | 14% | 12.172 | 81% | 40.1 |
| T600 | 14% | 0.099 | 46% | 0.94 | 35% | 3.32 | - | - | 5% | 106.0 | |
| 500 | UT | - | - | 4% | 0.81 | 17% | 6.91 | - | - | 89% | 33.00 |
| T600 | 6% | 0.090 | 64% | 1.25 | 24% | 5.15 | - | - | 6% | 122.6 | |
| 700 | UT | - | - | 4% | 0.85 | 10% | 4.91 | - | - | 86% | 22.3 |
| T600 | 16% | 0.100 | 27% | 0.43 | 48% | 2.27 | 5% | 13.1 | 4% | 115.2 | |
| 1000 | UT | 9% | 0.083 | 4% | 1.18 | - | - | 21% | 9.8 | 66% | 38.0 |
| T600 | 28% | 0.126 | 60% | 2.06 | 7% | 6.84 | - | - | 5% | 108.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proietti, N.; Capitani, D.; Di Tullio, V. Nuclear Magnetic Resonance, a Powerful Tool in Cultural Heritage. Magnetochemistry 2018, 4, 11. https://doi.org/10.3390/magnetochemistry4010011
Proietti N, Capitani D, Di Tullio V. Nuclear Magnetic Resonance, a Powerful Tool in Cultural Heritage. Magnetochemistry. 2018; 4(1):11. https://doi.org/10.3390/magnetochemistry4010011
Chicago/Turabian StyleProietti, Noemi, Donatella Capitani, and Valeria Di Tullio. 2018. "Nuclear Magnetic Resonance, a Powerful Tool in Cultural Heritage" Magnetochemistry 4, no. 1: 11. https://doi.org/10.3390/magnetochemistry4010011





