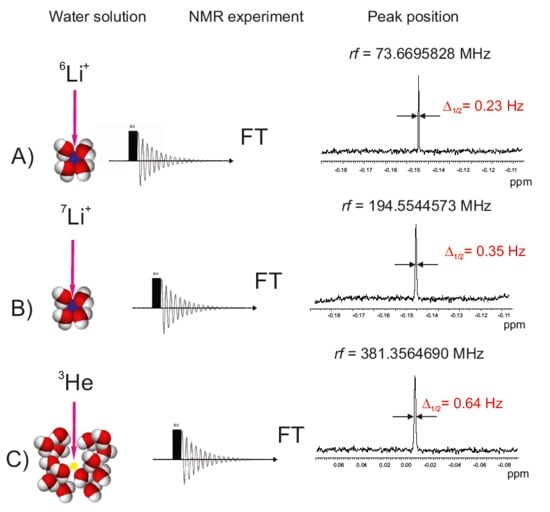
The Radiofrequency NMR Spectra of Lithium Salts in Water; Reevaluation of Nuclear Magnetic Moments for 6Li and 7Li Nuclei
Abstract
:1. Introduction
2. Results and Discussion
2.1. NMR Experiments in Water Solutions
2.2. ABMR Experiments for Atoms
2.3. Shielding Factors
3. Materials and Methods
4. Conclusions
Conflicts of Interest
References
- Rabi, I.I.; Zacharias, J.R.; Millman, S.; Kusch, P. A New Method of Measuring Nuclear Magnetic Moment. Phys. Rev. 1938, 53, 318. [Google Scholar] [CrossRef]
- Walhli, H.E. Some Improved Measurements of Nuclear Magnetic Dipole Moments by Means of Nuclear Magnetic Resonance; Spectroscopy Research Laboratory—Union Carbide and Carbon Corporation: Oak Ridge, TN, USA, 1954. [Google Scholar]
- Antušek, A.; Jackowski, K.; Jaszuński, M.; Makulski, W.; Wilczek, M. Nuclear magnetic dipole moments from NMR spectra. Chem. Phys. Lett. 2005, 411, 111–116. [Google Scholar] [CrossRef]
- Jaszuński, M.; Antušek, A.; Garbacz, P.; Jackowski, K.; Makulski, W.; Wilczek, M. The determination of accurate nuclear magnetic dipole moments and direct measurement of NMR shielding constants. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 67, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Baum, E.M.; Ernesti, M.C.; Knox, H.D.; Miller, T.R.; Watson, A.M. Nuclides and Isotopes. Chart of the Nuclides, 17th ed.; Bechtel: San Francisco, CA, USA, 2010. [Google Scholar]
- Puchalski, M.; Pachucki, K. Ground state hyperfine splitting in 6,7Li atoms and the nuclear structure. Phys. Rev. Lett. 2013, 111, 243001. [Google Scholar] [CrossRef] [PubMed]
- Goudsmit, S.; Young, L.A. The Nuclear Moment of Lithium. Nature 1930, 125, 461–462. [Google Scholar] [CrossRef]
- Granath, L.D. The Nuclear Spin and Magnetic Moment of Li7. Phys. Rev. 1932, 42, 44. [Google Scholar] [CrossRef]
- Rabi, I.I.; Millman, S.; Kush, P.; Zacharias, J.R. The Magnetic Moments of Li6, Li7 and F19. Phys. Rev. 1938, 53, 495. [Google Scholar] [CrossRef]
- Rabi, I.I.; Millman, S.; Kusch, P.; Zacharias, J.R. The Molecular Beam Resonance Method for Measuring Nuclear Magnetic Moments. Phys. Rev. 1939, 55, 526–535. [Google Scholar] [CrossRef]
- Lutz, O. The gI-factors and the magnetic moments of alkali nuclei and the shielding of Rb+ by water. Phys. Lett. A 1967, 25, 440–441. [Google Scholar] [CrossRef]
- Lutz, O. Untersuchungen über die magnetische Kernresonanz von Alkalikernen in wäßriger Lösung. Z. Naturforsch. A 1968, 23, 1202–1209. [Google Scholar] [CrossRef]
- Beckmann, A.; Böklen, K.D.; Elke, D. Precision Measurements of the Nuclear Magnetic Dipole Moments of 6Li, 7Li, 23Na, 39K and 41K. Z. Phys. 1974, 270, 173–186. [Google Scholar] [CrossRef]
- Raghavan, P. Table of nuclear moments. Atomic Data Nucl. Data Tables 1989, 42, 189–291. [Google Scholar] [CrossRef]
- Stone, N.J. Table of nuclear magnetic dipole and electric quadrupole moments. Atomic Data Nucl. Data Tables 2005, 90, 75–176. [Google Scholar] [CrossRef]
- Stone, N.J. Nuclear Data Section; IAEA, Vienna International Centre: Vienna, Austria, 2014. [Google Scholar]
- Antušek, A.; Kędziera, D.; Kaczmarek-Kędziera, A.; Jaszuński, M. Coupled cluster study of NMR shielding of alkali metal ions in water complexes and magnetic moments of alkali metal nuclei. Chem. Phys. Lett. 2012, 532, 1–8. [Google Scholar] [CrossRef]
- Friedman, H.L. Ionic Solution Theory: Based on Cluster Expansion Methods; Interscience Pub.: Miami, FL, USA, 1962; ISBN-13: 978-1124075259. [Google Scholar]
- Rudziński, A.; Puchalski, M.; Pachucki, K. Relativistic, QED, and nuclear mass effects in the magnetic shielding of 3He. J. Chem. Phys. 2009, 130, 244102. [Google Scholar] [CrossRef] [PubMed]
- Seydoux, R.; Diehl, P.; Mazitov, R.K.; Jokisaari, J. Chemical Shifts in Magnetic Resonance of the 3He Nucleus in Liquid Solvents and Comparison with Other Noble Gases. J. Magn. Reson. A 1993, 101, 78–83. [Google Scholar] [CrossRef]
- Jaszuński, M.; Repisky, M.; Demissie, T.B.; Komorovsky, S.; Malkin, E.; Ruud, K.; Garbacz, P.; Jackowski, K.; Makulski, W. Spin-rotation and NMR shielding constantsin HCl. J. Chem. Phys. 2013, 139, 234302. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Dinadayalane, T.C.; Leszczynski, J.; Sastry, G.N. Comprehensive Study on the Solvation of Mono- and Divalent Metal Cations: Li+, Na+, K+, Be2+, Mg2+ and Ca2+. J. Phys. Chem. A 2008, 112, 12944–12953. [Google Scholar] [CrossRef] [PubMed]
- Llanio-Trujillo, J.L.; Marques, J.M.C.; Pereira, F.B. New insights on lithium-cation microsolvation by solvents forming hydrogen-bonds: Water versus methanol. Comput. Theor. Chem. 2013, 1021, 124–134. [Google Scholar] [CrossRef]
- Rodriguez, O., Jr.; Lisy, J.M. Infrared spectroscopy of Li+(CH4)n, n = 1–9, clusters. Chem. Phys. Lett. 2011, 502, 145–149. [Google Scholar] [CrossRef]
- Mason, P.E.; Ansell, S.; Neilson, G.W.; Rempe, S.B. Neutron Scattering Studies of the Hydration Structure of Li+. J. Phys. Chem. B 2015, 119, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, C.; Zhang, X.; Ju, S. Solvation structure and dynamic of Li+ ion in liquid water, methanol and ethanol: A comparison study. Chem. Phys. 2014, 433, 89–97. [Google Scholar] [CrossRef]
- Alam, T.M.; Hart, D.; Rempe, S.L.B. Computing the 7Li NMR chemical shielding of hydrated Li+ using cluster calculations and time-averaged configurations from ab initio molecular dynamics simulations. Phys. Chem. Chem. Phys. 2011, 13, 13629–13637. [Google Scholar] [CrossRef] [PubMed]
- Mason, J. (Ed.) Multinuclear NMR; Plenum Press: New York, NY, USA, 1987; p. 56. ISBN 978-1-4613-1783-8. [Google Scholar]
- Mohr, P.J.; Newell, D.B.; Taylor, B.N. CODATA recommended values of the fundamental physical constants: 2014. Rev. Mod. Phys. 2016, 88, 035009. [Google Scholar] [CrossRef]
- AL-Khafiji, K.S.; Selman, A.M.; Al-Shebly, S.A.K. Calculation of the Standard Deviation and Nuclear Magnetic Shielding Constant for Lithium Atom. J. Kerbala Univ. 2008, 6, 107–110. [Google Scholar]
- Ormand, F.T.; Matsen, F.A. Nuclear Magnetic Shielding Constants for Several 2-, 3-, and 4-Electron Atoms and Ions. J. Chem. Phys. 1959, 30, 368–371. [Google Scholar] [CrossRef]
- Malli, G.; Froese, C. Nuclear Magnetic Shielding Constants Calculated from Numerical Hartree-Fock Wave Functions. Int. J. Quantum Chem. 1967, 1, 95–98. [Google Scholar] [CrossRef]
- Guan, X.-X.; Wang, Z.-W. Calculation of the Zeeman effect in the 2S1/2, n2P1/2, and n2P3/2 (n = 2, 3, 4, and 5) states of lithium atom. Phys. Lett. A 1998, 244, 120–126. [Google Scholar] [CrossRef]
- Cockrell, R.C. Ab Initio Nuclear Structure Calculations for Light Nuclei, 2012. Ph.D Thesis, Iowa State University, Ames, IA, USA, 30 January 2012. [Google Scholar]
- Borremans, D.; Balabanski, D.L.; Blaum, K.; Geithner, W.; Gheysen, S.; Himpe, P.; Kowalska, M.; Lassen, J.; Lievens, P.; Mallion, S.; et al. New measurement and reevaluation of the nuclear magnetic and quadrupole moments of 8Li and 9Li. Phys. Rev. C 2005, 044309. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1482260960. [Google Scholar]
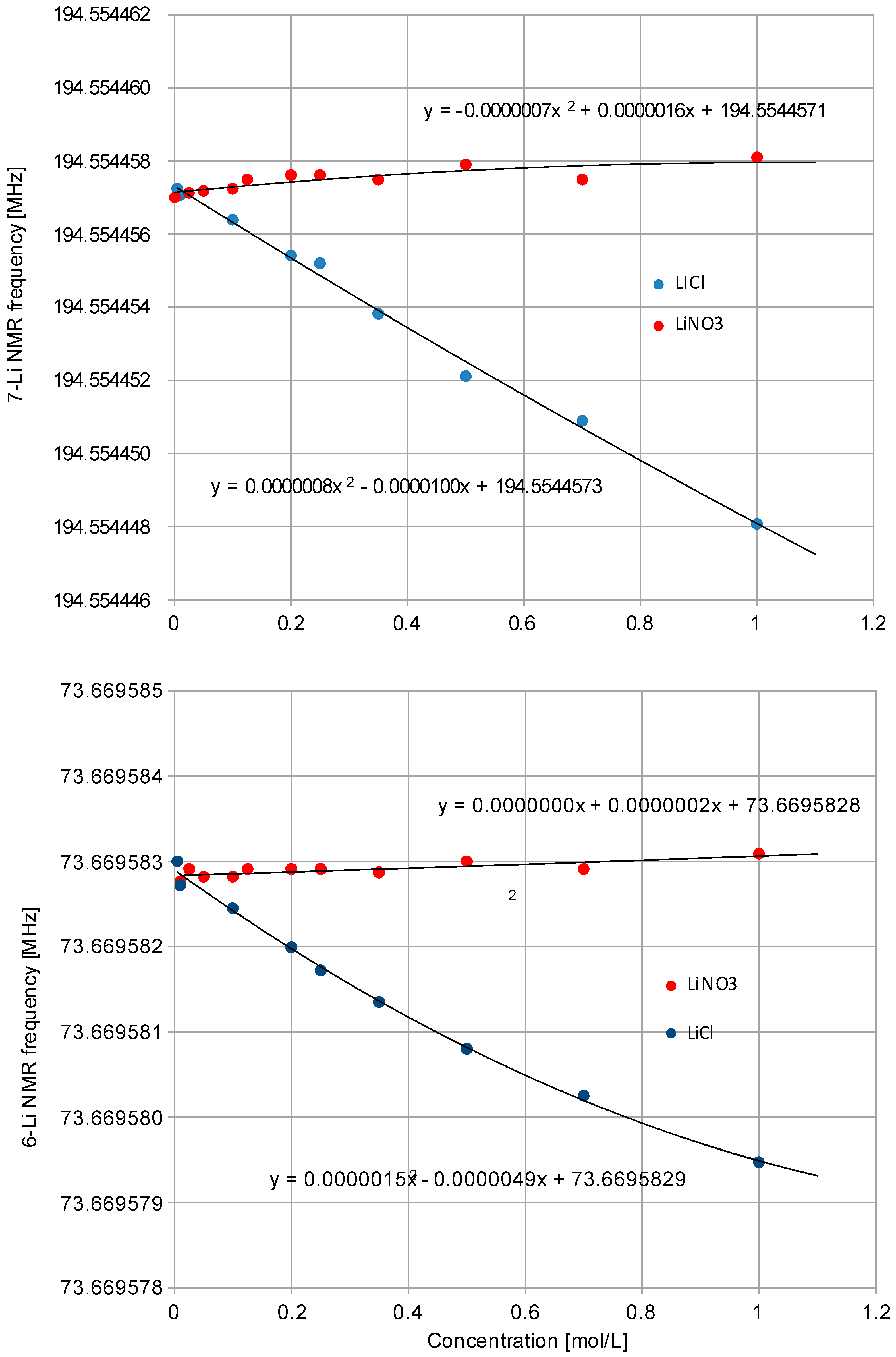


| Water Solution | Nuclide | ν0 (Radiofrq.) MHz | δ/ppm | δ1/ppm mL mol−1 | σ/ppm | Reference |
|---|---|---|---|---|---|---|
| δ2/ppm mL mol−2 | ||||||
| LiCl | ||||||
| (6Li+)aq. | 73.6695828(2) | −0.1472 | −0.0632 | 90.89(300) (a) | [17] | |
| 0.0148 | 91.69(300) (b) | |||||
| (7Li+)aq. | 194.5544573(2) | −0.1469 | −0.0632 | 90.89(300) (a) | [17] | |
| 0.0148 | 91.69(300) (b) | |||||
| 35Cl− | 49.0491386(10) | 4.7125 | 0.9358 | 998.28(500) | [21] (This work) | |
| −0.0461 | ||||||
| 3He | 381.3564690(5) | −2.7675 | −0.0478 | 59.729(1) | [19] (This work) | |
| 0.0102 | ||||||
| LiNO3 | ||||||
| (6Li+)aq. | 73.6695829(2) | −0.147 | −0.003 | 90.89(300) (a) | [17] | |
| −0.0059 | 91.69(300) (b) | |||||
| (7Li+)aq. | 194.5544571(2) | −0.147 | −0.003 | 90.89(300) (a) | [17] | |
| 0.0059 | 91.69(69) (b) | |||||
| 14NO3− | 36.1752096(10) | −5.595 | −0.107 | −132.14 | [4] (This work) | |
| 0.0165 | ||||||
| 3He | 381.3564691(5) | −2.7676 | −0.0045 | 59.729(1) | (This work) | |
| −0.004 | ||||||
| * Lock system tuned to ν(D2O) = 76.8464 MHz | ||||||
| μ(7Li)/μN | Method/Reference | Nucleus | σ(7Li+)aq./ppm |
|---|---|---|---|
| Theory/[17] | 90.89 ÷ 91.69(300) | ||
| 3.2564169(98) | NMR/(This work) | 35Cl− | 91.16 |
| 14NO3− | 90.36 | ||
| 3.2564195(98) | NMR/(This work) | 35Cl− | 91.53 |
| 14NO3− | 90.73 | ||
| 3.2564157(30) | ABMR/[13], (This work) | 35Cl− | 90.76 |
| 14NO3− | 89.96 | ||
| 3.2564625(4) | NMR/[15] | 35Cl− | 105.13 |
| 14NO3− | 104.33 | ||
| μ(6Li)/μN | σ(6Li+)aq./ppm | ||
| Theory/[17] | 90.89 ÷ 91.69(300) | ||
| 0.8220453(25) | NMR/(This work) | 35Cl− | 91.09 |
| 14NO3− | 90.30 | ||
| 0.8220459(25) | NMR/(This work) | 35Cl− | 91.82 |
| 14NO3− | 91.03 | ||
| 0.8220445(10) | ABMR*/[13], (This work) | 35Cl− | 90.12 |
| 14NO3− | 89.32 | ||
| 0.822567(3) | NMR/[15] | 35Cl− | 725.27 |
| 14NO3− | 724.47 |
| Nuclide | Iπ | Q Barn | Abundance % | μ/μN | Diamagnetic Correction | gI Factor | γI × 107 | Reference |
|---|---|---|---|---|---|---|---|---|
| 6Li | 1+ | 0.00082(2) | 7.59(4) | 0.8220453(25) | 1.00009089 | 0.822045(3) | 3.93712(1) | (This work) |
| 0.8220459(25) | 1.00009169 | |||||||
| 0.8220445(10) | 1.000101472 | [13] | ||||||
| 0.832; 0.835 | [34] | |||||||
| 0.843(5); 0.843(2) | ||||||||
| 7Li | 3/2− | 0.0406(8) | 92.41(4) | 3.2564169(98) | 1.00009089 | 2.170945(7) | 10.39756(3) | (This work) |
| 3.2564195(98) | 1.00009169 | |||||||
| 3.2564157(2) | 1.000101472 | [13] | ||||||
| 2.993; 3.036 | [34] | |||||||
| 3.01(2); 3,02(2) | ||||||||
| 35Cl | 3/2+ | 0.0850(11) | 75.78(4) | 0.821721(5) | 0.547814(3) | 2.62371(1) | [21] | |
| 14N | 1+ | 0.02001(10) | 99.632(7) | 0.4035729(45) | 0.403573(5) | 1.93288(2) | [4] | |
| 3He | 1/2+ | 0.000137 | 2.127625308(25) | 1.00005973 | 4.25525061(5) | 20.3801680(2) | [29] | |
| 2H(D) | 1+ | 0.00286(2) | 0.0156 | 0.8574382311(48) | 0.857438231(5) | 4.1066289(1) | [29] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makulski, W. The Radiofrequency NMR Spectra of Lithium Salts in Water; Reevaluation of Nuclear Magnetic Moments for 6Li and 7Li Nuclei. Magnetochemistry 2018, 4, 9. https://doi.org/10.3390/magnetochemistry4010009
Makulski W. The Radiofrequency NMR Spectra of Lithium Salts in Water; Reevaluation of Nuclear Magnetic Moments for 6Li and 7Li Nuclei. Magnetochemistry. 2018; 4(1):9. https://doi.org/10.3390/magnetochemistry4010009
Chicago/Turabian StyleMakulski, Włodzimierz. 2018. "The Radiofrequency NMR Spectra of Lithium Salts in Water; Reevaluation of Nuclear Magnetic Moments for 6Li and 7Li Nuclei" Magnetochemistry 4, no. 1: 9. https://doi.org/10.3390/magnetochemistry4010009
APA StyleMakulski, W. (2018). The Radiofrequency NMR Spectra of Lithium Salts in Water; Reevaluation of Nuclear Magnetic Moments for 6Li and 7Li Nuclei. Magnetochemistry, 4(1), 9. https://doi.org/10.3390/magnetochemistry4010009