Enhanced Coercivity of Low-Density Barium Hexaferrite Magnets from Paste-Injection Molding
Abstract
:1. Introduction
2. Materials and Methods
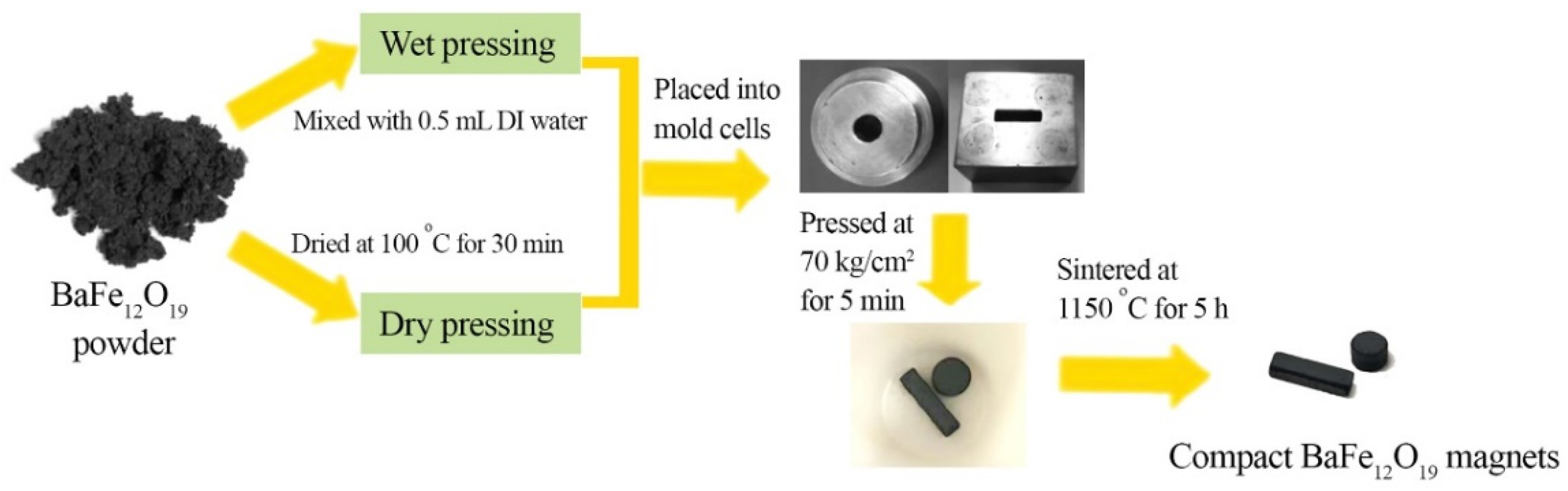
2.1. BaFe12O19 Magnets from Powder Compaction
2.2. BaFe12O19 Magnets from Paste-Injection Molding
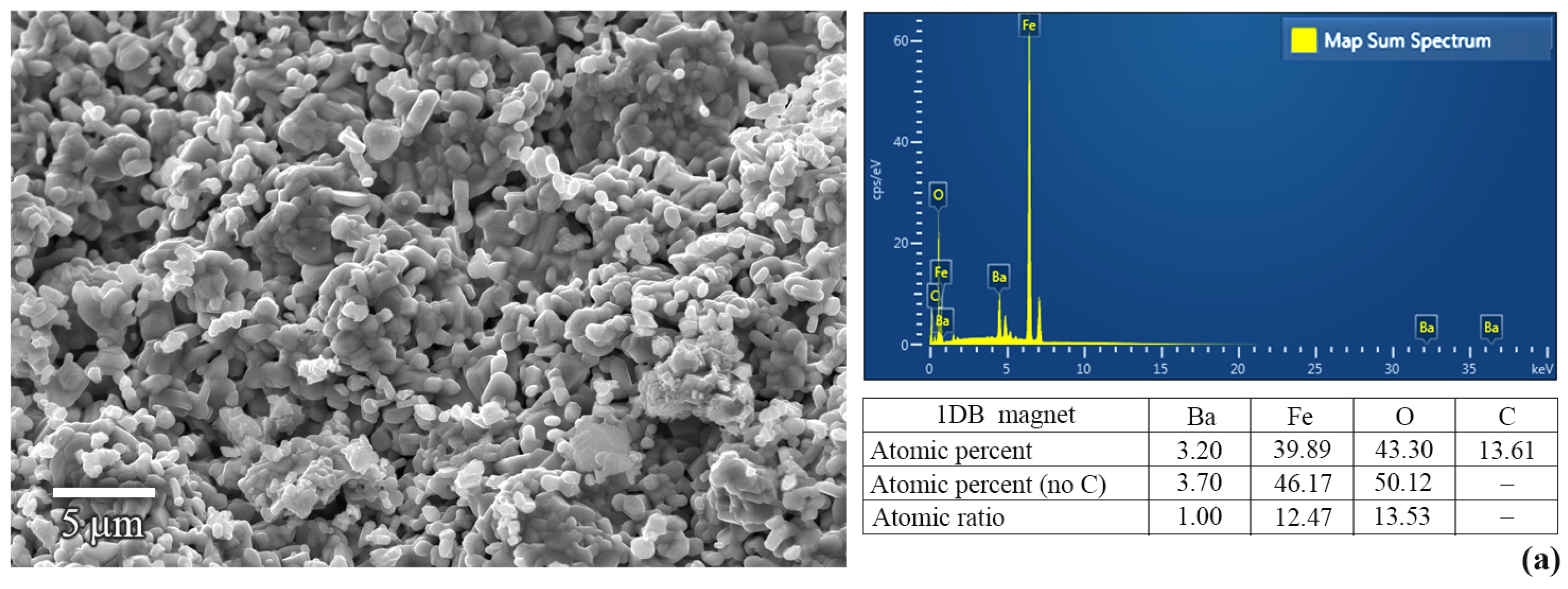
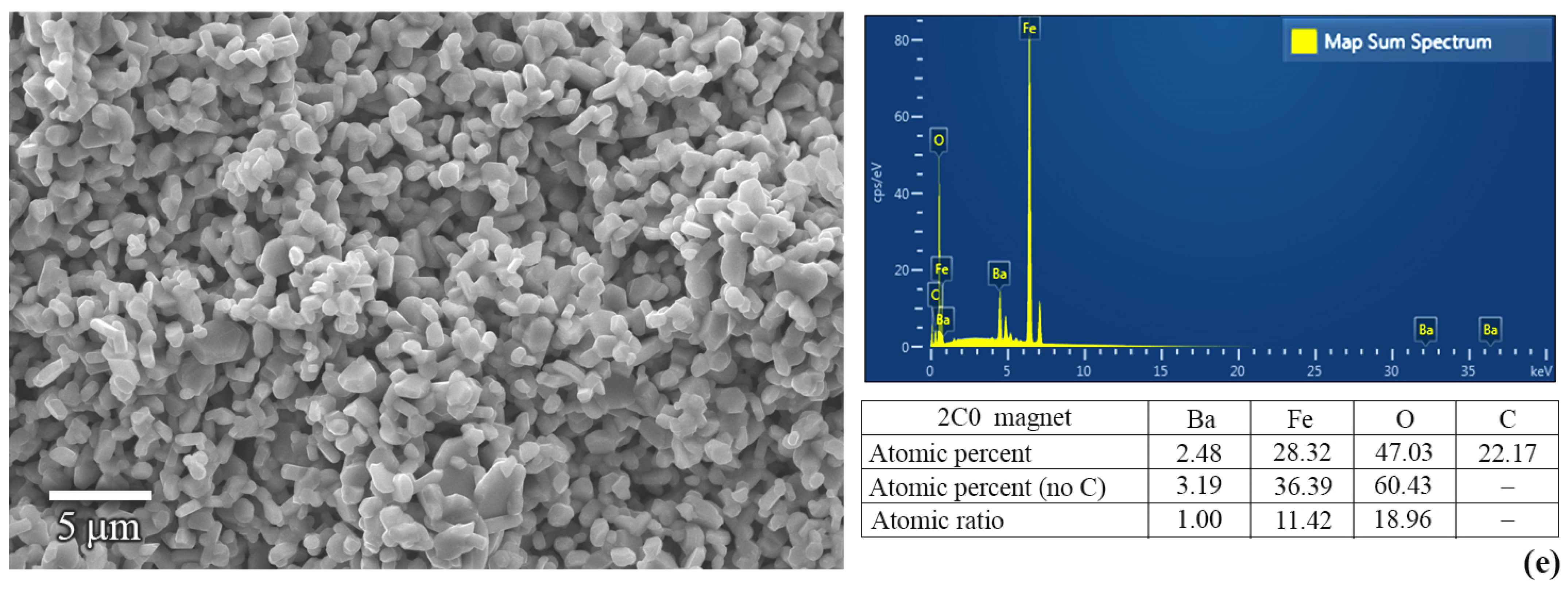
2.3. Characterizations of BaFe12O19 Magnets
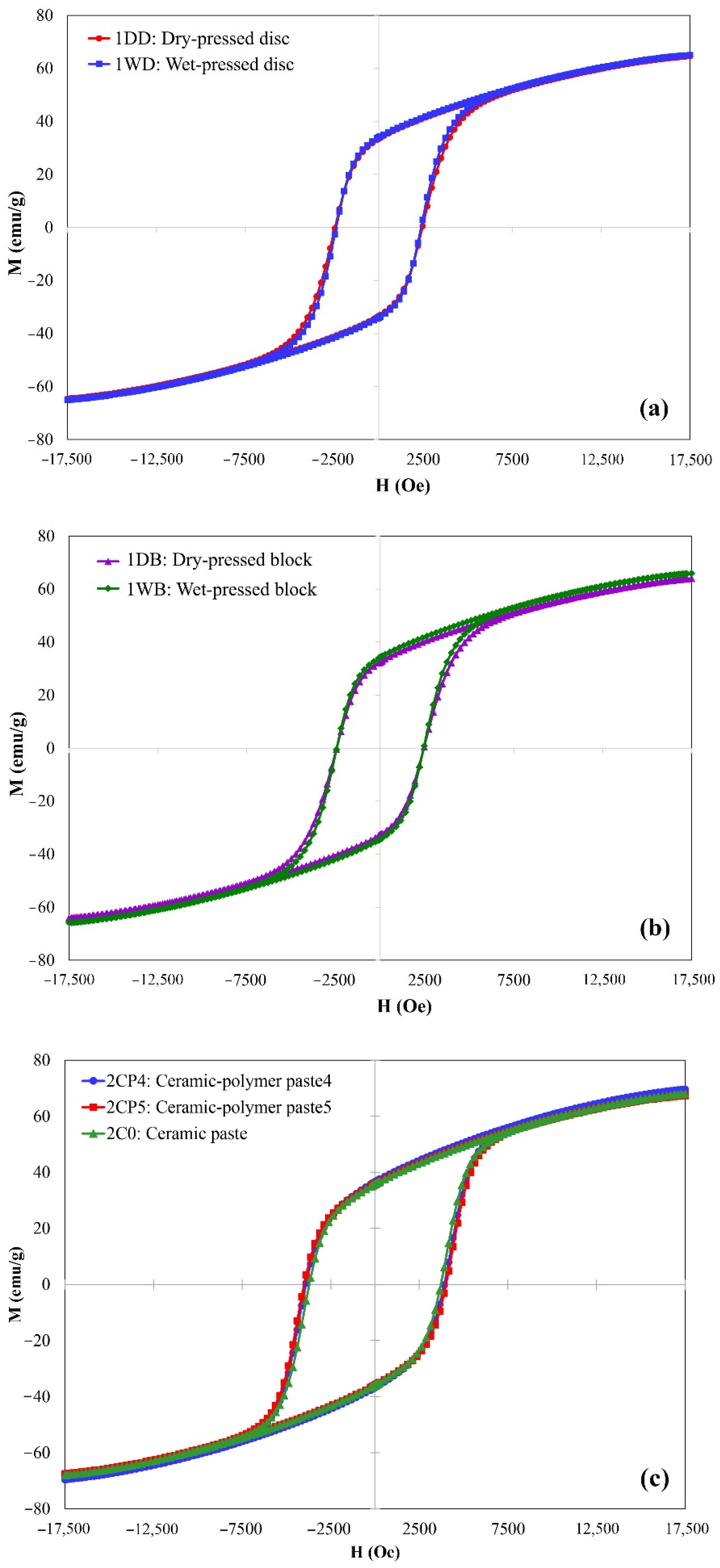
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pullar, R. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- de Julián Fernández, C.; Sangregorio, C.; de la Figuera, J.; Belec, B.; Makovec, D.; Quesada, A. Progress and prospects of hard hexaferrites for permanent magnet applications. J. Phys. D Appl. Phys. 2021, 54, 153001. [Google Scholar] [CrossRef]
- Frenea-Robin, M.; Marchalot, J. Basic principles and recent advances in magnetic cell separation. Magnetochemistry 2022, 8, 11. [Google Scholar] [CrossRef]
- Granados-Miralles, C.; Jenuš, P. On the potential of hard ferrite ceramics for permanent magnet technology: A review on sintering strategies. J. Phys. D Appl. Phys. 2021, 54, 303001. [Google Scholar] [CrossRef]
- Brightlin, B.C.; Balamurugan, S. The effect of post annealing treatment on the citrate sol–gel derived nanocrystalline BaFe12O19 powder: Structural, morphological, optical and magnetic properties. Appl. Nanosci. 2016, 6, 1199–1210. [Google Scholar] [CrossRef] [Green Version]
- Faisal, M.; Saeed, A.; Larik, F.A.; Ghumro, S.A.; Rasheed, S.; Channar, P.A. WOWS sol–gel based synthesis and structural, morphological, electrical and magnetic characterization of Co-Sm doped M-type barium hexaferrite materials. J. Electron. Mater. 2018, 47, 7011–7022. [Google Scholar] [CrossRef]
- Asghar, G.; Asri, S.; Khusro, S.N.; Tariq, G.H.; Awan, M.S.; Irshad, M.; Safeen, A.; Iqbal, Y.; Shah, W.H.; Anis-Ur-Rehman, M. Enhanced magnetic properties of barium hexaferrite. J. Electron. Mater. 2020, 49, 4318–4323. [Google Scholar] [CrossRef]
- Charoensuk, T.; Thongsamrit, W.; Ruttanapun, C.; Jantaratana, P.; Sirisathitkul, C. Loading effect of sol-gel derived barium hexaferrite on magnetic polymer composites. Nanomaterials 2021, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Kristiantoro; Idayanti; Dedi; Sudrajat; Mulyadi; Gustinova. Influence of compaction pressure on magnetic characteristics, density, and hardness of barium hexaferrite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 620, 012106. [Google Scholar] [CrossRef] [Green Version]
- Timofeev, A.V.; Kostishin, V.G.; Makeev, D.B.; Chitanov, D.N. Magnetic properties of barium hexaferrite compacted nanopowders. Tech. Phys. 2019, 64, 1484–1487. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Chernukha, A.S.; Gudkova, S.A.; Zhivulin, V.E.; Trofimov, E.A.; Tarasova, A.Y.; Zherebtsov, D.A.; Kalandija, M.; Trukhanov, A.V.; Trukhanov, S.V.; et al. Morphology and magnetic properties of pressed barium hexaferrite BaFe12O19 materials. J. Magn. Magn. Mater. 2018, 459, 131–135. [Google Scholar] [CrossRef]
- Chaudhary, V.; Mantri, S.A.; Ramanujan, R.V.; Banerjee, R. Additive manufacturing of magnetic materials. Prog. Mater. Sci. 2020, 114, 100688. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Zhao, D.; Mao, X.; Jiang, W.; Ge, S.S. Net-shaped barium and strontium ferrites by 3D printing with enhanced magnetic performance from milled powders. J. Magn. Magn. Mater. 2020, 493, 165664. [Google Scholar] [CrossRef]
- Anjum, S.; Sehar, F.; Mustafa, Z.; Awan, M.S. Enhancement of structural and magnetic properties of M-type hexaferrite permanent magnet based on synthesis temperature. Appl. Phys. A 2018, 124, 49. [Google Scholar] [CrossRef]
- Azis, R.A.S.; Che Muda, N.N.; Hassan, J.; Shaari, A.H.; Ibrahim, I.R.; Mustaffa, M.S.; Sulaiman, S.; Matori, K.A.; Fen, Y.W. Effect of ratio in ammonium nitrate on the structural, microstructural, magnetic, and AC conductivity properties of BaFe12O19. Materials 2018, 11, 2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalini, M.G.; Subha, A.; Sahu, B.; Sahoo, S.C. Phase evolution and temperature dependent magnetic properties of nanosrystalline barium hexaferrite. J. Mater. Sci. Mater. Electron. 2019, 30, 13647–13654. [Google Scholar] [CrossRef]
- Lohmaah, A.; Chokprasombat, K.; Pinitsoontorn, S.; Sirisathitkul, C. Magnetic properties and morphology copper-substituted barium hexaferrites from sol-gel auto-combustion synthesis. Materials 2021, 14, 5873. [Google Scholar] [CrossRef] [PubMed]



| Samples | Fabrication Method | Density (g/cm3) | Magnetic Properties | |||
|---|---|---|---|---|---|---|
| Hc (Oe) | Mr (emu/g) | Ms (emu/g) | Mr/Ms | |||
| 1DD | Dry-pressed disc | 3.63 | 2454 | 33.48 | 69.94 | 0.48 |
| 1DB | Dry-pressed bar | 3.60 | 2453 | 32.63 | 68.48 | 0.48 |
| 1WD | Wet-pressed disc | 3.77 | 2389 | 33.97 | 70.01 | 0.48 |
| 1WB | Wet-pressed bar | 3.46 | 2450 | 34.29 | 70.85 | 0.48 |
| 2CP1-4 | Ceramic–polymer paste * | 1.90 ± 0.02 | 3868 ± 42 | 35.24 ± 0.57 | 72.55 ± 2.13 | 0.49 |
| 2CP5 | Ceramic–polymer paste | 2.35 | 4002 | 35.80 | 72.59 | 0.49 |
| 2C0 | Ceramic paste | 2.55 | 3714 | 35.81 | 73.38 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongsamrit, W.; Jantaratana, P.; Charoensuk, T.; Sirisathitkul, C. Enhanced Coercivity of Low-Density Barium Hexaferrite Magnets from Paste-Injection Molding. Magnetochemistry 2022, 8, 46. https://doi.org/10.3390/magnetochemistry8040046
Thongsamrit W, Jantaratana P, Charoensuk T, Sirisathitkul C. Enhanced Coercivity of Low-Density Barium Hexaferrite Magnets from Paste-Injection Molding. Magnetochemistry. 2022; 8(4):46. https://doi.org/10.3390/magnetochemistry8040046
Chicago/Turabian StyleThongsamrit, Wannisa, Pongsakorn Jantaratana, Thanida Charoensuk, and Chitnarong Sirisathitkul. 2022. "Enhanced Coercivity of Low-Density Barium Hexaferrite Magnets from Paste-Injection Molding" Magnetochemistry 8, no. 4: 46. https://doi.org/10.3390/magnetochemistry8040046






