Recovery of Cobalt, Nickel, and Lithium from Spent Lithium-Ion Batteries with Gluconic Acid Leaching Process: Kinetics Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Input Materials
2.2. Experimental Procedures
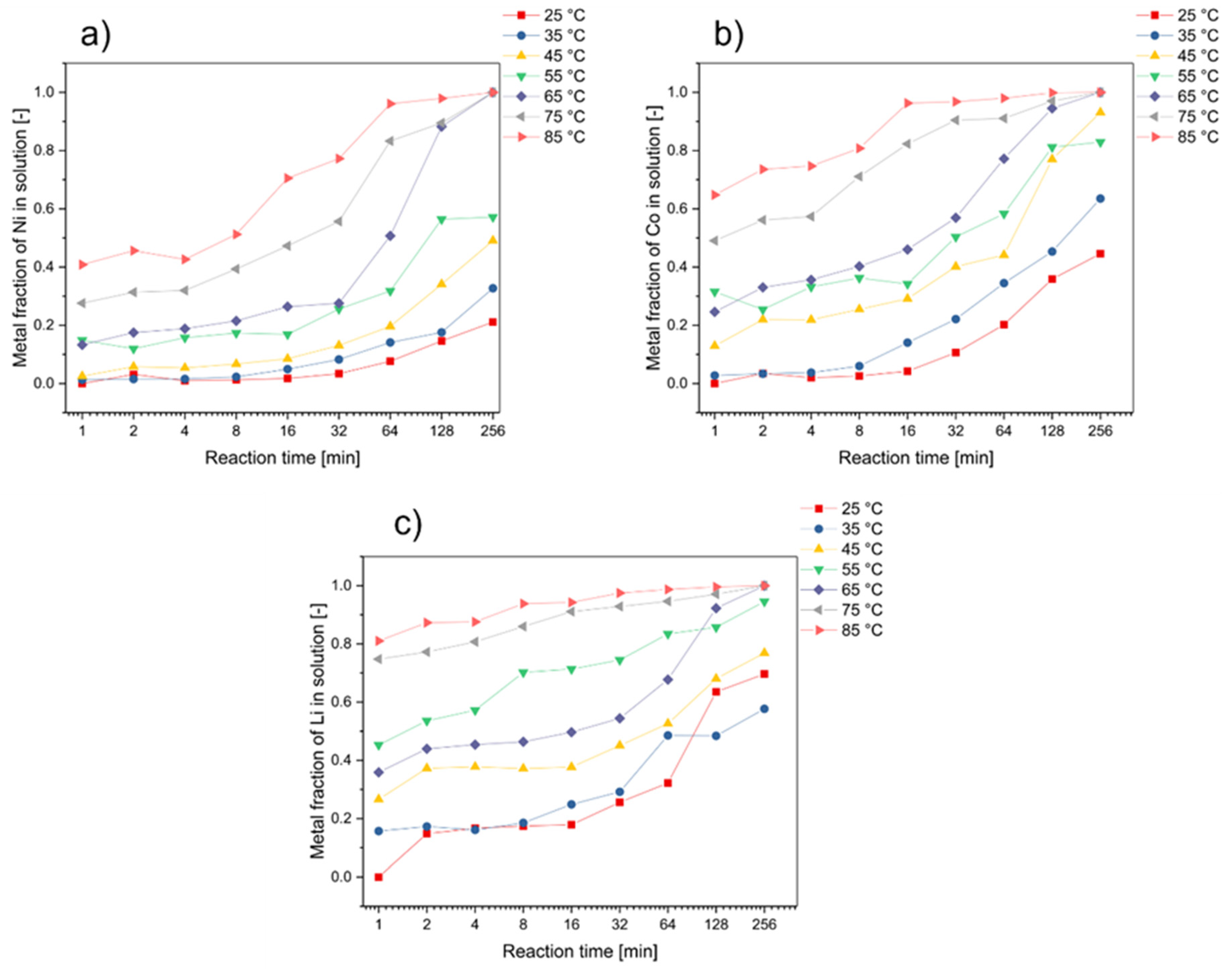
3. Results and Discussion
3.1. Effect of Reaction Temperature
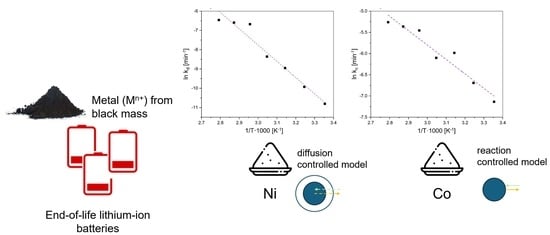
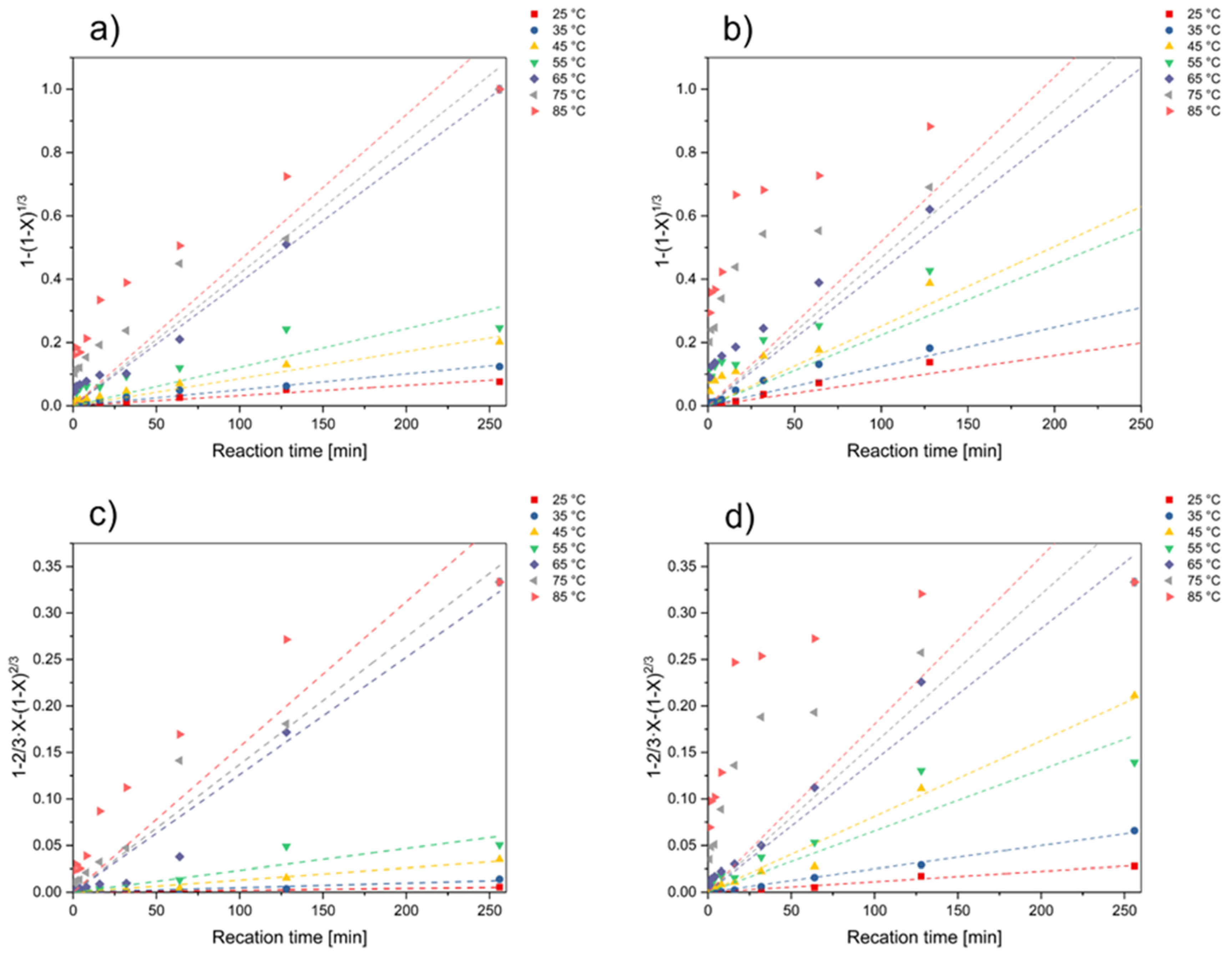
3.2. Kinetic Analysis
- The gluconic acid molecule (leaching agent) diffuses through the bulk solution to the solid-liquid interface.
- This molecule diffuses through the diffusion layer to the unreacted core surface.
- The gluconic acid molecule reaches this solid-liquid interface and reacts with solid core components, and metal ions are dissolved and released into the solution.
- The metal ion as the product will diffuse through the diffusion layer to the solid-liquid interface.
- The product diffuses into a bulk solution.
- X—Fractional conversion (based on mole ratios of respective elements) [-]
- b’—Stoichiometric coefficient [-]; kg… Rate constant of film diffusion [min−1]
- cA—Bulk concentration of the fluid [mol·m−3]
- ρB—Molar density of B in the solid [mol·m−3]
- r0—Radius of unreacted core [mm]
- t—Reaction time [min]
- kr—Rate constant of surface reaction [min−1]
- kd—Apparent reaction rate constant [min−1]
- DB—Effective diffusion coefficient [m2·s−1]
3.3. Calculation of Activation Energy
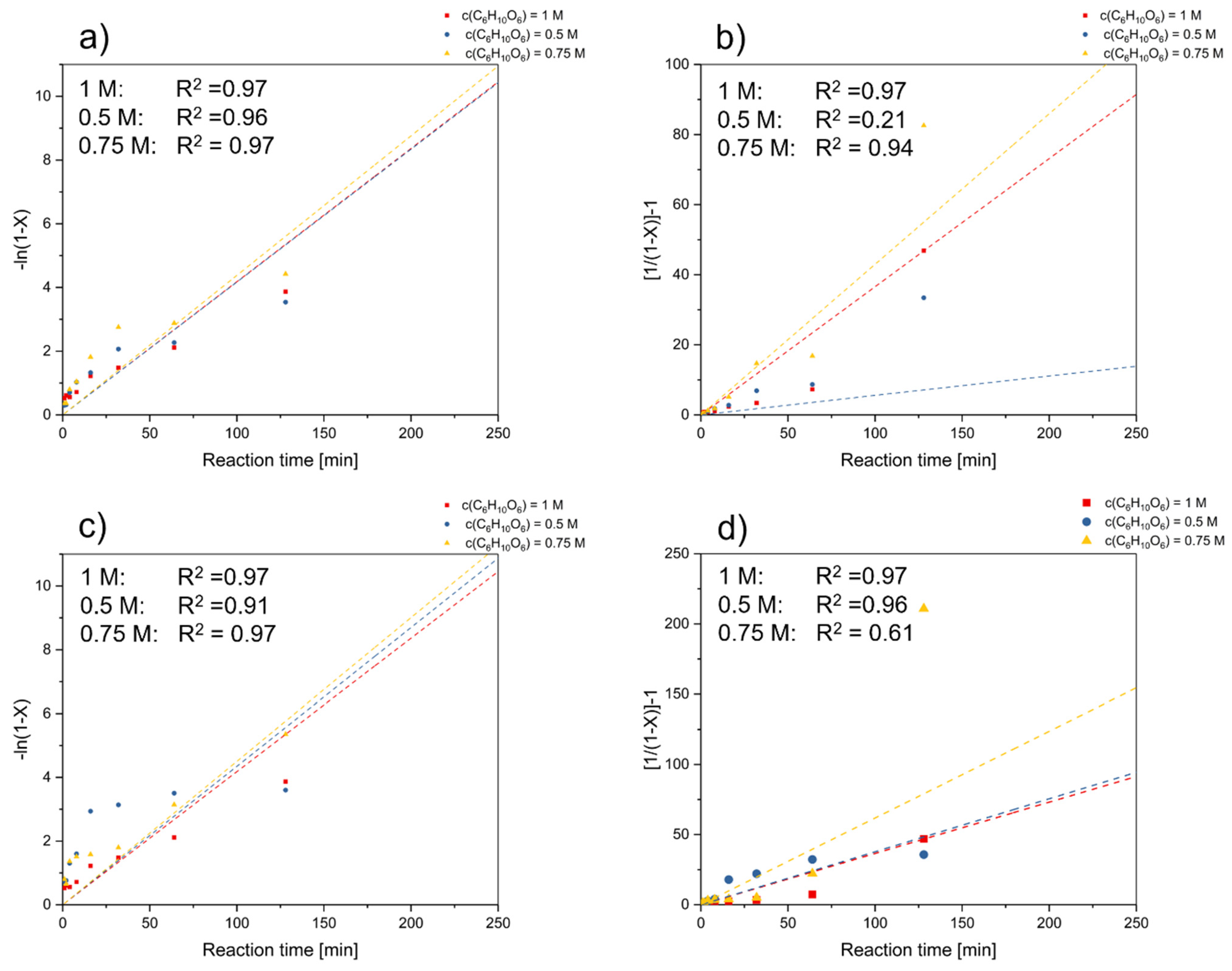
3.4. Determination of Reaction Order
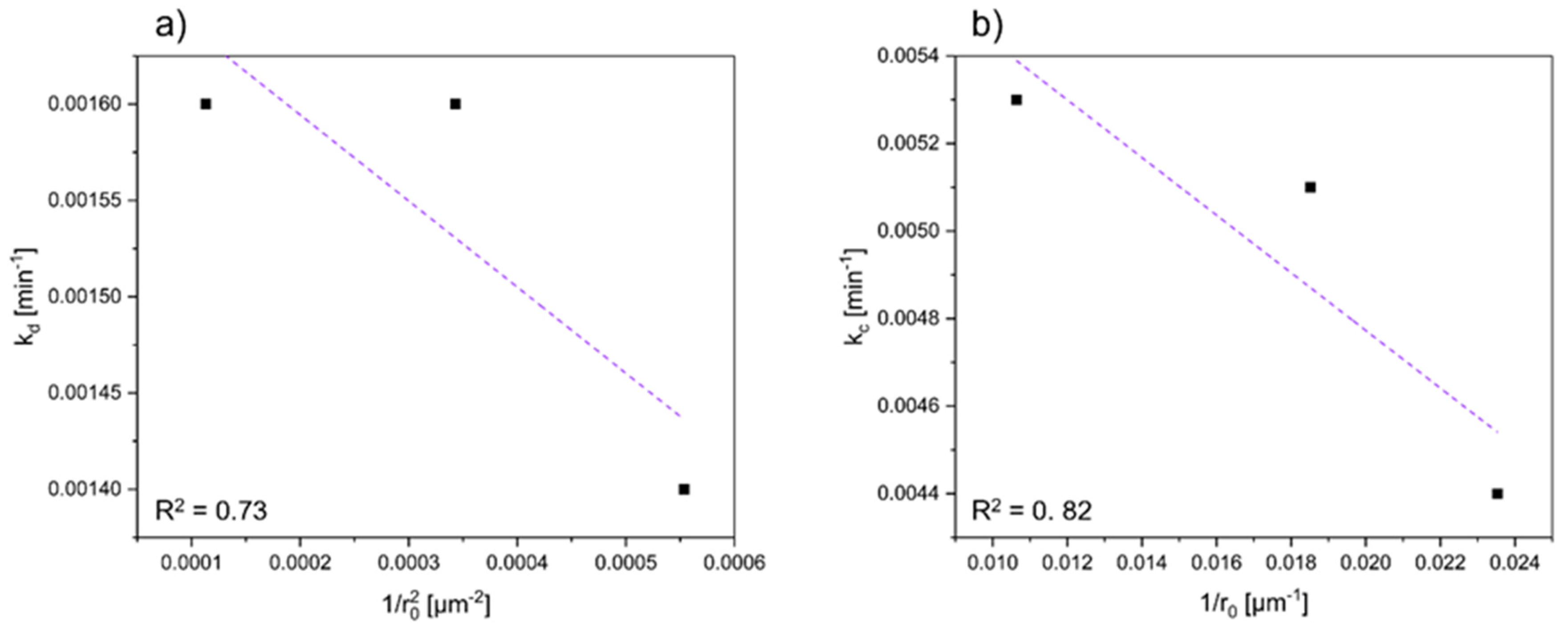
3.5. Influence of Mean Particle Sizes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meshram, P.; Mishra, A.; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef] [PubMed]
- Kresse, C.; Bastian, D.; Bookhagen, B. Lithium-Ionen-Batterierecycling in Deutschland und Europa; Bundesanstalt für Geowissenschaften und Rohstoffe: Hannover, Germany, 2022. [Google Scholar]
- Zechmeister, A.; Anderl, M.; Bartel, A.; Frei, E.; Gugele, B.; Gössl, M.; Mayer, S.; Heinfellner, H.; Heller, C.; Heuberet, A.; et al. Klimaschutzbericht 2022; Report; Umweltbundesamt: Wien, Austria, 2022. [Google Scholar]
- Statista: Annual Greenhouse Gas Emissions in the European Union (EU-27) from 1990 to 2021, by Sector. Available online: https://www.statista.com/statistics/1171183/ghg-emissions-sector-european-union-eu/ (accessed on 6 June 2023).
- IEA. Global Electric Vehicle Outlook 2022; IEA: Paris, France, 2022. [Google Scholar]
- Presse—Und Informationsamt der Bundesregierung: EU-Umweltrat: Nur Noch CO2-Frei Fahren. Available online: https://www.bundesregierung.de/breg-de/schwerpunkte/europa/verbrennermotoren-2058450 (accessed on 29 March 2022).
- Vertretung in Deutschland: EU-Staaten Stimmen—Endgültig—Für Emissionsfreie Autos ab 2035. Available online: https://germany.representation.ec.europa.eu/news/eu-staaten-stimmen-endgultig-fur-emissionsfreie-autos-ab-2035-2023-03-28_de (accessed on 3 April 2023).
- Europäische Kommission. Der Europäische Gründe Deal—Mitteilung der Kommission an das Europäische Parlament, den Europäischen Rat, den Rat, den Europäischen Wirtschafts- und Sozialausschuss und den Ausschuss der Regionen—Der Europäische Grüne Deal; Europäische Kommission: Brussels, Belgium, 2019. [Google Scholar]
- Europäische Kommission. Die Europäische Batterie-Allianz Macht Fortschritte: Gründung einer Europäischen Batterieakademie zur Verbesserung der Fähigkeiten für ein Rasch Wachsendes Batterieökosystem in Europa; Europäische Kommission: Brussels, Belgium, 2022. [Google Scholar]
- Europäische Kommission. Verordnung des Europäischen Parlaments über Batterien und Altbatterien, zur Aufhebung der Richtlinie 2006/66/EG und zur Änderung der Verordnung (EU) 2019/1020; Europäische Kommission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Critical Raw Materials: Ensuring Secure and Sustainable Supply Chains for EU’s Green and Digital Future; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- 2023/0081 (COD); Verordnung des Europäischen Parlaments und des Rates zur Schaffung eines Rahmens für Maßnahmen zur Stärkung des Europäischen Ökosystems der Fertigung von Netto-Null-Technologieprodukten (Netto-Null-Industrie-Verordnung). European Commission: Brussels, Belgium, 2023.
- Blengini, G.A.; Latunussa, C.E.L.; Eynard, U.; de Matos, C.T.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s List of Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Sidiq, A.L.; Floweri, O.; Karunawan, J.; Abdillah, O.B.; Santosa, S.P.; Iskandar, F. NCM cathode active materials reproduced from end-of-life Li-ion batteries using a simple and green hydrometallurgical recycling process. Mater. Res. Bull. 2022, 153, 111901. [Google Scholar] [CrossRef]
- Becker, R.; Fanghänel, E.; Habicher, W.-D.; Knölker, H.-J.; Metz, P.; Schwetick, K. Organikum—Organisch-Chemisches Grundpraktikum; Wiley-VCH Verlag: Weinheim, Germany, 2015. [Google Scholar]
- Lerchbammer, R.; Gerold, E.; Antrekowitsch, H. Gluconic acid leaching of spent lithium-ion batteries as an environmentally friendly approach to achieve high leaching efficiencies in the recycling of NMC active material. Metals 2023, 13, 1330. [Google Scholar] [CrossRef]
- Singh, O.V.; Kumar, R. Biotechnological production of gluconic acid: Future implications. Appl. Microbiol. Biotechnol. 2007, 75, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadis, S.; Morgunov, I.G. Gluconic acid production. Recent Pat. Biotechnol. 2007, 1, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ding, X.; Cai, C.; Peng, G.; Hu, J.; Yang, X.; Chen, S.; Liu, L.; Hou, H.; Liang, S.; et al. Acetate acid and glucose assisted subcritical reaction for metal recovery from spent lithium ion batteries. J. Clean. Prod. 2022, 369, 133281. [Google Scholar] [CrossRef]
- Yao, L.; Xi, Y.; Han, H.; Li, W.; Wang, C.; Feng, Y. LiMn2O4 prepared from waste lithium ion batteries through sol-gel process. J. Alloys Compd. 2021, 868, 159222. [Google Scholar] [CrossRef]
- Choi, J.-W.; Cho, C.-W.; Yun, Y.-S. Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance. J. Hazard. Mater. 2022, 423, 127214. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, J.; Gao, L.; Lin, H.; Lin, J. Modeling and simulation of enzymatic gluconic acid production using immobilized enzyme and CSTR-PFTR circulation reaction system. Biotechnol. Lett. 2018, 40, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Zhang, X.; Yang, E.; Tu, Y. A green process to recover valuable metals from the spent ternary lithium-ion batteries. Sep. Purif. Technol. 2022, 299, 121782. [Google Scholar] [CrossRef]
- Gok, O.; Anderson, C.G.; Ciceli, G.; Cocen, E.I. Leaching kinetics of copper from chalcopyrite concentrate in nitrous-sulfuric acid. Physiochem. Probl. Miner. Process. 2013, 50, 399–413. [Google Scholar]
- Yuliusman, S.; Nurqomariah, A.; Fajaryanto, R. Recovery of cobalt and nickel from spent lithium ion batteries with citric acid using leaching process: Kinetics study. E3S Web Conf. 2018, 67, 03008. [Google Scholar] [CrossRef]
- Yuliusman, S.; Fajaryanto, R.; Nurqomariah, A. Acid leaching and kinetics study of cobalt recovery from spent lithium-ion batteries with nitric acid. E3S Web Conf. 2018, 67, 03025. [Google Scholar] [CrossRef]
- Zheng, Y.; Long, H.L.; Zhou, L.; Wu, Z.S.; Zhou, X.; You, L.; Yang, L.; Liu, J.W. Leaching Procedure an Kinetic Studies of Cobalt in Cathode Materials from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. Int. J. Environ. Res. 2016, 10, 159–168. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 2nd ed.; John Wiley: New York, NY, USA, 1972. [Google Scholar]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, G.; Cheng, Y.; Liu, M.; Ji, J. Effect of Hydrogen Peroxide on the Recovery of Valuable Metals from Spent LiNi0.6Co0.2Mn0.2O2 Batteries. Energy Technol. 2022, 10, 220039. [Google Scholar] [CrossRef]
- Nicol, M.J. The role and use of hydrogen peroxide as an oxidant in the leaching of minerals 1. Acid Solut. Hydrometall. 2020, 193, 105328. [Google Scholar] [CrossRef]
- Sun, F.; Chen, T.; Zou, X.; Liu, H.; Chu, Z.; Shu, D.; Wang, H.; Huang, F.; Chen, D. A quantitative analysis of hydroxyl radical generation as H2O2 encounters siderite: Kinetics and effect of parameters. Appl. Geochem. 2021, 126, 104893. [Google Scholar] [CrossRef]
- Fan, B.; Chen, X.; Zhou, T.; Zhang, J.; Xu, B. A Sustainable process for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. Res. 2016, 34, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E. Hydrometallurgical Extraction and Reclamation; Ellis Horwood Ltd.: Chichester, UK, 1982; pp. 46–47. [Google Scholar]
- Baba, A.A.; Adekola, F.A.; Bale, R.B. Study of dissolution kinetics of a Nigerian cassiterite ore by hydrochloric acid. Sci. Focus 2009, 14, 198–207. [Google Scholar]
- Olanipekun, E.O. A kinetics study of the leaching of a Nigerian ilmenite ore by hydrochloric acid. Hydrometallurgy 1999, 53, 1–10. [Google Scholar] [CrossRef]
- Aydogan, S.; Aras, A.; Uçar, G.; Erdemoğlu, M. Dissolution kinetics of galena in acetic acid solutions with hydrogen peroxide. Hydrometallurgy 2007, 89, 189–195. [Google Scholar] [CrossRef]



| Element | Li | Al | Mn | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|---|
| g/100 g BM | 3.38 | 5.18 | 3.2 | 2.39 | 14.0 | 9.8 | 1.45 |
| Nickel | Surface Chemical Reaction | Diffusion through Product Layer | ||
|---|---|---|---|---|
| kr [min−1] | R2 | kd [min−1] | R2 | |
| Temperature [°C] | ||||
| 25 | 0.00032 | 0.97001 | 0.000020 | 0.97672 |
| 35 | 0.00050 | 0.97334 | 0.000049 | 0.95229 |
| 45 | 0.00086 | 0.96416 | 0.000130 | 0.98362 |
| 55 | 0.00122 | 0.82538 | 0.000234 | 0.91091 |
| 65 | 0.00390 | 0.99031 | 0.001260 | 0.97795 |
| 75 | 0.00418 | 0.93207 | 0.001370 | 0.97818 |
| 85 | 0.00460 | 0.84863 | 0.001560 | 0.89762 |
| Cobalt | Surface Chemical Reaction | Diffusion through Product Layer | ||
| kr [min−1] | R2 | kd [min−1] | R2 | |
| Temperature [°C] | ||||
| 25 | 0.000795 | 0.95444 | 0.000112 | 0.98040 |
| 35 | 0.001240 | 0.94991 | 0.000250 | 0.99603 |
| 45 | 0.002520 | 0.93979 | 0.000813 | 0.98779 |
| 55 | 0.002240 | 0.77820 | 0.000657 | 0.90657 |
| 65 | 0.004270 | 0.93907 | 0.001420 | 0.97884 |
| 75 | 0.004680 | 0.93907 | 0.001600 | 0.79229 |
| 85 | 0.005190 | 0.62942 | 0.001800 | 0.62508 |
| Lithium | Surface Chemical Reaction | Diffusion through Product Layer | ||
| kr [min−1] | R2 | kd [min−1] | R2 | |
| Temperature [°C] | ||||
| 25 | 0.001520 | 0.89476 | 0.000357 | 0.95108 |
| 35 | 0.001230 | 0.75774 | 0.000233 | 0.90290 |
| 45 | 0.001870 | 0.72296 | 0.000487 | 0.92073 |
| 55 | 0.003090 | 0.63294 | 0.001060 | 0.77527 |
| 65 | 0.004150 | 0.91846 | 0.001360 | 0.98077 |
| 75 | 0.004790 | 0.62489 | 0.001660 | 0.64111 |
| 85 | 0.005180 | 0.55799 | 0.001810 | 0.54256 |
| Metal | Slope | Ea [kJ·mole−1] | R2 |
|---|---|---|---|
| Ni | −8.43734 | 70.148045 | 0.95657 |
| Co | −3.3955 | 28.230187 | 0.93381 |
| Particle Size Category [µm] | Mean Particle Size [µm] | Ni | Co | ||
|---|---|---|---|---|---|
| kd [min−1] | R2 | kr [min−1] | R2 | ||
| 40–45 | 42.5 | 0.0014 | 0.9424 | 0.0044 | 0.7847 |
| 45–63 | 54 | 0.0016 | 0.8855 | 0.0051 | 0.6288 |
| 63–125 | 94 | 0.0016 | 0.8438 | 0.0053 | 0.5694 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerold, E.; Lerchbammer, R.; Antrekowitsch, H. Recovery of Cobalt, Nickel, and Lithium from Spent Lithium-Ion Batteries with Gluconic Acid Leaching Process: Kinetics Study. Batteries 2024, 10, 120. https://doi.org/10.3390/batteries10040120
Gerold E, Lerchbammer R, Antrekowitsch H. Recovery of Cobalt, Nickel, and Lithium from Spent Lithium-Ion Batteries with Gluconic Acid Leaching Process: Kinetics Study. Batteries. 2024; 10(4):120. https://doi.org/10.3390/batteries10040120
Chicago/Turabian StyleGerold, Eva, Reinhard Lerchbammer, and Helmut Antrekowitsch. 2024. "Recovery of Cobalt, Nickel, and Lithium from Spent Lithium-Ion Batteries with Gluconic Acid Leaching Process: Kinetics Study" Batteries 10, no. 4: 120. https://doi.org/10.3390/batteries10040120