Variable Porous Electrode Compression for Redox Flow Battery Systems
Abstract
:1. Introduction
2. Results
2.1. Reactant Concentration and Limiting Current Density
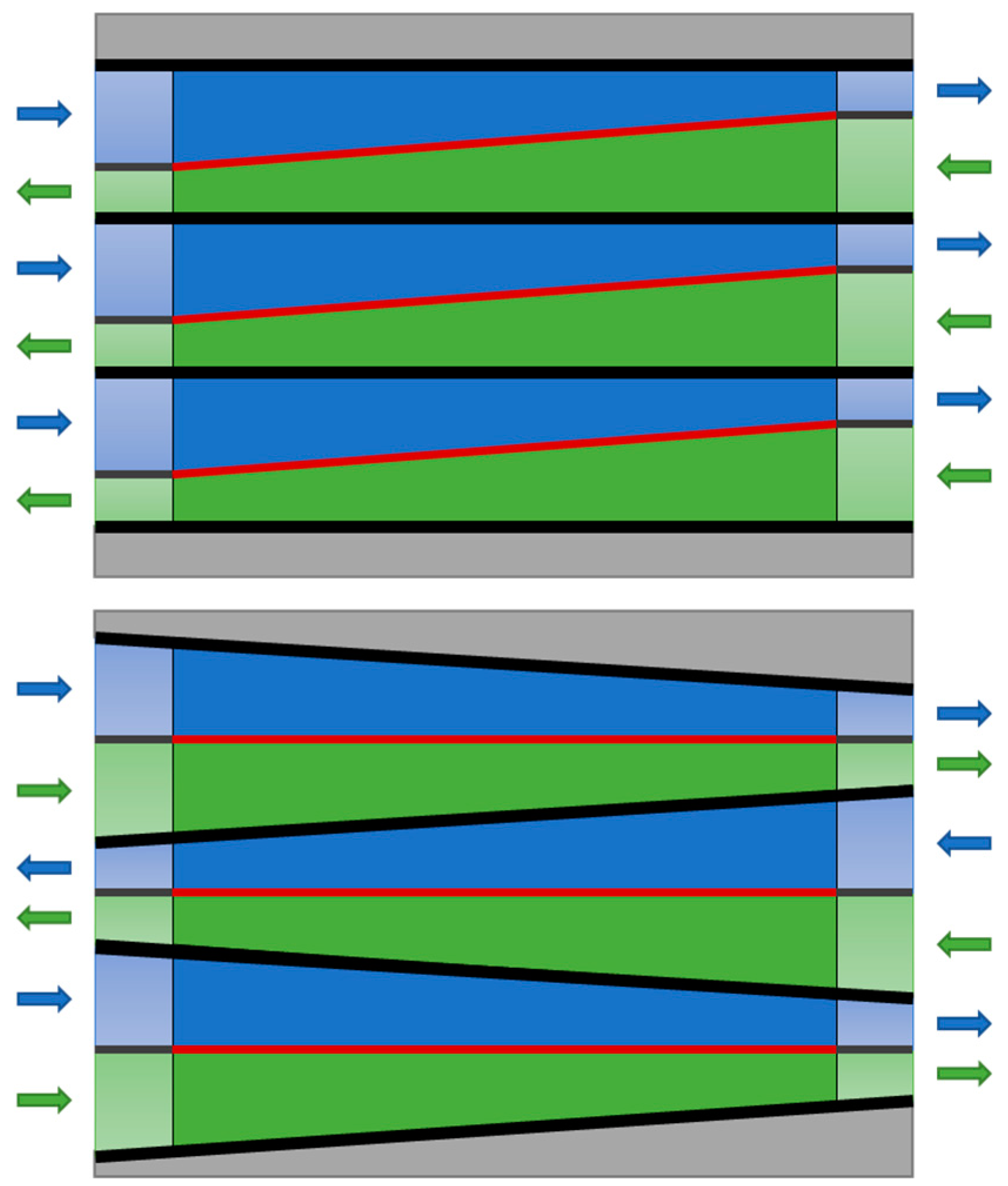
2.2. Effect of Cell Dimensions
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- Ryan, J.; Eckhouse, B. The Age of the Giant Battery is Almost upon Us, Bloomberg. Available online: https://www.bloomberg.com/news/articles/2017-02-21/big-batteries-coming-of-age-prompt-bankers-to-place-their-bets (accessed on 28 September 2018).
- Conca, J. Vanadium-Flow Batteries: The Energy Storage Breakthrough We’ve Needed. Available online: https://www.forbes.com/sites/jamesconca/2016/12/13/vanadium-flow-batteries-the-energy-storage-breakthrough-weve-needed/#7077b6885bde (accessed on 28 September 2018).
- Skyllas-Kazacos, M.; Menictas, C.; Lim, T. Redox flow batteries for medium to large scale energy storage. In Electricity Transmission, Distribution and Storage Systems; Melhem, Z., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 398–441. [Google Scholar]
- Tang, A.; Bao, J.; Skyllas-Kazacos, M. Studies on pressure losses and flow rate optimization in vanadium redox flow battery. J. Power Sources 2014, 248, 154–162. [Google Scholar] [CrossRef]
- Shah, A.A.; Watt-Smith, M.J.; Walsh, F.C. A dynamic performance model for redox-flow batteries involving soluble species. Electrochim. Acta 2008, 53, 8087–8100. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.A.; Al-Fetlawi, H.; Walsh, F.C. Dynamic modelling of hydrogen evolution effects in the all-vanadium redox flow battery. Electrochim. Acta 2010, 55, 1125–1139. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.A.; Tangirala, R.; Singh, R.; Wills, R.G.A.; Walsh, F.C. A Dynamic Unit Cell Model for the All-Vanadium Flow Battery. J. Electrochem. Soc. 2011, 158, A671–A677. [Google Scholar] [CrossRef]
- You, D.; Zhang, H.; Chen, J. A simple model for the vanadium redox battery. Electrochim. Acta 2009, 54, 6827–6836. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, H.; Xing, F. A three-dimensional model for negative half cell of the vanadium redox flow battery. Electrochim. Acta 2011, 58, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Knehr, K.W.; Agar, E.; Dennison, C.R.; Kalidindi, A.R.; Kumbur, E.C. A Transient Vanadium Flow Battery Model Incorporating Vanadium Crossover and Water Transport through the Membrane. J. Electrochem. Soc. 2012, 159, A1446–A1459. [Google Scholar] [CrossRef]
- Ke, X.; Alexander, J.I.D.; Prahl, J.M.; Savinell, R.F. Flow distribution and maximum current density studies in redox flow batteries with a single passage of the serpentine flow channel. J. Power Sources 2014, 270, 646–657. [Google Scholar] [CrossRef]
- Jyothi Latha, T.; Jayanti, S. Ex-situ experimental studies on serpentine flow field design for redox flow battery systems. J. Power Sources 2014, 248, 140–146. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S.; Leung, P.K. Numerical investigations of flow field designs for vanadium redox flow batteries. Appl. Energy 2013, 105, 47–56. [Google Scholar] [CrossRef]
- Zheng, Q.; Xing, F.; Li, X.; Liu, T.; Lai, Q.; Ning, G.; Zhang, H. Dramatic performance gains of a novel circular vanadium flow battery. J. Power Sources 2015, 277, 104–109. [Google Scholar] [CrossRef]
- Yue, M.; Zheng, Q.; Zhang, H.; Li, X.; Ma, X. Flow field design and optimization of high power density vanadium flow batteries: A novel trapezoid flow battery. AIChE J. 2017, 64, 13–18. [Google Scholar] [CrossRef]
- Park, S.K.; Shim, J.; Yang, J.H.; Jin, C.S.; Lee, B.S.; Lee, Y.S.; Shin, K.H.; Jeon, J.D. The influence of compressed carbon felt electrodes on the performance of a vanadium redox flow battery. Electrochim. Acta 2014, 116, 447–452. [Google Scholar] [CrossRef]
- Oh, K.; Won, S.; Ju, H. Numerical study of the effects of carbon felt electrode compression in all-vanadium redox flow batteries. Electrochim. Acta 2015, 181, 13–23. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, H.; Xing, F.; Ma, X.; Li, X.; Ning, G. A three-dimensional model for thermal analysis in a vanadium flow battery. Appl. Energy 2014, 113, 1675–1685. [Google Scholar] [CrossRef]


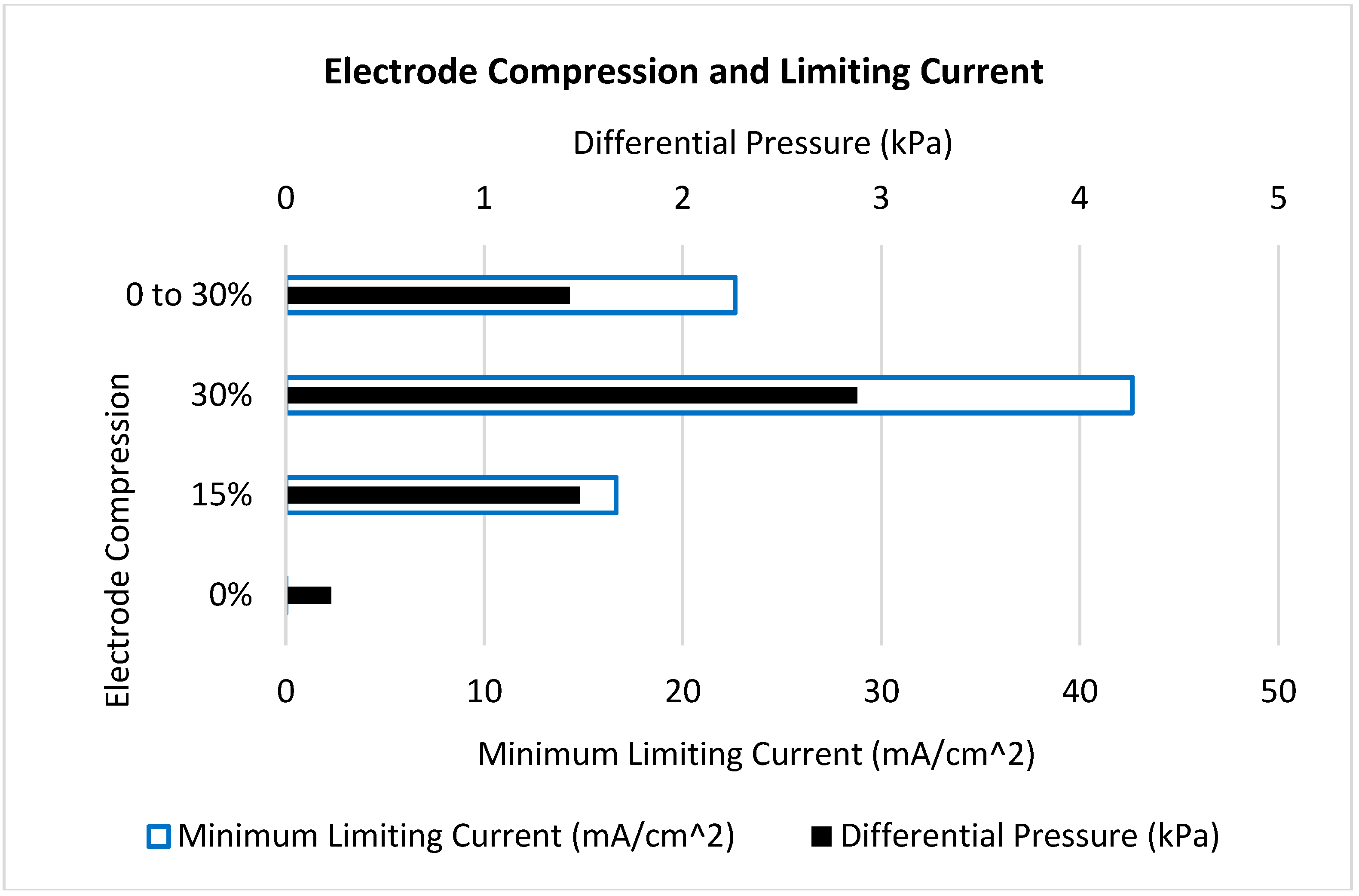




| Case | Geometry | Electrode Compression | Min. V3+ Concentration (mol m−3) | Min. Limiting Current (mA cm−2) | Differential Pressure (kPa) | Cell Voltage (V) |
|---|---|---|---|---|---|---|
| 1 | Uniform | 0% | 0 | 0 | 0.23 | 1.92 |
| 2 | Uniform | 15% | 46 | 17 | 1.48 | 1.72 |
| 3 | Uniform | 30% | 110 | 43 | 2.88 | 1.59 |
| 4 | Reducing | 0 to 30% | 58 | 23 | 1.43 | 1.69 |
| Dependent Variable | Improvement |
|---|---|
| Minimum V3+ Concentration (mol m−3) | 25% |
| Minimum Limiting Current (mA cm−2) | 36% |
| Differential Pressure (kPa) | 3% |
| Cell Voltage (V) | 1% |
| Thickness | Compression | Conductivity | Porosity | |
|---|---|---|---|---|
| mm | mm | % | S/m | |
| 4.0 | 0.0 | 0% | 5.9 | 0.95 |
| 3.6 | 0.4 | 10% | 14.3 | 0.90 |
| 3.2 | 0.8 | 20% | 20.0 | 0.89 |
| 2.8 | 1.2 | 30% | 50.0 | 0.87 |
| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Inlet velocity | 26 × 10−3 | m/s | |
| Outlet pressure | 0 | Pa | |
| Temperature | 280 | K | |
| Current density | 1600 | A m−2 | |
| State of Charge | 90 | - | |
| Cell width | 0.05 | m | |
| Cell length | 0.08 | m | |
| Membrane thickness | 0.123 × 10−3 | m |
| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Dynamic viscosity (negative electrolyte) | 0.0025 | Pa s | |
| Dynamic viscosity (positive electrolyte) | 0.005 | Pa s | |
| Density (negative electrolyte) | 1300 | kg m−3 | |
| Density (positive electrolyte) | 1350 | kg m−3 | |
| Mean pore radius | 50.3 × 10−6 | m | |
| Kozeny-Carman constant | 180 | – |
| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| V2+ diffusion coefficient | 2.4 × 10−10 | m2 s−1 | |
| V3+ diffusion coefficient | 2.4 × 10−10 | m2 s−1 | |
| VO2+ diffusion coefficient | 3.9 × 10−10 | m2 s−1 | |
| VO2+ diffusion coefficient | 3.9 × 10−10 | m2 s−1 | |
| Proton diffusion coefficient | 9.312 × 10−9 | m2 s−1 | |
| Initial vanadium concentration | 1500 | mol m−3 | |
| Initial proton concentration (negative) | 4500 | mol m−3 | |
| Initial proton concentration (positive) | 6000 | mol m−3 | |
| Standard reaction rate constant (negative) | 1.7 × 10−7 | m s−1 | |
| Standard reaction rate constant (positive) | 6.8 × 10−7 | m s−1 | |
| Anodic transfer coefficient | 0.5 | – | |
| Cathodic transfer coefficient | 0.5 | – | |
| Equilibrium potential: V2+/V3+ | −0.255 | V | |
| Equilibrium potential: VO2+/VO2+ | 1.004 | V |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurieff, N.; Timchenko, V.; Menictas, C. Variable Porous Electrode Compression for Redox Flow Battery Systems. Batteries 2018, 4, 53. https://doi.org/10.3390/batteries4040053
Gurieff N, Timchenko V, Menictas C. Variable Porous Electrode Compression for Redox Flow Battery Systems. Batteries. 2018; 4(4):53. https://doi.org/10.3390/batteries4040053
Chicago/Turabian StyleGurieff, Nicholas, Victoria Timchenko, and Chris Menictas. 2018. "Variable Porous Electrode Compression for Redox Flow Battery Systems" Batteries 4, no. 4: 53. https://doi.org/10.3390/batteries4040053







