Conversion of Spent Coffee Beans to Electrode Material for Vanadium Redox Flow Batteries
Abstract
:1. Introduction
2. Materials and Methods
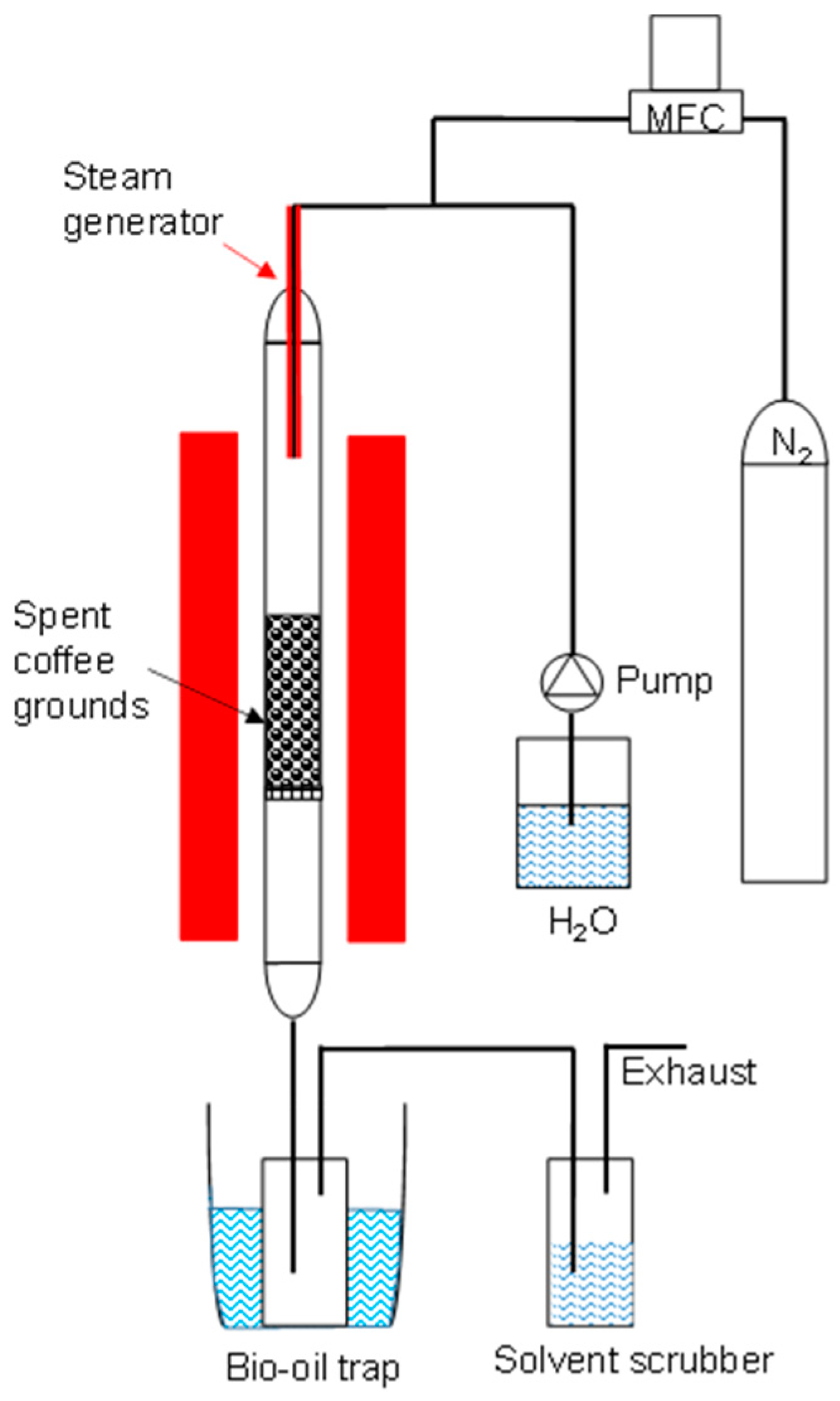
2.1. Preparation of Biochar and Activated Carbon
2.2. Characterization of Carbon Properties
2.3. Electrochemical Characterization of Carbon Material Based Electrodes
3. Results and Discussion
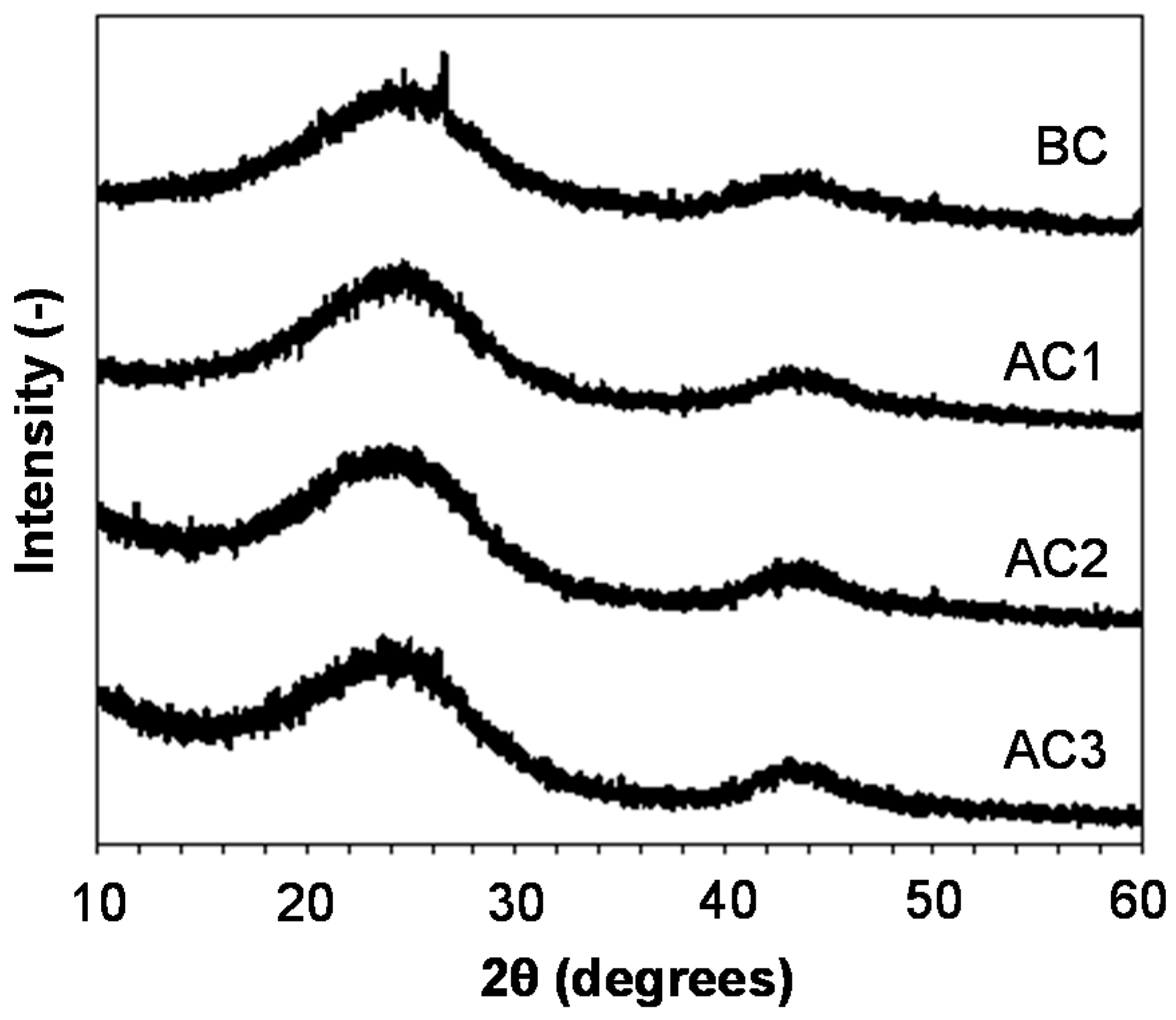
3.1. Carbon Properties
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, T.; Savinell, R.F. Flow Batteries. Electrochem. Soc. Interface 2010, 19, 54–56. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Chen, N.; Li, M.; Wang, C.; Ehrenberg, H.; Bie, X.; Wei, Y.; Chen, G.; Du, F. Na3V2(PO4)3/C composite as the intercalation-type anode material for sodium-ion batteries with superior rate capability and long-cycle life. J. Mater. Chem. A 2015, 3, 8636–8642. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Grossmith, F. Efficient Vanadium Redox Flow Cell. J. Electrochem. Soc. 1987, 2950–2953. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; McCann, J.F. Vanadium Redox Flow Batteries (VRBs) for Medium- and Large-Scale Energy Storage; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar]
- Park, M.; Ryu, J.; Cho, J. Nanostructured Electrocatalysts for All-Vanadium Redox Flow Batteries. Chem. Asian J. 2015, 10, 2096–2110. [Google Scholar] [CrossRef] [PubMed]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the all-vanadium redox flow battery for energy storage: A review of technological, financial and policy aspects. Int. J. Energy Res. 2011, 36, 1105–1120. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Yan, C. Multi-walled carbon nanotubes used as an electrode reaction catalyst for VO2+/VO2+ for a vanadium redox flow battery. Carbon 2011, 49, 3463–3470. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.-F.; Liu, Y.; Zhang, H.; Ren, Y.; Sun, C.-J.; Lu, W.; Zhou, Y.; Stanciu, L.; Stach, E.A.; et al. Graphene-modified nanostructured vanadium pentoxide hybrids with extraordinary electrochemical performance for Li-ion batteries. Nat. Commun. 2015, 6, 6127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Huang, J.-Q.; Qian, W.-Z.; Zhang, Y.-Y.; Wei, F. The Road for Nanomaterials Industry: A Review of Carbon Nanotube Production, Post-Treatment, and Bulk Applications for Composites and Energy Storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett. 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Jain, A.; Aravindan, V.; Jayaraman, S.; Ling, W.C.; Lim, T.M.; Srinivasan, M.P.; Yan, Q.; Madhavi, S. Bio-mass derived mesoporous carbon as superior electrode in all vanadium redox flow battery with multicouple reactions. J. Power Sources 2015, 274, 846–850. [Google Scholar] [CrossRef]
- Maharjan, M.; Bhattarai, A.; Ulaganathan, M.; Wai, N.; Oo, M.O.; Wang, J.-Y.; Lim, T.M. High surface area bio-waste based carbon as a superior electrode for vanadium redox flow battery. J. Power Sources 2017, 362, 50–56. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Zhu, Z.; Lu, G.Q. Nanoporous carbon electrode from waste coffee beans for high performance supercapacitors. Electrochem. Commun. 2008, 10, 1594–1597. [Google Scholar] [CrossRef]
- Li, X.; Strezov, V.; Kan, T. Energy recovery potential analysis of spent coffee grounds pyrolysis products. J. Anal. Appl. Pyrolysis 2014, 110, 79–87. [Google Scholar] [CrossRef]
- Pacioni, T.R.; Soares, D.; Domenico, M.D.; Rosa, M.F.; Moreira, R.D.; José, H.J. Bio-syngas production from agro-industrial biomass residues by steam gasification. Waste Manag. 2016, 58, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Nabais, J.M.V.; Laginhas, C.; Carrott, M.M.L.R.; Carrott, P.J.M.; Amorós, J.E.C.; Gisbert, A.V.N. Surface and porous characterisation of activated carbons made from a novel biomass precursor, the esparto grass. Appl. Surf. Sci. 2013, 265, 919–924. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-S.; Jang, Y.S.; Jeong, H.K. Bamboo-based activated carbon for supercapacitor applications. Curr. Appl. Phys. 2014, 14, 1616–1620. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Menictas, C.; Lim, T. Redox flow batteries for medium- to large-scale energy storage. In Electricity Transmission, Distribution and Storage Systems; Melhem, Z., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 398–441. [Google Scholar]
- Zhong, S.; Kazacos, M.; Burford, R.P.; Skyllas-Kazacos, M. Fabrication and activation studies of conducting plastic composite electrodes for redox cells. Power Sources 1991, 29–43. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Gu, M.; Nie, Z.; Wei, X.; Wang, C.; Sprenkle, V. Nanorod Niobium Oxide as Powerful Catalysts for an All Vanadium Redox Flow Battery; Nano Letters: Washington, DC, USA, 2013; pp. 158–165. [Google Scholar]
- Taylor, S.M.; Pătru, A.; Perego, D.; Fabbri, E.; Schmidt, T.J. Influence of Carbon Material Properties on Activity and Stability of the Negative Electrode in Vanadium Redox Flow Batteries: A Model Electrode Study. ACS Appl. Energy Mater. 2018, 1, 1166–1174. [Google Scholar] [CrossRef]
- Garg, B. Introduction to Flow Batteries: Theory and Applications; Stanford University: Stanford, CA, USA, 2016. [Google Scholar]
- Kennedy, L.J.; Vijaya, J.J.; Sekaran, G. Electrical Conductivity Study of Porous Carbons Derived from Rice Husk. Mater. Chem. Phys. 2004, 91, 471–476. [Google Scholar] [CrossRef]





| Sample | Yield (%) | Burn-Off (%) | Micropore Volume (mL g−1) | Total Pore Volume (mL g−1) | BET Specific Surface Area (m2 g−1) |
|---|---|---|---|---|---|
| BC | 26.1 | 0 | <0.01 | <0.01 | <1 |
| AC1 | 20.5 | 21 | 0.28 | 0.29 | 541 |
| AC2 | 11.8 | 55 | 0.47 | 0.55 | 817 |
| AC3 | 5.9 | 77 | 0.60 | 0.75 | 1113 |
| Sample | C | H | N | Ash |
|---|---|---|---|---|
| Spent coffee beans | 54.5 | 8.9 | 1.8 | - |
| BC | 81.2 | 1.3 | 2.1 | 5.7 |
| AC1 | 82.3 | 2.2 | 1.4 | 3.3 |
| AC2 | 83.0 | 2.9 | 1.0 | 3.9 |
| AC3 | 83.1 | 2.4 | 0.7 | 4.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krikstolaityte, V.; Joshua, O.E.Y.; Veksha, A.; Wai, N.; Lisak, G.; Lim, T.M. Conversion of Spent Coffee Beans to Electrode Material for Vanadium Redox Flow Batteries. Batteries 2018, 4, 56. https://doi.org/10.3390/batteries4040056
Krikstolaityte V, Joshua OEY, Veksha A, Wai N, Lisak G, Lim TM. Conversion of Spent Coffee Beans to Electrode Material for Vanadium Redox Flow Batteries. Batteries. 2018; 4(4):56. https://doi.org/10.3390/batteries4040056
Chicago/Turabian StyleKrikstolaityte, Vida, Oh En Yao Joshua, Andrei Veksha, Nyunt Wai, Grzegorz Lisak, and Tuti Mariana Lim. 2018. "Conversion of Spent Coffee Beans to Electrode Material for Vanadium Redox Flow Batteries" Batteries 4, no. 4: 56. https://doi.org/10.3390/batteries4040056




