Nanostructured Manganese Dioxide for Hybrid Supercapacitor Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Physical-Chemical Characterization of MnO2 Samples
2.2. Preparation of Electrodes
2.3. Electrochemical Characterization
3. Results and Discussion
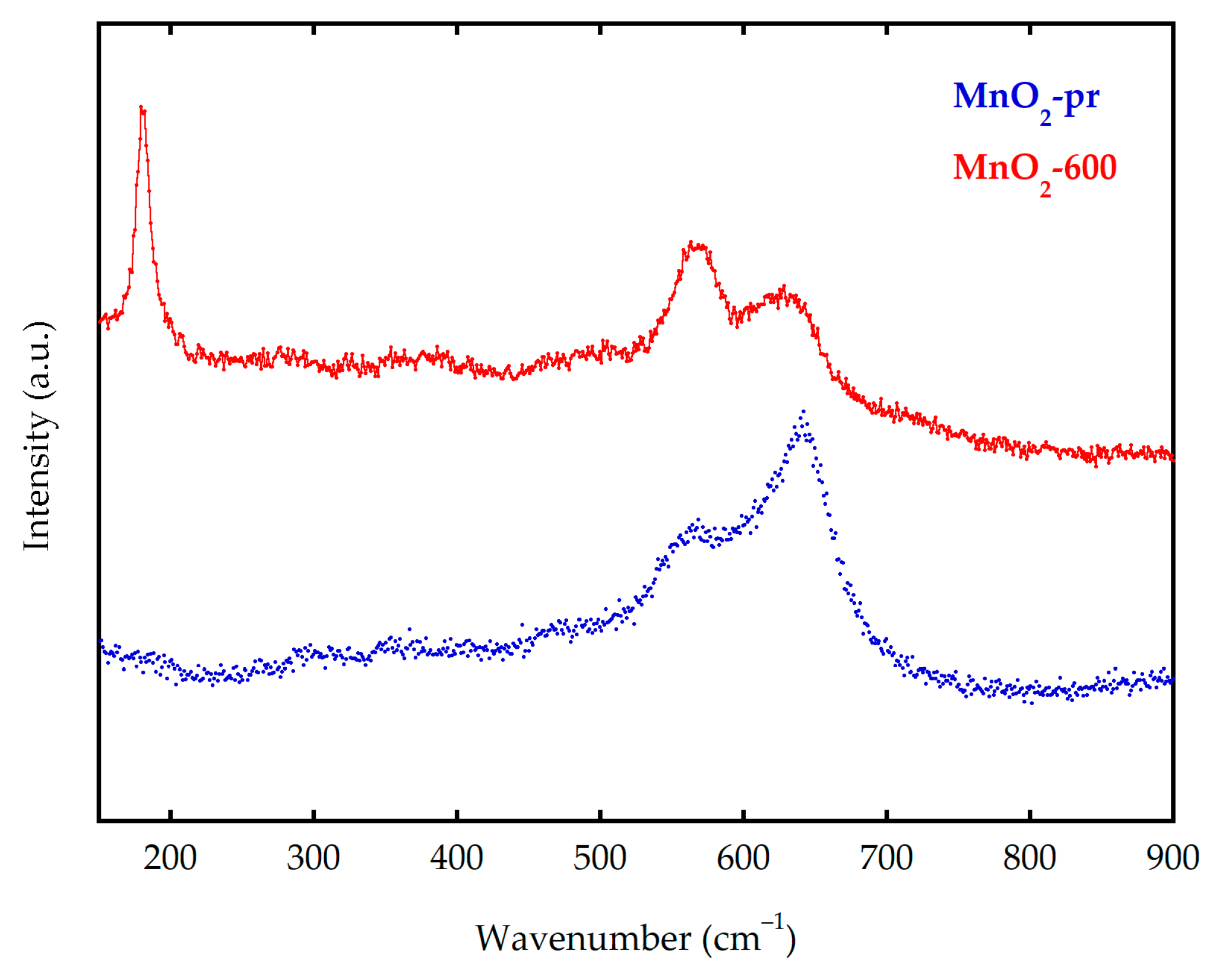
3.1. Structural Characterization of MnO2 Composite Electrodes
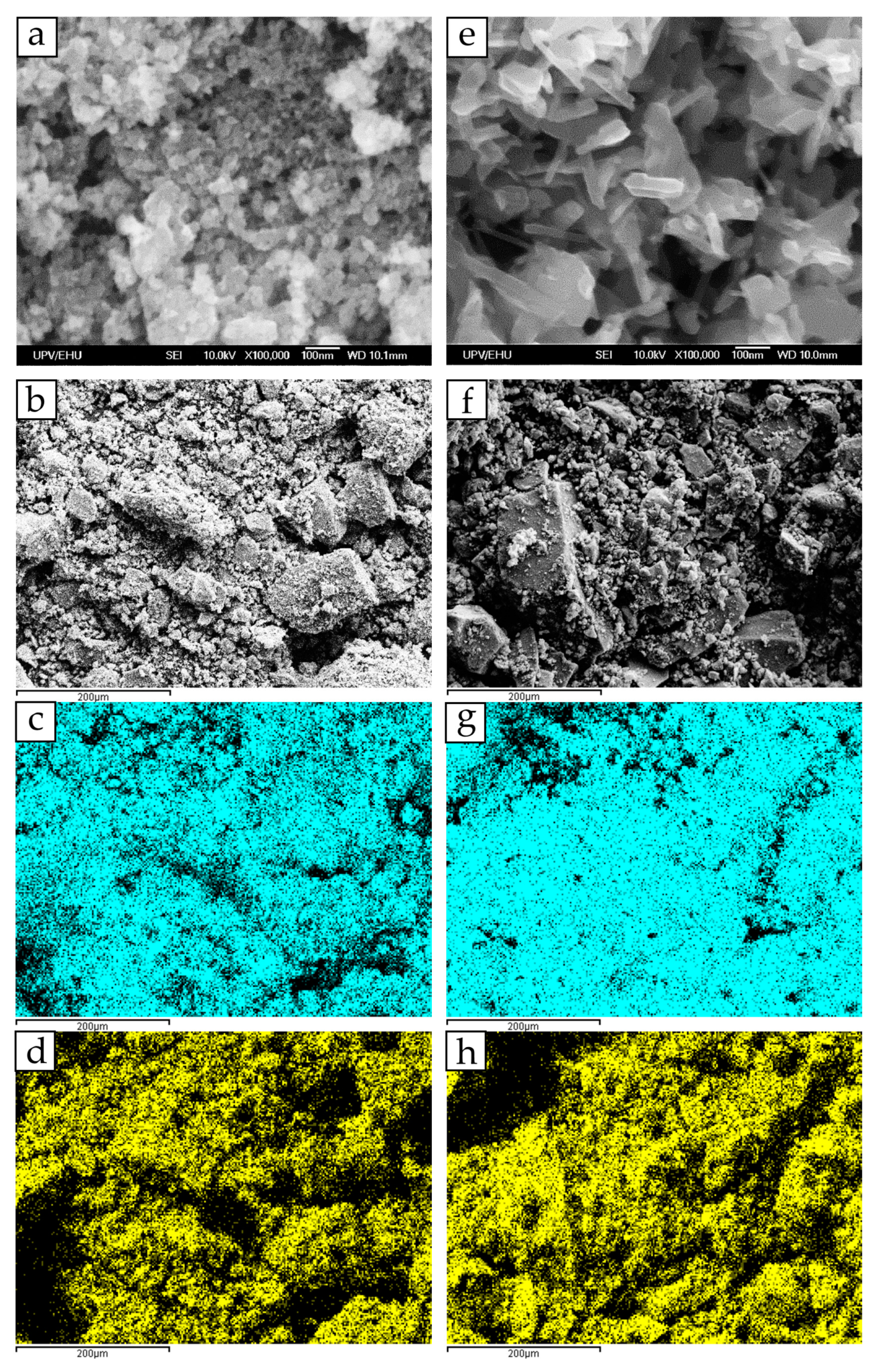
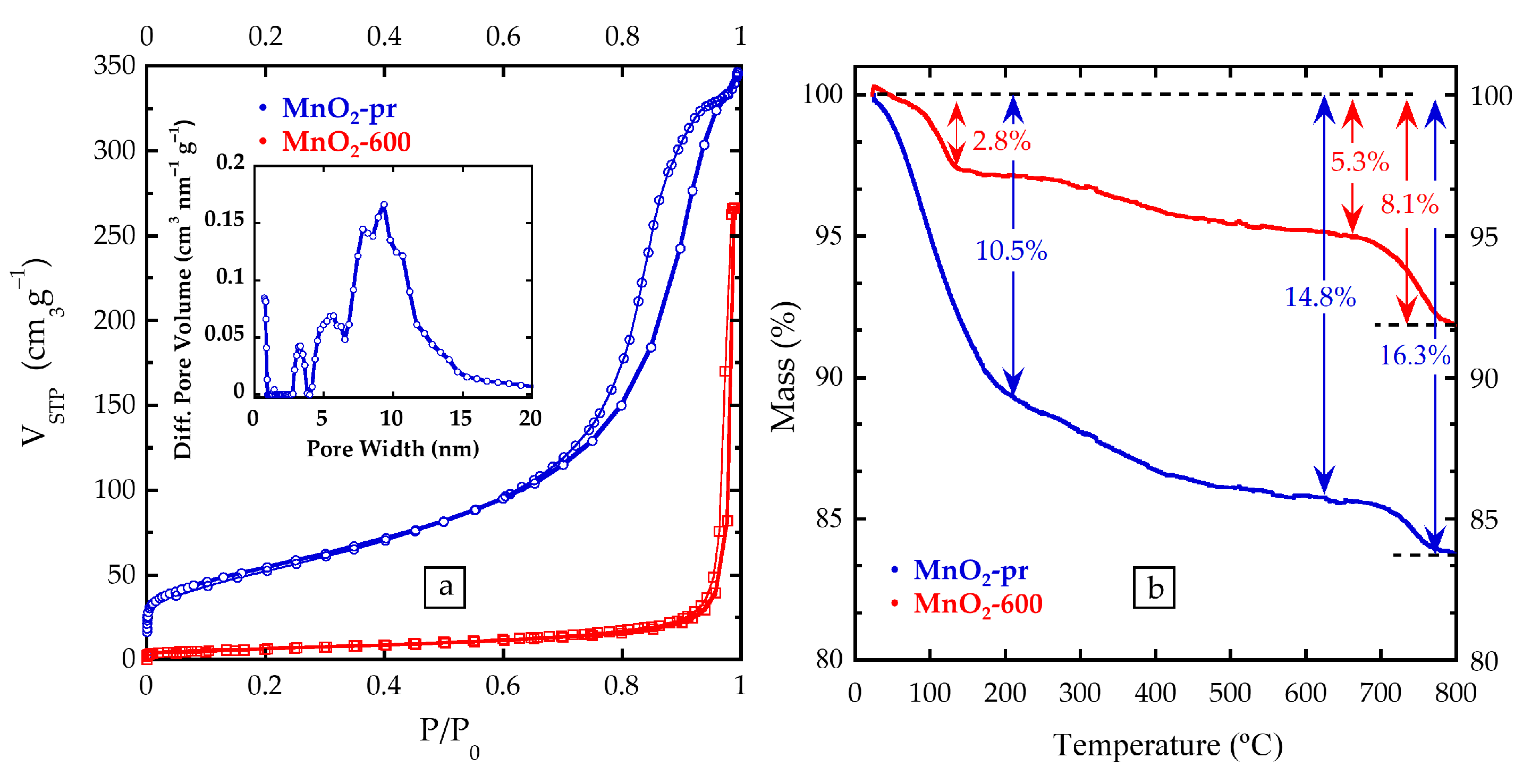
3.2. Morphology and Specific Surface Area
3.3. Compositional Characterization of MnO2: Thermogravimetric Analysis and Inductively Coupled Plasma-Atomic Emission Spectroscopy
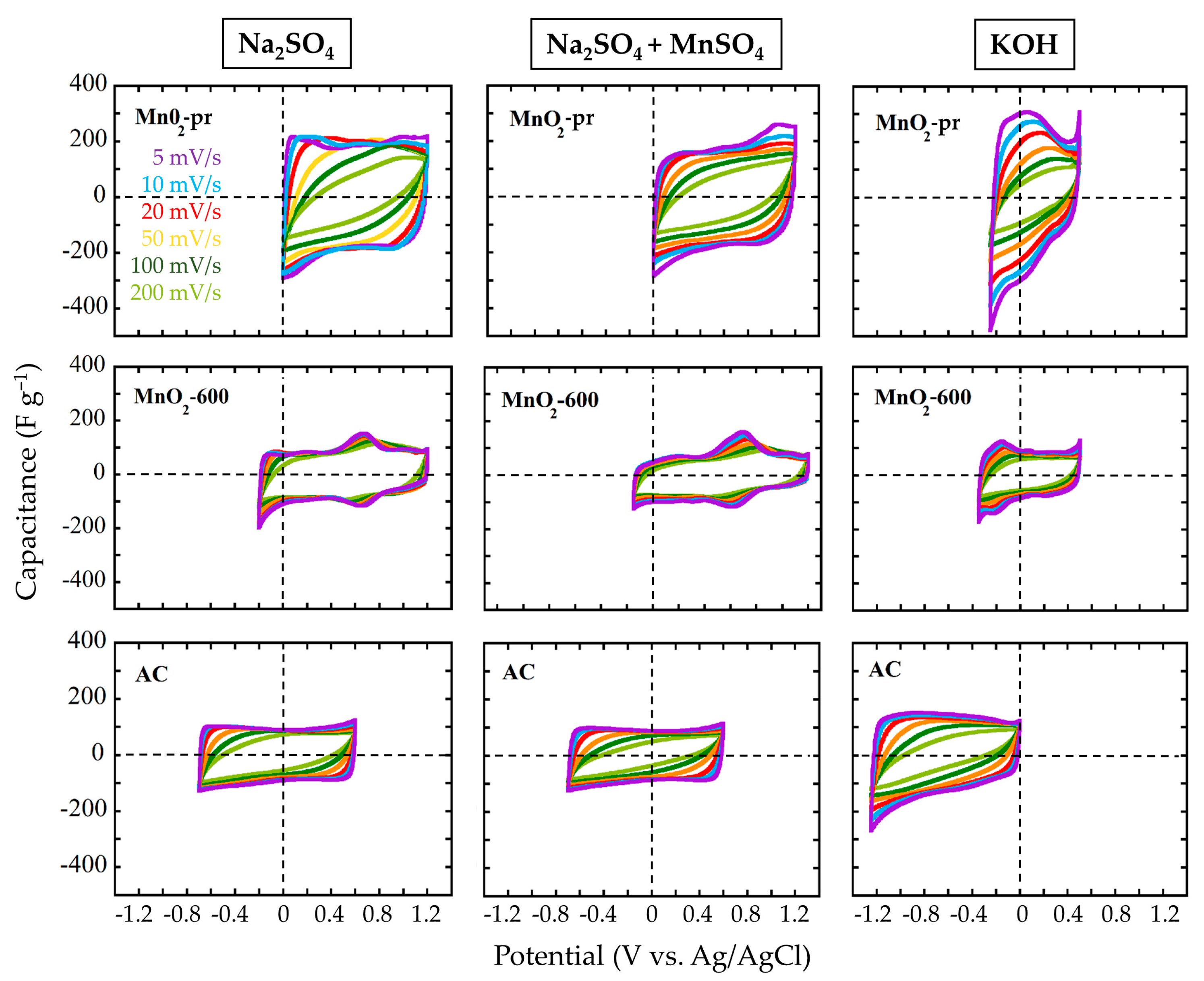
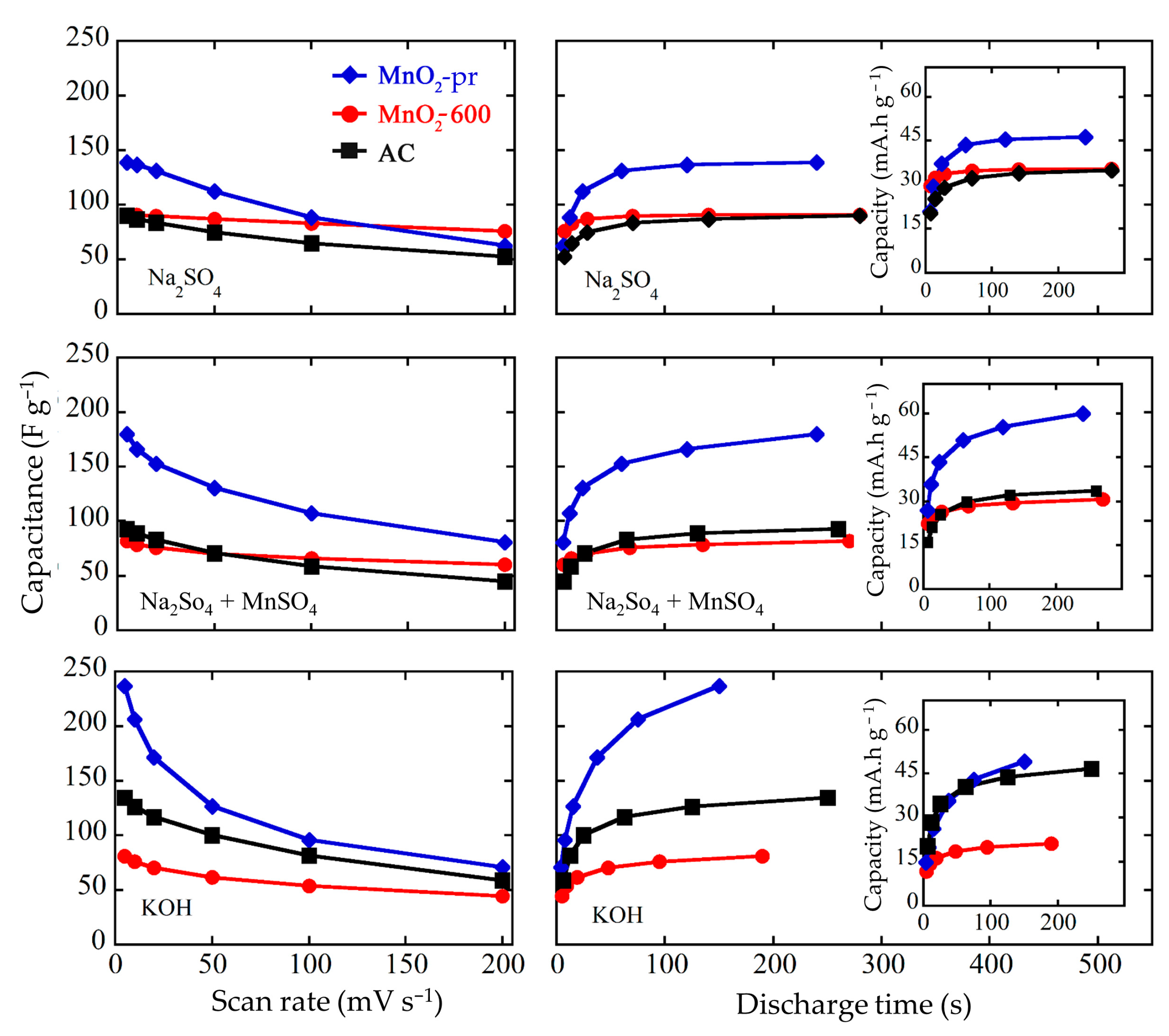
3.4. Electrochemical Characterization of MnO2 Electrodes
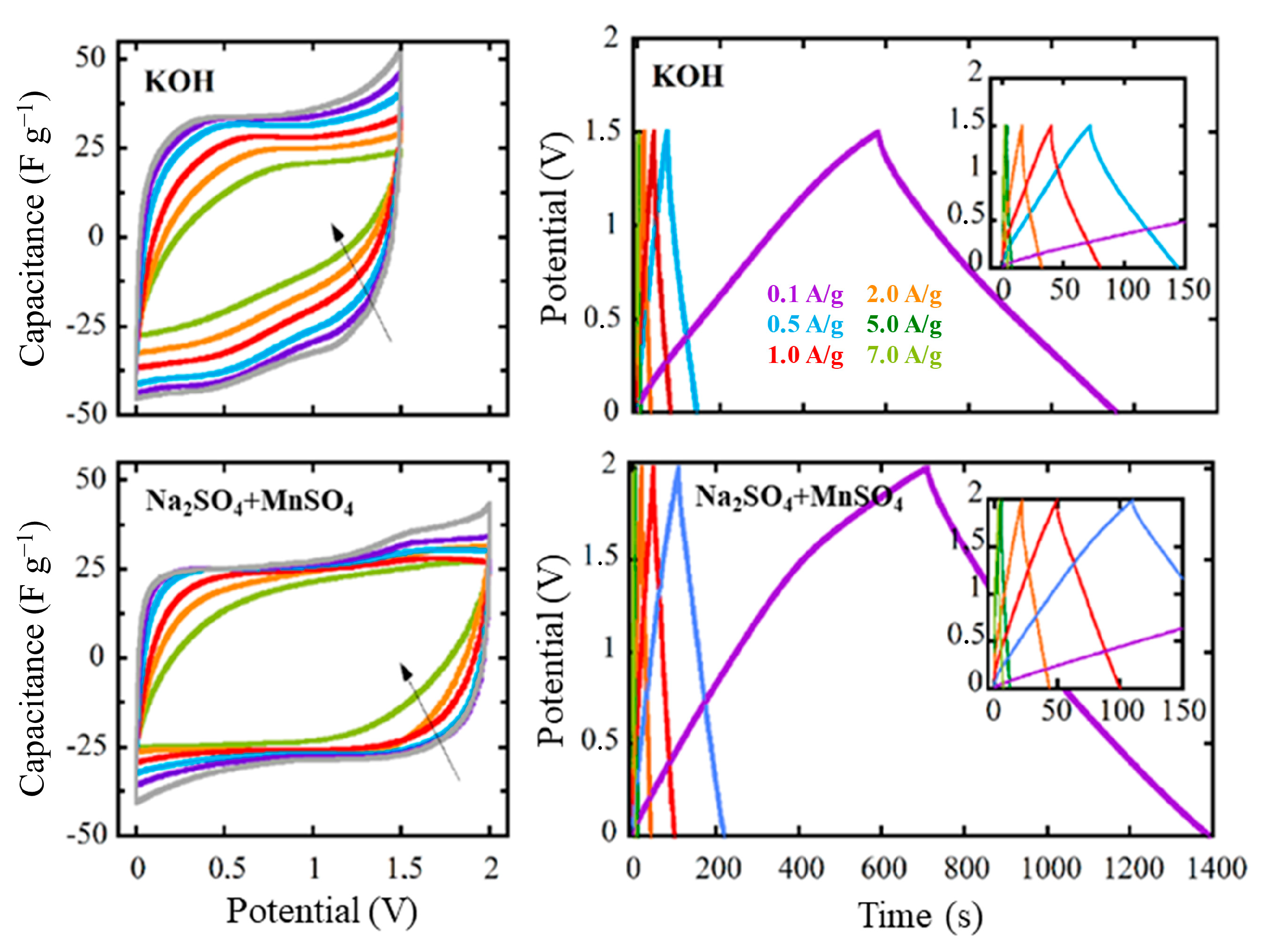
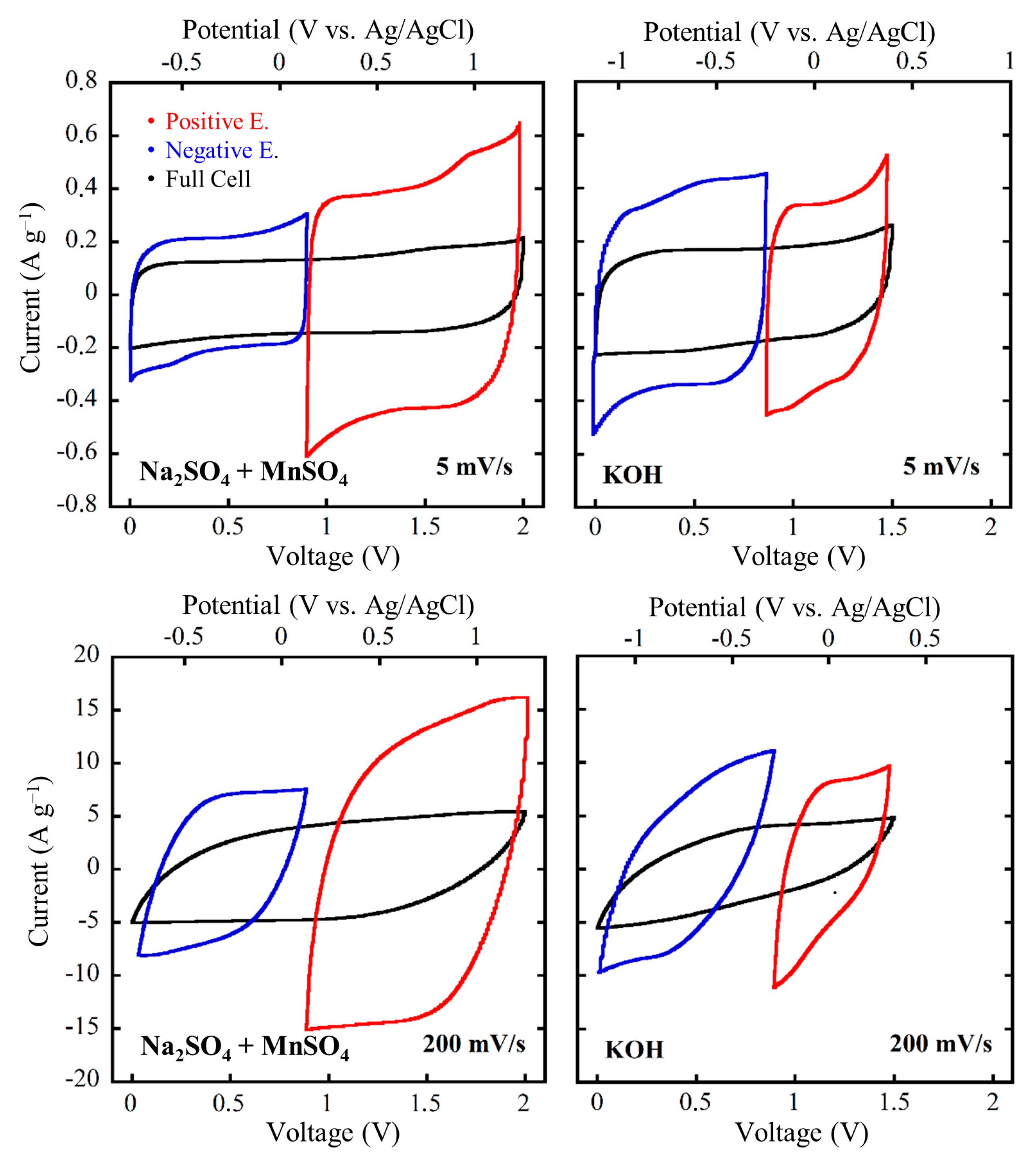
3.5. Electrochemical Characterization of the Final Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kondrat, S.; Georgi, N.; Fedorov, M.V.; Kornyshev, A.A. A Superionic State in Nano-Porous Double-Layer Capacitors: Insights from Monte Carlo Simulations. Proc. Phys. Chem. Chem. Phys. 2011, 13, 11359–11366. [Google Scholar] [CrossRef] [PubMed]
- Shanti, S.; Ravi, S. Electrochemical Study of Al Doped MnO2 Nanorods Over Stainless Steel Substrate. Int. J. Chemtech. Res. 2014, 6, 2066–2068. [Google Scholar]
- Cui, Z.; Kang, L.; Li, L.; Wang, L.; Wang, K. A Hybrid Neural Network Model with Improved Input for State of Charge Estimation of Lithium-Ion Battery at Low Temperatures. Renew. Energy 2022, 198, 1328–1340. [Google Scholar] [CrossRef]
- Li, D.; Yang, D.; Li, L.; Wang, L.; Wang, K. Electrochemical Impedance Spectroscopy Based on the State of Health Estimation for Lithium-Ion Batteries. Energies 2022, 15, 6665. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Z.; Chen, Y.; He, H.; Li, X.; Qin, T.; Wang, Y. High-Performance Supercapacitors of Ruthenium-Based Nanohybrid Compounds. J. Alloys Compd. 2020, 842, 155798. [Google Scholar] [CrossRef]
- Goda, E.S.; Lee, S.; Sohail, M.; Yoon, K.R. Prussian Blue and Its Analogues as Advanced Supercapacitor Electrodes. J. Energy Chem. 2020, 50, 206–229. [Google Scholar] [CrossRef]
- Dubal, D.P.; Wu, Y.P.; Holze, R. Supercapacitors: From the Leyden Jar to Electric Busses. ChemTexts 2016, 2, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mamun, A.-A.; Liu, Z.; Rizzo, D.M.; Onori, S. An Integrated Design and Control Optimization Framework for Hybrid Military Vehicle Using Lithium-Ion Battery and Supercapacitor as Energy Storage Devices. IEEE Trans. Transp. Electrif. 2019, 5, 239–251. [Google Scholar] [CrossRef]
- Zhan, Y.; Guo, Y.; Zhu, J.; Li, L. Power and Energy Management of Grid/PEMFC/Battery/Supercapacitor Hybrid Power Sources for UPS Applications. Int. J. Electr. Power Energy Syst. 2015, 67, 598–612. [Google Scholar] [CrossRef] [Green Version]
- Smolinka, T.; Ojong, E.T. Electrochemical Energy Storage for Renewable Sources and Grid Balancing. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 103–128. [Google Scholar]
- Pan, H.; Li, J.; Feng, Y.P. Carbon Nanotubes for Supercapacitor. Nanoscale Res. Lett. 2010, 5, 654–668. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Li, F.; Cheng, H.M. Carbon Nanotubes and Graphene for Flexible Electrochemical Energy Storage: From Materials to Devices. Adv. Mater. 2016, 28, 4306–4337. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ni, M.; Ren, X.; Tian, Y.; Li, N.; Su, Y.; Zhang, X. Graphene in Supercapacitor Applications. Curr. Opin. Colloid Interface Sci. 2015, 20, 416–428. [Google Scholar] [CrossRef]
- Ghosh, A.; Lee, Y.H. Carbon-Based Electrochemical Capacitors. ChemSusChem 2012, 5, 480–499. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, G.S.; de Oliveira, H.P.; Larsson, S.H.; Thyrel, M.; Lima, E.C. A Short Review on the Electrochemical Performance of Hierarchical and Nitrogen-Doped Activated Biocarbon-Based Electrodes for Supercapacitors. Nanomaterials 2021, 11, 1–17. [Google Scholar]
- dos Reis, G.S.; Larsson, S.H.; de Oliveira, H.P.; Thyrel, M.; Lima, E.C. Sustainable Biomass Activated Carbons as Electrodes for Battery and Supercapacitors—A Mini-Review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef]
- Hu, W.; Xiang, R.; Lin, J.; Cheng, Y.; Lu, C. Lignocellulosic Biomass-Derived Carbon Electrodes for Flexible Supercapacitors: An Overview. Materials 2021, 14, 4571. [Google Scholar] [CrossRef]
- Choi, N.S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. 2012, 51, 9994–10024. [Google Scholar] [CrossRef]
- Xiong, W.; Liu, M.; Gan, L.; Lv, Y.; Li, Y.; Yang, L.; Xu, Z.; Hao, Z.; Liu, H.; Chen, L. A Novel Synthesis of Mesoporous Carbon Microspheres for Supercapacitor Electrodes. J. Power Sources 2011, 196, 10461–10464. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P. Preparation of High-Surface-Area Activated Carbons from Coconut Shell; Elsevier: Amsterdam, The Netherlands, 1999; Volume 27. [Google Scholar]
- Lu, X.; Shen, C.; Zhang, Z.; Barrios, E.; Zhai, L. Core-Shell Composite Fibers for High-Performance Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 4041–4049. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Xu, C.; Ma, X.; Huang, G.; Lu, C.; Li, Z. Mechanisms of Porous Carbon-Based Supercapacitors. ChemNanoMat 2021, 7, 1273–1290. [Google Scholar] [CrossRef]
- Gummow, R.J.; de Kock, A.; Thackeray, M.M. Improved Capacity Retention in Rechargeable 4 V Lithium/Lithium-Manganese Oxide (Spinel) Cells. Solid State Ion. 1994, 69, 59–67. [Google Scholar] [CrossRef]
- Shin, J.; Seo, J.K.; Yaylian, R.; Huang, A.; Meng, Y.S. A Review on Mechanistic Understanding of MnO2 in Aqueous Electrolyte for Electrical Energy Storage Systems. Int. Mater. Rev. 2020, 65, 356–387. [Google Scholar] [CrossRef]
- Qi, H.; Bo, Z.; Yang, S.; Duan, L.; Yang, H.; Yan, J.; Cen, K.; Ostrikov, K. (Ken) Hierarchical Nanocarbon-MnO2 Electrodes for Enhanced Electrochemical Capacitor Performance. Energy Storage Mater. 2019, 16, 607–618. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Sol-Gel MnO2 as an Electrode Material for Electrochemical Capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Ohzuku, T.; Kitigawa, M.; Hirai, T. Electrochemistry of Manganese Dioxide in Lithium Nonaqueous Cell. J. Electrochem. Soc. 1990, 137, 769–775. [Google Scholar] [CrossRef]
- Shrestha, D.; Maensiri, S.; Wongpratat, U.; Lee, S.W.; Nyachhyon, A.R. Shorea Robusta Derived Activated Carbon Decorated with Manganese Dioxide Hybrid Composite for Improved Capacitive Behaviors. J. Environ. Chem. Eng. 2019, 7, 1137–1146. [Google Scholar] [CrossRef]
- Lee, J.Y.; Liang, K.; An, K.H.; Lee, Y.H. Nickel Oxide/Carbon Nanotubes Nanocomposite for Electrochemical Capacitance. Synth. Met. 2005, 150, 153–157. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, J.; Liu, M.; Li, Y.; Ruan, J.; Li, Q.; Tian, J.; Zhu, X.; Zhang, X.; Wei, Y. Synthesis of Polyacrylamide Immobilized Molybdenum Disulfide (MoS2@PDA@PAM) Composites via Mussel-Inspired Chemistry and Surface-Initiated Atom Transfer Radical Polymerization for Removal of Copper (II) Ions. J. Taiwan Inst. Chem. Eng. 2018, 86, 174–184. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Chen, W.; Hedhili, M.N.; Cha, D.; Alshareef, H.N. Enhanced Rate Performance of Mesoporous Co3O4 Nanosheet Supercapacitor Electrodes by Hydrous RuO2 Nanoparticle Decoration. ACS Appl. Mater. Interfaces 2014, 6, 4196–4206. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.C. Graphene and Carbon Nanotube Composite Electrodes for Supercapacitors with Ultra-High Energy Density. Phys. Chem. Chem. Phys. 2011, 13, 17615–17624. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, P.; Wang, B.; Chen, Y.; Tian, S.; Wu, Y.; Holze, R. Electrochemical Performance of MnO2 Nanorods in Neutral Aqueous Electrolytes as a Cathode for Asymmetric Supercapacitors. J. Phys. Chem. C 2009, 113, 14020–14027. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Jayachandran, M.; Rose, A.; Maiyalagan, T.; Poongodi, N.; Vijayakumar, T. Effect of Various Aqueous Electrolytes on the Electrochemical Performance of α-MnO2 Nanorods as Electrode Materials for Supercapacitor Application. Electrochim. Acta 2020, 366, 137412. [Google Scholar] [CrossRef]
- Xu, C.; Kang, F.; Li, B.; Du, H. Recent Progress on Manganese Dioxide Based Supercapacitors. J. Mater. Res. 2010, 25, 1421–1432. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese Oxide-Based Materials as Electrochemical Supercapacitor Electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Han, G.; Liu, Y.; Zhang, L.; Kan, E.; Zhang, S.; Tang, J.; Tang, W. MnO2 Nanorods Intercalating Graphene Oxide/Polyaniline Ternary Composites for Robust High-Performance Supercapacitors. Sci. Rep. 2014, 4, 4824. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Jin, M.; Yao, F.; Kim, T.H.; Le, V.T.; Yue, H.; Gunes, F.; Li, B.; Ghosh, A.; Xie, S.; et al. Asymmetric Supercapacitors Based on Graphene/MnO2 Nanospheres and Graphene/MoO3 Nanosheets with High Energy Density. Adv. Funct. Mater. 2013, 23, 5074–5083. [Google Scholar] [CrossRef]
- Babakhani, B.; Ivey, D.G. Improved Capacitive Behavior of Electrochemically Synthesized Mn Oxide/PEDOT Electrodes Utilized as Electrochemical Capacitors. Electrochim. Acta 2010, 55, 4014–4024. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Shaijumon, M.M.; Gowda, S.R.; Ajayan, P.M. Multisegmented Au-MnO2/Carbon Nanotube Hybrid Coaxial Arrays for High-Power Supercapacitor Applications. J. Phys. Chem. C 2010, 114, 658–663. [Google Scholar] [CrossRef]
- Xu, M.; Kong, L.; Zhou, W.; Li, H. Hydrothermal Synthesis and Pseudocapacitance Properties of α-MnO2 Hollow Spheres and Hollow Urchins. J. Phys. Chem. C 2007, 111, 19141–19147. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Zhang, Y.; Zhang, D.; Du, W.; Zhang, X.; Wang, B. MnO2/Carbon Composites for Supercapacitor: Synthesis and Electrochemical Performance. Front. Mater. 2020, 7, 2. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Brief communication: Supercapacitor Behavior with KCl Electrolyte. J. Solid. State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Brousse, T.; Toupin, M.; Dugas, R.; Athouël, L.; Crosnier, O.; Bélanger, D. Crystalline MnO[Sub 2] as Possible Alternatives to Amorphous Compounds in Electrochemical Supercapacitors. J. Electrochem. Soc. 2006, 153, A2171. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, D.; Zhai, T.; Liu, Z.; Huang, Y.; Xie, S.; Tong, Y. Facile Synthesis of Large-Area Manganese Oxide Nanorod Arrays as a High-Performance Electrochemical Supercapacitor. Energy Environ. Sci. 2011, 4, 2915–2921. [Google Scholar] [CrossRef]
- Wang, J.-W.; Chen, Y.; Chen, B.-Z. A Synthesis Method of MnO2/Activated Carbon Composite for Electrochemical Supercapacitors. J. Electrochem. Soc. 2015, 162, A1654–A1661. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.W.; Lee, H.Y. Expansion of Active Site Area and Improvement of Kinetic Reversibility in Electrochemical Pseudocapacitor Electrode. Electrochem. Solid-State Lett. 2001, 4, A19–A22. [Google Scholar] [CrossRef]
- Chen, P.C.; Shen, G.; Shi, Y.; Chen, H.; Zhou, C. Preparation and Characterization of Flexible Asymmetric Supercapacitors Based on Transition-Metal-Oxide Nanowire/Single-Walled Carbon Nanotube Hybrid Thin-Film Electrodes. ACS Nano 2010, 4, 4403–4411. [Google Scholar] [CrossRef]
- Ragupathy, P.; Park, D.H.; Campet, G.; Vasan, H.N.; Hwang, S.J.; Choy, J.H.; Munichandraiah, N. Remarkable Capacity Retention of Nanostructured Manganese Oxide upon Cycling as an Electrode Material for Supercapacitor. J. Phys. Chem. C 2009, 113, 6303–6309. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Dong, Z.; Wu, X.; Situ, Y.; Huang, H. Tuning Mn 2+ Additive in the Aqueous Electrolyte for Enhanced Cycling Stability of Birnessite Electrodes. Electrochim. Acta 2019, 298, 678–684. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M.; Baddour-Hadjean, R.; Franger, S.; Bach, S.; Pereira-Ramos, J.P. Raman Spectra of Birnessite Manganese Dioxides. Solid State Ion. 2003, 159, 345–356. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice Vibrations of Manganese Oxides: Part I. Periodic Structures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Gao, T.; Fjellvåg, H.; Norby, P. A Comparison Study on Raman Scattering Properties of α- and β-MnO2. Anal. Chim. Acta 2009, 648, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Buciuman, F.; Patcas, F.; Craciun, R.; Zahn, D.R.T. Vibrational Spectroscopy of Bulk and Supported Manganese Oxides. Phys. Chem. Chem. Phys. 1998, 1, 185–190. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ghodbane, O.; Pascal, J.L.; Fraisse, B.; Favier, F. Structural in Situ Study of the Thermal Behavior of Manganese Dioxide Materials: Toward Selected Electrode Materials for Supercapacitors. ACS Appl. Mater. Interfaces 2010, 2, 3493–3505. [Google Scholar] [CrossRef]
- Yang, X.; Makita, Y.; Liu, Z.H.; Sakane, K.; Ooi, K. Structural Characterization of Self-Assembled MnO2 Nanosheets from Birnessite Manganese Oxide Single Crystals. Chem. Mater. 2004, 16, 5581–5588. [Google Scholar] [CrossRef]
- Valente, J.S.; Frías, D.; Navarro, P.; Montes, M.; Delgado, J.J.; Fregoso-Israel, E.; Torres-García, E. Manganese Cryptomelane-Type Oxides: A Thermo-Kinetic and Morphological Study. Appl. Surf. Sci. 2008, 254, 3006–3013. [Google Scholar] [CrossRef]
- Qu, Q.; Li, L.; Tian, S.; Guo, W.; Wu, Y.; Holze, R. A Cheap Asymmetric Supercapacitor with High Energy at High Power: Activated Carbon//K0.27MnO2·0.6H2O. J. Power Sources 2010, 195, 2789–2794. [Google Scholar] [CrossRef]
- Goikolea, E.; Mysyk, R. Nanotechnology in Electrochemical Capacitors. In Emerging Nanotechnologies in Rechargable Energy Storage Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–169. [Google Scholar]
- Bichat, M.P.; Raymundo-Piñero, E.; Béguin, F. High Voltage Supercapacitor Built with Seaweed Carbons in Neutral Aqueous Electrolyte. Carbon 2010, 48, 4351–4361. [Google Scholar] [CrossRef]
- Sharma, R.K.; Oh, H.S.; Shul, Y.G.; Kim, H. Carbon-Supported, Nano-Structured, Manganese Oxide Composite Electrode for Electrochemical Supercapacitor. J. Power Sources 2007, 173, 1024–1028. [Google Scholar] [CrossRef]
- Li, S.; Gao, L.; Wang, L.; Wang, M.; Shen, Y. Hierarchical MnO2 Located on Carbon Nanotubes for Enhanced Electrochemical Performance. ChemElectroChem 2018, 5, 1525–1531. [Google Scholar] [CrossRef]
- Wen, J.; Chen, X.; Huang, M.; Yang, W.; Deng, J. Core-Shell-Structured MnO2 @carbon Spheres and Nitrogen-Doped Activated Carbon for Asymmetric Supercapacitors with Enhanced Energy Density. J. Chem. Sci. 2020, 132, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Guo, L.; Han, D.; Li, B.; Gong, Z. Manganese Oxide/Hierarchical Porous Carbon Nanocomposite from Oily Sludge for High-Performance Asymmetric Supercapacitors. Electrochim. Acta 2018, 265, 71–77. [Google Scholar] [CrossRef]
- Li, M.; Chen, Q.; Zhan, H. Ultrathin Manganese Dioxide Nanosheets Grown on Partially Unzipped Nitrogen-Doped Carbon Nanotubes for High-Performance Asymmetric Supercapacitors. J. Alloys Compd. 2017, 702, 236–243. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Romero, J.; Ruiz de Larramendi, I.; Goikolea, E. Nanostructured Manganese Dioxide for Hybrid Supercapacitor Electrodes. Batteries 2022, 8, 263. https://doi.org/10.3390/batteries8120263
Rodriguez-Romero J, Ruiz de Larramendi I, Goikolea E. Nanostructured Manganese Dioxide for Hybrid Supercapacitor Electrodes. Batteries. 2022; 8(12):263. https://doi.org/10.3390/batteries8120263
Chicago/Turabian StyleRodriguez-Romero, Jon, Idoia Ruiz de Larramendi, and Eider Goikolea. 2022. "Nanostructured Manganese Dioxide for Hybrid Supercapacitor Electrodes" Batteries 8, no. 12: 263. https://doi.org/10.3390/batteries8120263






