An Experimentally Parameterized Equivalent Circuit Model of a Solid-State Lithium-Sulfur Battery
Abstract
:1. Introduction
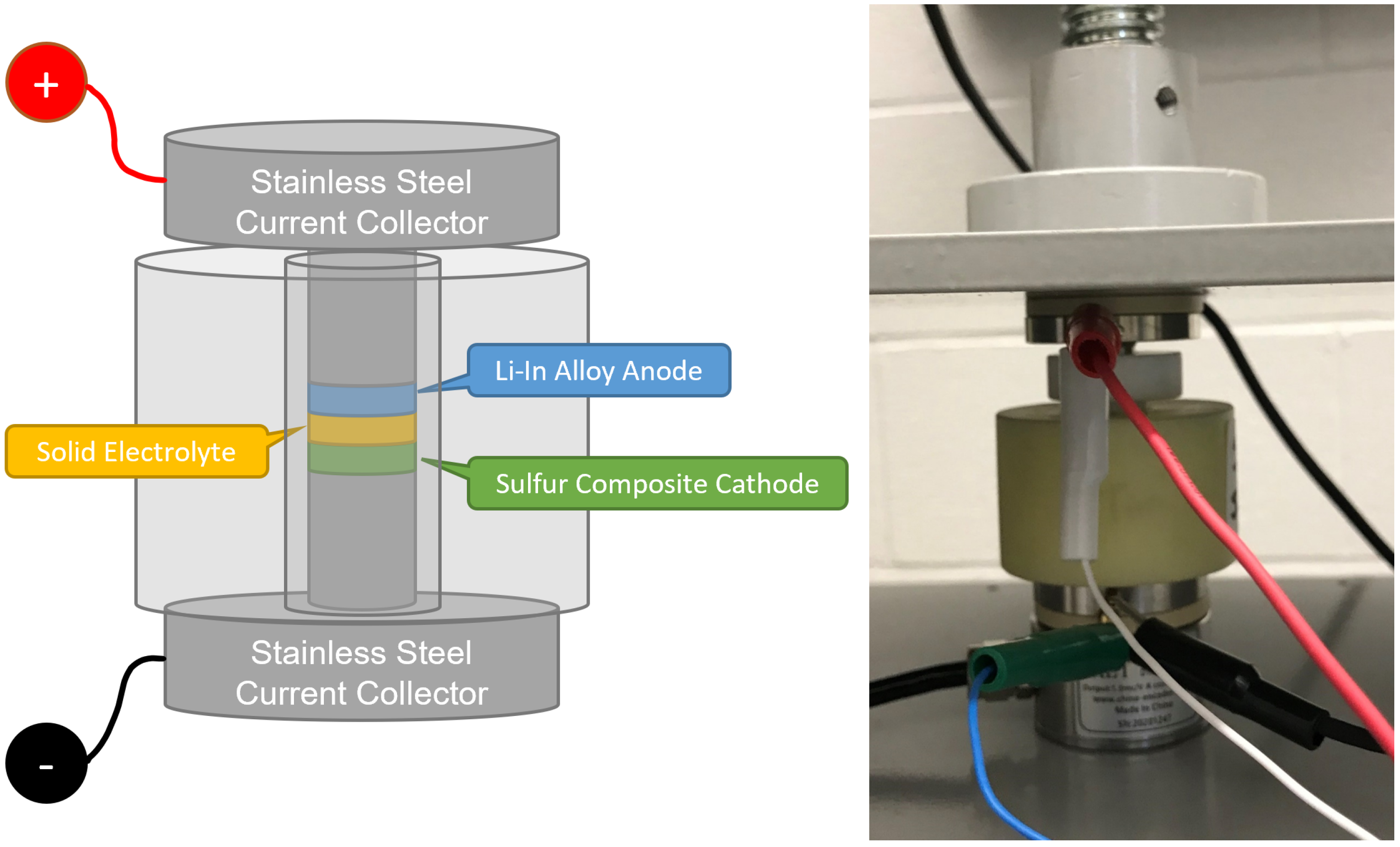
2. Cell Fabrication
2.1. Preparation of Solid Electrolytes
2.2. Preparation of Sulfur Cathode Composite
2.3. Solid-State Li-S Cell Fabrication
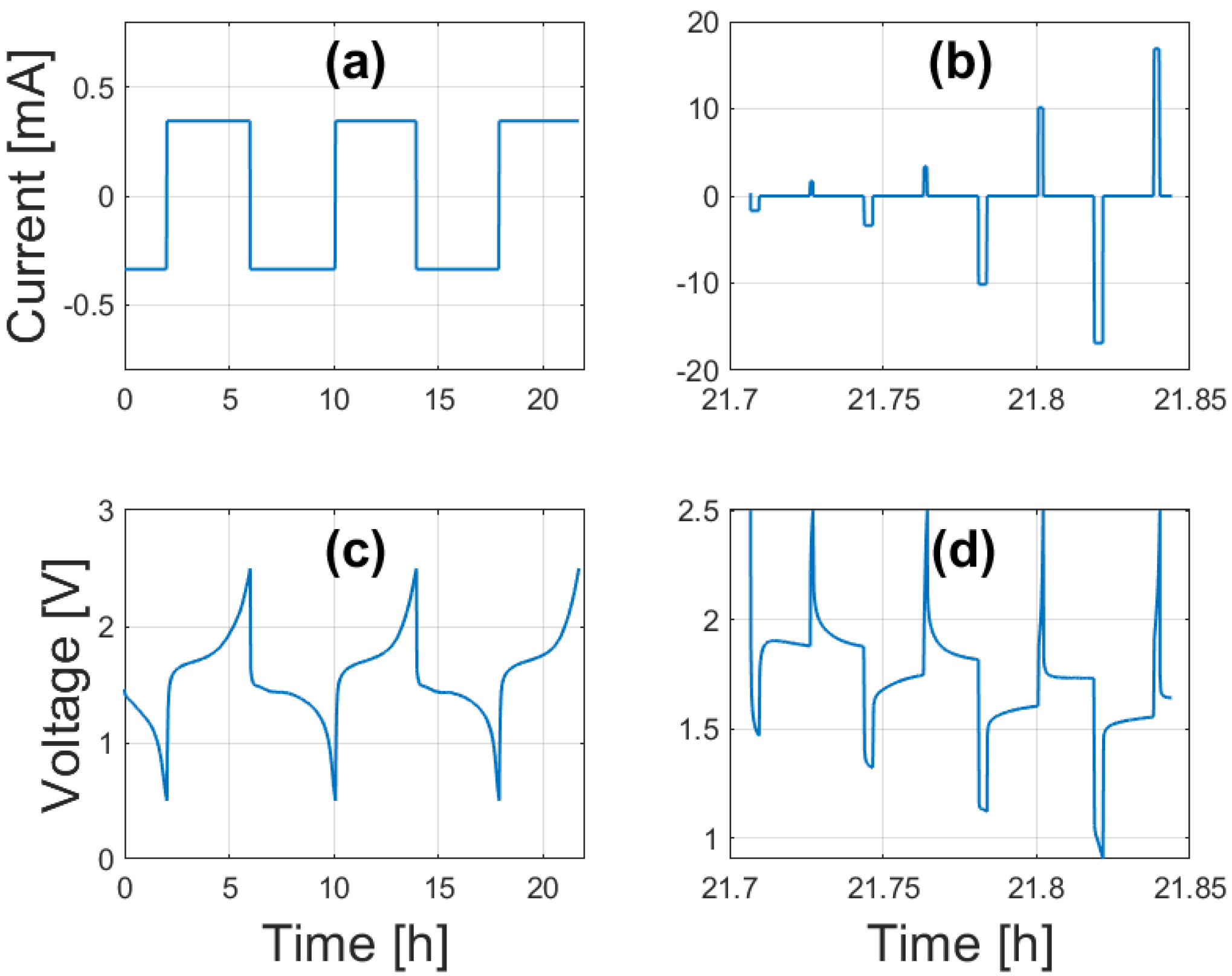
3. Cell Characterization
4. Equivalent Circuit Model
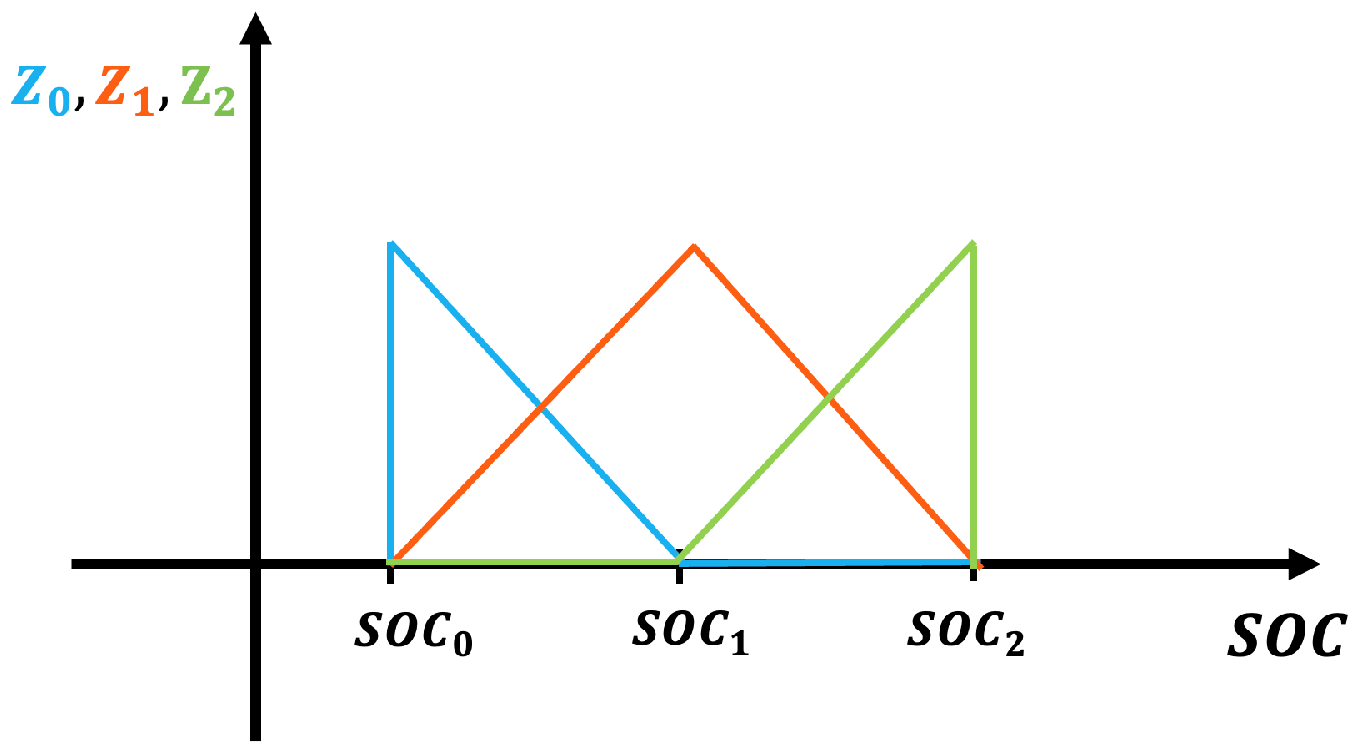
5. Model Parameterization
5.1. Outer Loop: Nonlinear Regression
5.2. Inner Loop: Linear Regression
- The regressor associated with is , evaluated at every sampling time.
- The regressor associated with is , evaluated at every sampling time.
- The regressor associated with is , evaluated at every sampling time, for the variant of the estimation algorithm where battery series resistance is assumed to be a function of SOC.
- The regressor associated with is , evaluated at every sampling time, for the variant of the estimation algorithm where battery series resistance is assumed to be a function of input current.
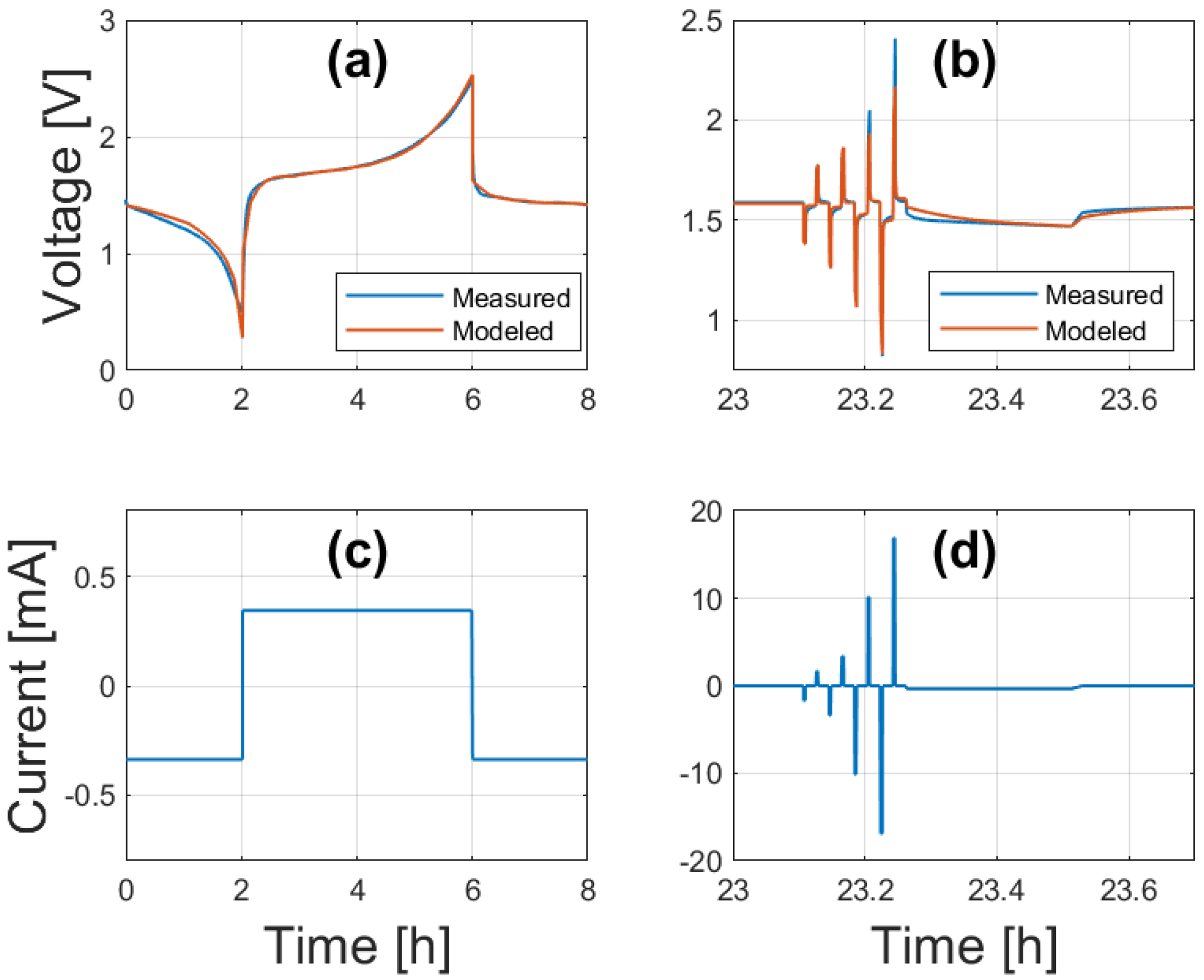
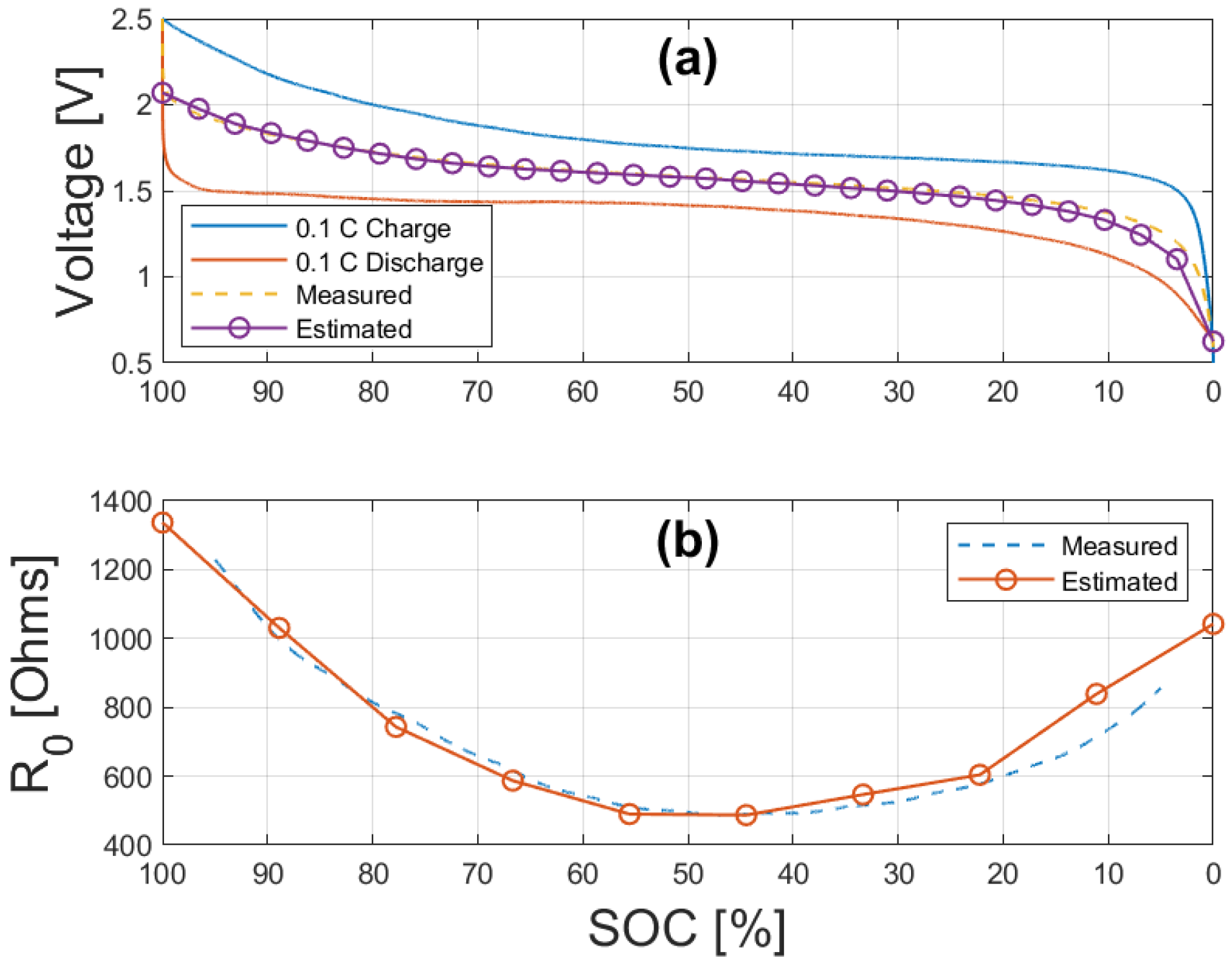
6. Parameterization Results
6.1. Sensitivity Analysis
6.2. Model Parameters
7. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Liu, X.; Li, B. Capacity Fade Analysis of Sulfur Cathodes in Lithium–Sulfur Batteries. Adv. Sci. 2016, 3, 1600101. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Otaki, M.; Wang, D.; Gao, Y.; Arthur, T.S.; Liu, S.; Wang, D. Confining Sulfur in Porous Carbon by Vapor Deposition to Achieve High-Performance Cathode for All-Solid-State Lithium–Sulfur Batteries. ACS Energy Lett. 2021, 6, 413–418. [Google Scholar] [CrossRef]
- Zheng, J.; Lv, D.; Gu, M.; Wang, C.; Zhang, J.G.; Liu, J.; Xiao, J. How to Obtain Reproducible Results for Lithium Sulfur Batteries? J. Electrochem. Soc. 2013, 160, A2288–A2292. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Silva, S.R.P.; Ding, B.; Zhang, P.; Shao, G. Lithium–Sulfur Batteries Meet Electrospinning: Recent Advances and the Key Parameters for High Gravimetric and Volume Energy Density. Adv. Sci. 2022, 9, 2103879. [Google Scholar] [CrossRef] [PubMed]
- Thomas Jefferson National Accelerator Facility—Office of Science Education The Element Sulfur, 2022. Available online: https://education.jlab.org/itselemental/ele016.html (accessed on 6 June 2022).
- Ohno, S.; Zeier, W.G. Toward Practical Solid-State Lithium–Sulfur Batteries: Challenges and Perspectives. Acc. Mater. Res. 2021, 2, 869–880. [Google Scholar] [CrossRef]
- Ji, X.; Nazar, L.F. Advances in Li–S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Ohno, S.; Rosenbach, C.; Dewald, G.F.; Janek, J.; Zeier, W.G. Linking Solid Electrolyte Degradation to Charge Carrier Transport in the Thiophosphate-Based Composite Cathode toward Solid-State Lithium-Sulfur Batteries. Adv. Funct. Mater. 2021, 31, 2010620. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969. [Google Scholar] [CrossRef]
- Busche, M.R.; Adelhelm, P.; Sommer, H.; Schneider, H.; Leitner, K.; Janek, J. Systematical electrochemical study on the parasitic shuttle-effect in lithium-sulfur-cells at different temperatures and different rates. J. Power Sources 2014, 259, 289–299. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Zheng, J.; Lv, M.; Song, H.; Du, L. Inhibition of Polysulfide Shuttles in Li–S Batteries: Modified Separators and Solid-State Electrolytes. Adv. Energy Mater. 2021, 11, 2000779. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Zhang, S.; Wang, Z.; Li, N.; Silva, S.R.P.; Shao, G. A flexible metallic TiC nanofiber/vertical graphene 1D/2D heterostructured as active electrocatalyst for advanced Li–S batteries. InfoMat 2021, 3, 790–803. [Google Scholar] [CrossRef]
- Abdulkadiroglu, B.; Bektas, H.; Eroglu, D. How to Model the Cathode Area in Lithium-Sulfur Batteries? ChemElectroChem 2022, 9, e202101553. [Google Scholar] [CrossRef]
- Kumaresan, K.; Mikhaylik, Y.; White, R.E. A Mathematical Model for a Lithium–Sulfur Cell. J. Electrochem. Soc. 2008, 155, A576. [Google Scholar] [CrossRef]
- Brieske, D.M.; Hassan, A.; Warnecke, A.; Sauer, D.U. Modeling of the temporal evolution of polysulfide chains within the lithium-sulfur battery. Energy Storage Mater. 2022, 47, 249–261. [Google Scholar] [CrossRef]
- Danner, T.; Latz, A. On the influence of nucleation and growth of S8 and Li2S in lithium-sulfur batteries. Electrochim. Acta 2019, 322, 134719. [Google Scholar] [CrossRef]
- Marinescu, M.; Zhang, T.; Offer, G.J. A zero dimensional model of lithium–sulfur batteries during charge and discharge. Phys. Chem. Chem. Phys. 2016, 18, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Cleary, T.; Li, G.; Wang, D.; Fathy, H. Parameter Identification and Sensitivity Analysis for Zero-Dimensional Physics-Based Lithium-Sulfur Battery Models. ASME Lett. Dyn. Syst. Control 2021, 1, 041001. [Google Scholar] [CrossRef]
- Knap, V.; Stroe, D.I.; Teodorescu, R.; Swierczynski, M.; Stanciu, T. Comparison of parametrization techniques for an electrical circuit model of Lithium-Sulfur batteries. In Proceedings of the 2015 IEEE 13th International Conference on Industrial Informatics (INDIN), Cambridge, UK, 22–24 July 2015; pp. 1278–1283. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical Impedance Spectroscopy Study of a Lithium/Sulfur Battery: Modeling and Analysis of Capacity Fading. J. Electrochem. Soc. 2013, 160, 553–558. [Google Scholar] [CrossRef]
- Cañas, N.A.; Hirose, K.; Pascucci, B.; Wagner, N.; Friedrich, K.A.; Hiesgen, R. Investigations of lithium–sulfur batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2013, 97, 42–51. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, Y.; Ju, Q.; Zhang, L.; Zhang, W.; Zhang, C. An Equivalent Circuit Model for Lithium-sulfur Batteries. Energy Procedia 2017, 105, 3533–3538. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Purkayastha, R.; O’Neill, L.; Waluś, S. Lithium–Sulfur Cell Equivalent Circuit Network Model Parameterization and Sensitivity Analysis. IEEE Trans. Veh. Technol. 2017, 66, 7711–7721. [Google Scholar] [CrossRef] [Green Version]
- Yen, Y.J.; Chung, S.H. Lean-electrolyte lithium–sulfur electrochemical cells with high-loading carbon nanotube/nanofiber–polysulfide cathodes. Chem. Commun. 2021, 57, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Hua, Q.; Zheng, L.; Dai, Z. Study of the discharge/charge process of lithium–sulfur batteries by electrochemical impedance spectroscopy. RSC Adv. 2020, 10, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Upreti, K.; Oyewole, I.; Lin, X.; Kim, Y. On Simplification of a Solid-State Battery Model for State Estimation. In Proceedings of the 2019 IEEE Conference on Control Technology and Applications (CCTA), Hong Kong, China, 19–21 August 2019; pp. 487–492. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, S.X.; Couto, L.D.; Viswanathan, V. PDE Observer for All-Solid-State Batteries via an Electrochemical Model. In Proceedings of the 2021 IEEE Conference on Control Technology and Applications (CCTA), San Diego, CA, USA, 9–11 August 2021; pp. 51–56. [Google Scholar] [CrossRef]
- Sultanova, L.; Figiel, Ł. Microscale diffusion-mechanics model for a polymer-based solid-state battery cathode. Comput. Mater. Sci. 2021, 186, 109990. [Google Scholar] [CrossRef]
- Song, X.; Lu, Y.; Wang, F.; Zhao, X.; Chen, H. A coupled electro-chemo-mechanical model for all-solid-state thin film Li-ion batteries: The effects of bending on battery performances. J. Power Sources 2020, 452, 227803. [Google Scholar] [CrossRef]
- Kim, Y.; Lin, X.; Abbasalinejad, A.; Kim, S.U.; Chung, S.H. On state estimation of all solid-state batteries. Electrochim. Acta 2019, 317, 663–672. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Estevez, L.; Kumar, J. Kinetics of all-solid-state sulfur cathodes. Energy Storage Mater. 2020, 27, 232–243. [Google Scholar] [CrossRef]
- Fan, B.; Guan, Z.; Wang, H.; Wu, L.; Li, W.; Zhang, S.; Xue, B. Electrochemical processes in all-solid-state Li-S batteries studied by electrochemical impedance spectroscopy. Solid State Ion. 2021, 368, 115680. [Google Scholar] [CrossRef]
- Kobayashi, T.; Imade, Y.; Shishihara, D.; Homma, K.; Nagao, M.; Watanabe, R.; Yokoi, T.; Yamada, A.; Kanno, R.; Tatsumi, T. All solid-state battery with sulfur electrode and thio-LISICON electrolyte. J. Power Sources 2008, 182, 621–625. [Google Scholar] [CrossRef]
- Propp, K.; Marinescu, M.; Auger, D.J.; O’Neill, L.; Fotouhi, A.; Somasundaram, K.; Offer, G.J.; Minton, G.; Longo, S.; Wild, M.; et al. Multi-temperature state-dependent equivalent circuit discharge model for lithium-sulfur batteries. J. Power Sources 2016, 328, 289–299. [Google Scholar] [CrossRef] [Green Version]
- Bi, C.X.; Zhao, M.; Hou, L.P.; Chen, Z.X.; Zhang, X.Q.; Li, B.Q.; Yuan, H.; Huang, J.Q. Anode Material Options Toward 500 Wh/kg Lithium–Sulfur Batteries. Adv. Sci. 2021, 9, 2103910. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Ware, S.; Hansen, C.; Jones, J.P.; Hennessy, J.; Bugga, R.; See, K. Fluoride in the SEI Stabilizes the Li Metal Interface in Li-S Batteries with Solvate Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 18865–18875. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhang, X.; Gao, H.; Li, L.; Zhang, Z. Novel LixSiSy/Nafion as an artificial SEI film to enable dendrite-free Li metal anodes and high stability Li–S batteries. J. Mater. Chem. A 2020, 8, 8979–8988. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Q.; Chu, F.; Hu, J.; Cui, Y.; Yin, C.; Li, C. Sericin protein as a conformal protective layer to enable air-endurable Li metal anodes and high-rate Li-S batteries. J. Power Sources 2019, 419, 72–81. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.H.; Cho, S.J.; Gwon, J.G.; Cho, H.J.; Jang, M.; Lee, S.Y.; Lee, S.Y. Nanomat Li–S batteries based on all-fibrous cathode/separator assemblies and reinforced Li metal anodes: Towards ultrahigh energy density and flexibility. Energy Environ. Sci. 2019, 12, 177–186. [Google Scholar] [CrossRef]
- Li, L.; Zhou, G.; Yin, L.; Koratkar, N.; Li, F.; Cheng, H.M. Stabilizing sulfur cathodes using nitrogen-doped graphene as a chemical immobilizer for LiS batteries. Carbon 2016, 108, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Schröder, D.; Arlt, T.; Manke, I.; Koerver, R.; Pinedo, R.; Weber, D.A.; Sann, J.; Zeier, W.G.; Janek, J. (Electro)chemical expansion during cycling: Monitoring the pressure changes in operating solid-state lithium batteries. J. Mater. Chem. A 2017, 5, 9929–9936. [Google Scholar] [CrossRef]
- Christophersen, J.P. Battery Test Manual For Electric Vehicles, Revision 3; Idaho National Lab. (INL): Idaho Falls, ID, USA, 2015. [Google Scholar] [CrossRef]



| Avg. Sensitivity | |||||
|---|---|---|---|---|---|
| [mV/Order] | |||||
| RC Pairs | 1 | 2 | 3 | 4 | 0.375 |
| RMS Voltage Error [mV] | 12.40 | 11.50 | 11.40 | 10.90 | |
| Parameters | 1 | 3 | 5 | 7 | 1.25 |
| RMS Voltage Error [mV] | 18.73 | 15.12 | 11.47 | 9.98 | |
| SOC Breakpoints | 2 | 5 | 10 | 15 | 0.158 |
| RMS Voltage Error [mV] | 11.55 | 11.47 | 9.716 | 9.498 |
| [Ohms] | [Ohms] | [F] | [F] | b [mA] | Q [mAh] |
|---|---|---|---|---|---|
| 8.760 | 194.690 | 0.372 | 1.658 | 4.942 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cleary, T.; Nozarijouybari, Z.; Wang, D.; Wang, D.; Rahn, C.; Fathy, H.K. An Experimentally Parameterized Equivalent Circuit Model of a Solid-State Lithium-Sulfur Battery. Batteries 2022, 8, 269. https://doi.org/10.3390/batteries8120269
Cleary T, Nozarijouybari Z, Wang D, Wang D, Rahn C, Fathy HK. An Experimentally Parameterized Equivalent Circuit Model of a Solid-State Lithium-Sulfur Battery. Batteries. 2022; 8(12):269. https://doi.org/10.3390/batteries8120269
Chicago/Turabian StyleCleary, Timothy, Zahra Nozarijouybari, Daiwei Wang, Donghai Wang, Christopher Rahn, and Hosam K. Fathy. 2022. "An Experimentally Parameterized Equivalent Circuit Model of a Solid-State Lithium-Sulfur Battery" Batteries 8, no. 12: 269. https://doi.org/10.3390/batteries8120269







