Ionic Liquid-Laden Zn-MOF-74-Based Solid-State Electrolyte for Sodium Batteries
Abstract
:1. Introduction
- i.
- Increase the energy density, which could be solved by developing batteries that use metallic lithium anodes. Lithium metal batteries are expected to be the next generation of energy storage devices since lithium anodes show a high theoretical capacity (3860 mA h g–1), the lowest redox potential (−3.04 V vs. the standard hydrogen electrode) and a low density (0.59 g cm–3). If safety and volume expansion issues associated with the use of metallic lithium are alleviated, it is certain that lithium metal batteries will provide a higher energy density than traditional graphite anode-based systems [5,6].
- ii.
- Properly address safety issues due to the use of liquid organic electrolytes. The use of liquid electrolytes based on the dissolution of a lithium salt (such as LiPF6) in a carbonated organic solvent carries significant risks. These organic liquid electrolytes are flammable and, with the heating of the battery during cycling, they can be related to the explosion or combustion of the device. This fact is aggravated when these toxic solvents leak, so the device must be tightly sealed. These solvent leaks contribute to a reduction in the useful life of the LIB [7].
- iii.
- Finally, lithium is considered a critical element due to its low abundance as well as problems related to accessibility, mining and sociopolitical conditions. Thus, lithium exploitation may not cover the future demand, and it is crucial to look for alternatives to LIBs, such as technologies based on other metallic elements like sodium. Both alkaline elements exhibit some chemical characteristics that, together with the high abundance of the heavier element, have driven the development of sodium-ion batteries (NIBs) as a promising alternative to LIBs [8,9].
2. Materials and Methods
2.1. Materials
2.2. Synthesis Procedures
2.2.1. Synthesis of Zn-MOF-74
2.2.2. Sodium Enrichment of the Ionic Liquid [EMIm][TFSI]
2.2.3. Insertion of Na[EMIm][TFSI] into Zn-MOF-74
2.3. Physicochemical Characterization Techniques
2.4. Electrochemical Characterization Techniques
3. Results and Discussion
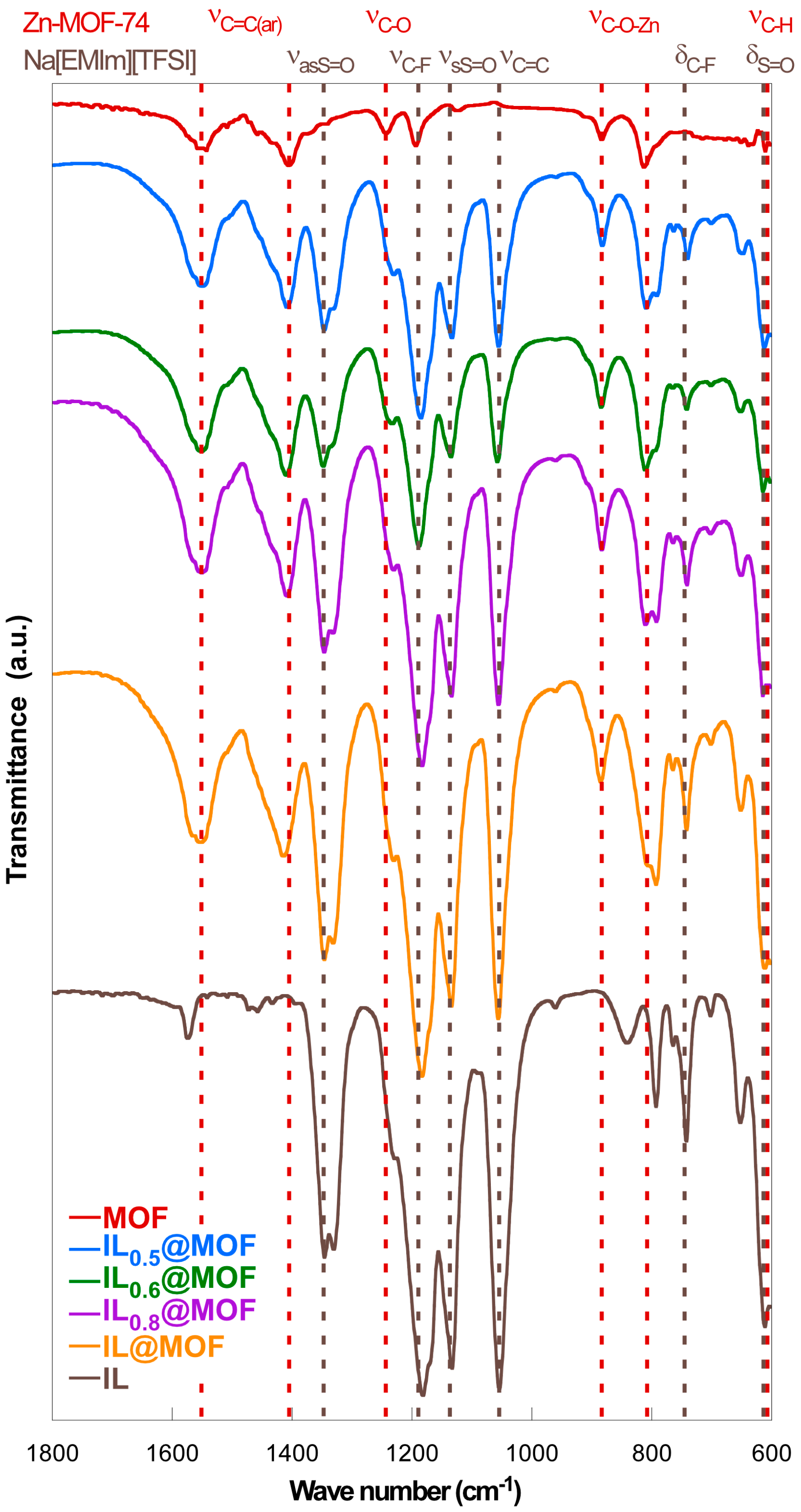
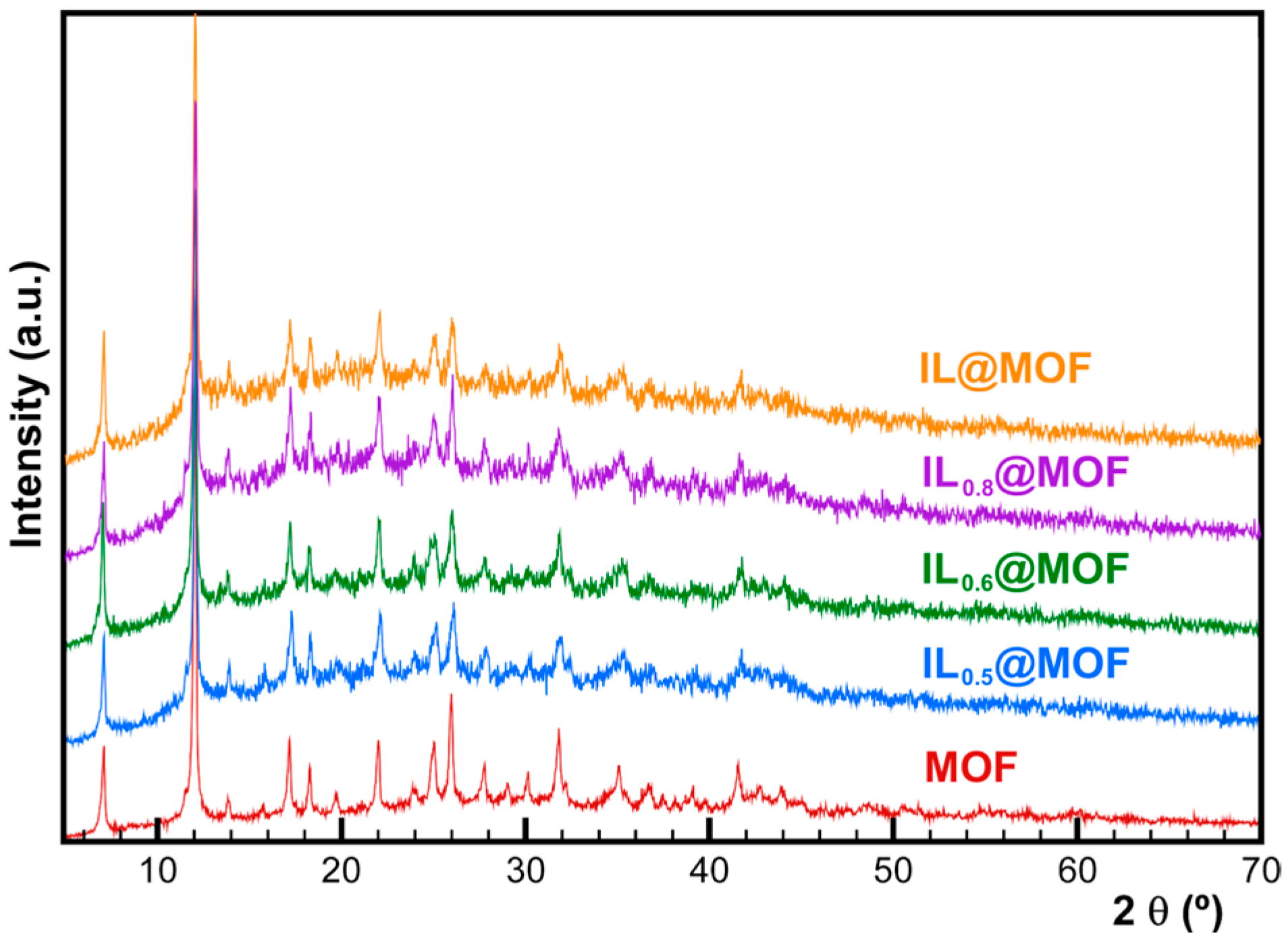
3.1. Physicochemical Characterization
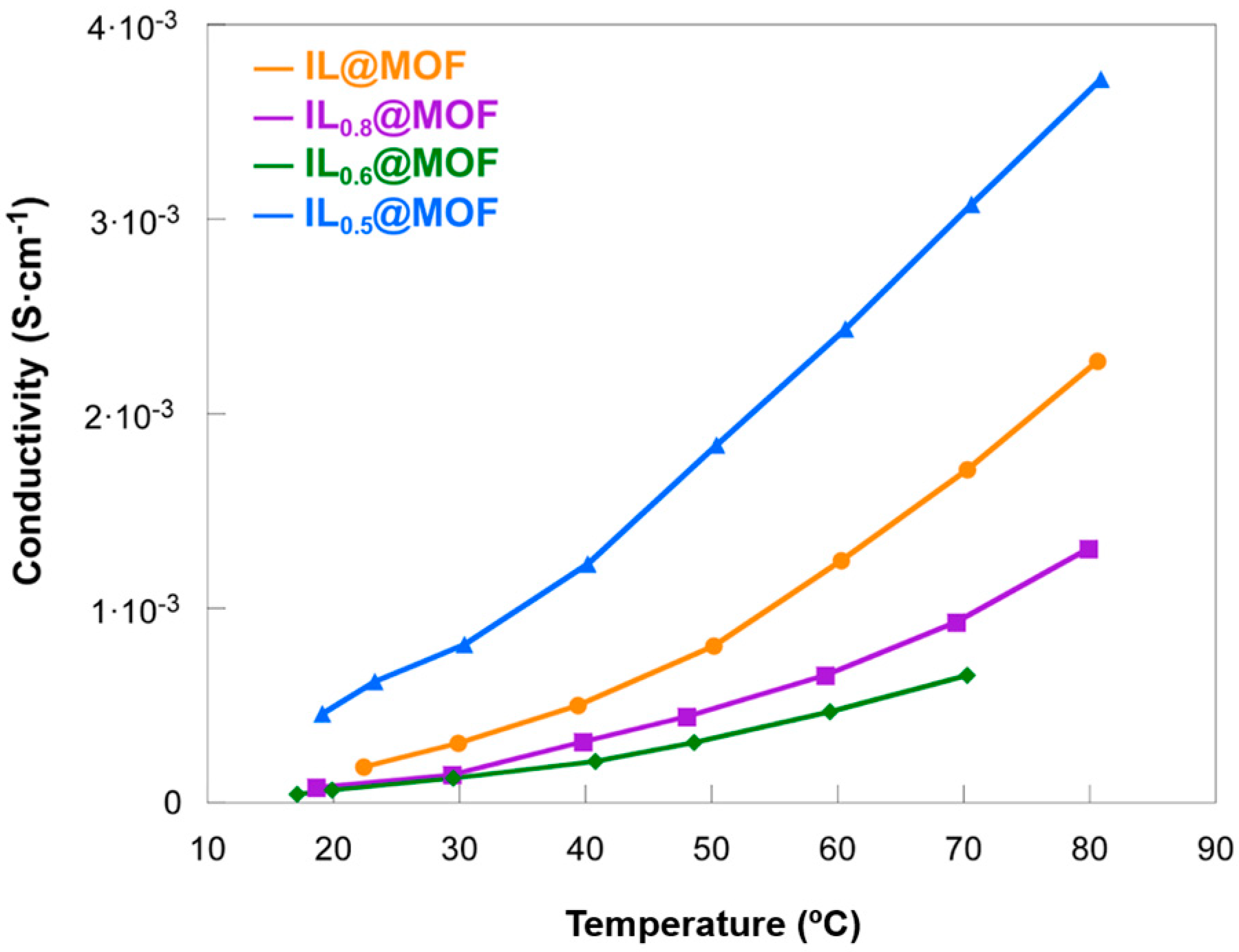
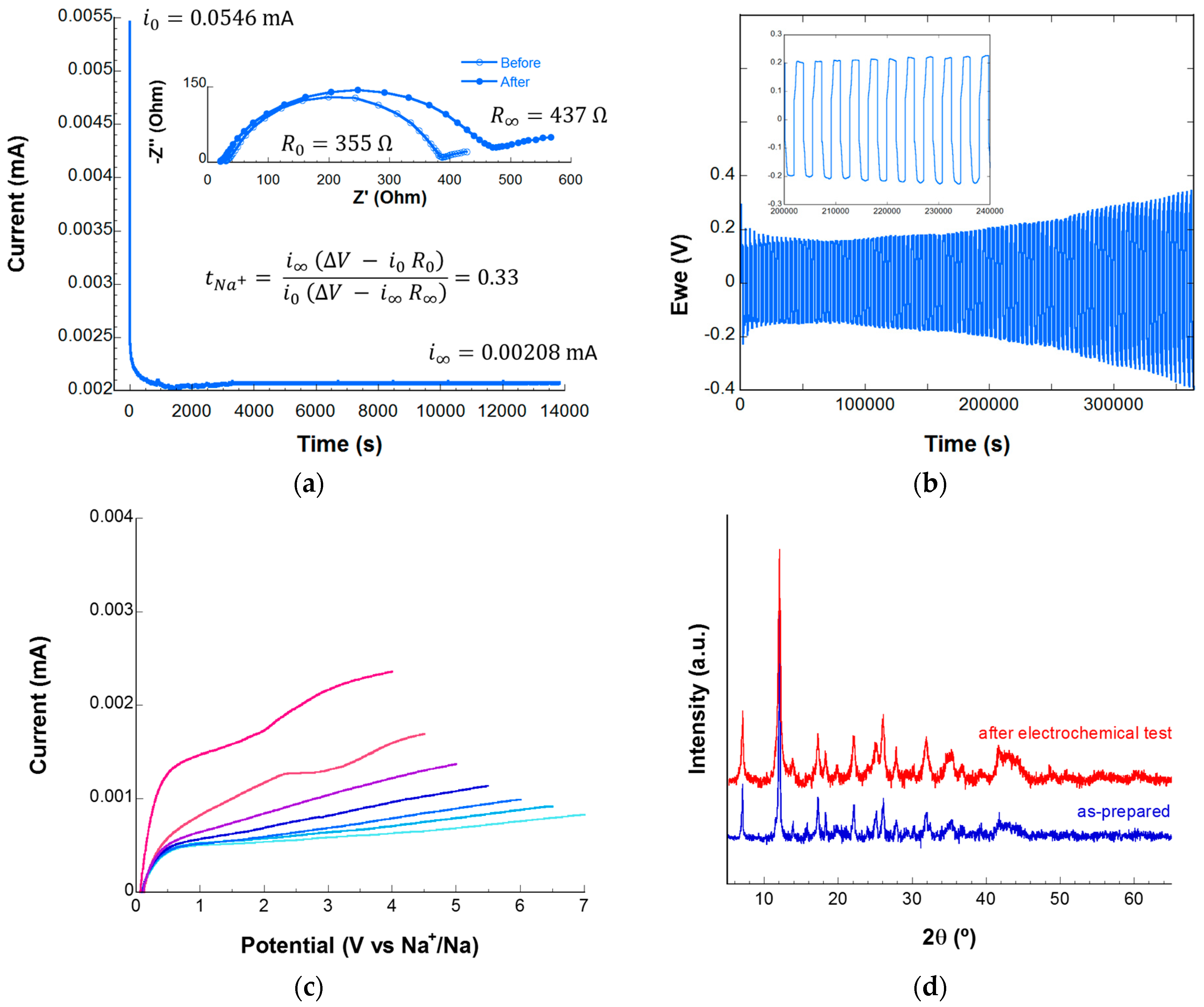
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sternberg, A.; Bardow, A. Power-to-What?-Environmental Assessment of Energy Storage Systems. Energy Environ. Sci. 2015, 8, 389–400. [Google Scholar] [CrossRef]
- Wang, D.; Liu, N.; Chen, F.; Wang, Y.; Mao, J. Progress and Prospects of Energy Storage Technology Research: Based on Multidimensional Comparison. J. Energy Storage 2024, 75, 109710. [Google Scholar] [CrossRef]
- Gutsch, M.; Leker, J. Global Warming Potential of Lithium-Ion Battery Energy Storage Systems: A Review. J. Energy Storage 2022, 52, 105030. [Google Scholar] [CrossRef]
- Hossain, M.H.; Chowdhury, M.A.; Hossain, N.; Islam, M.A.; Mobarak, M.H. Advances of Lithium-Ion Batteries Anode Materials—A Review. Chem. Eng. J. Adv. 2023, 16, 100569. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, Y.; Luo, D.; Or, T.; Liu, Y.; Yang, L.; Dou, H.; Liang, J.; Nie, Y.; Wang, X.; et al. 2D Materials for All-Solid-State Lithium Batteries. Adv. Mater. 2022, 34, 2108079. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Phani, N.; Aetukuri, B.; Nanda, J. Solid State Lithium Metal Batteries—Issues and Challenges at the Lithium-Solid Electrolyte Interface. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100999. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Goikolea, E.; Palomares, V.; Wang, S.; Ruiz de Larramendi, I.; Guo, X.; Wang, G.; Rojo, T. Na-Ion Batteries—Approaching Old and New Challenges. Adv. Energy Mater. 2020, 10, 2002055. [Google Scholar] [CrossRef]
- Feng, J.; An, Y.; Ci, L.; Xiong, S. Nonflammable Electrolyte for Safer Non-Aqueous Sodium Batteries. J. Mater. Chem. A 2015, 3, 14539–14544. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lu, G.; Li, W.; Tao, Q.; Shi, C.; Jin, H.; Chen, G.; Wang, S. Fundamentals of Electrolytes for Solid-State Batteries: Challenges and Perspectives. Front. Mater. 2020, 7, 111. [Google Scholar] [CrossRef]
- He, Y.; Qiao, Y.; Chang, Z.; Zhou, H. The Potential of Electrolyte Filled MOF Membranes. Energy Environ. Sci. 2019, 12, 2327–2344. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Yue, L.; Liang, J.; Li, T.; Liu, Q.; Luo, Y.; Kong, X.; Lu, S.; Shi, X.; et al. Electrochemistry Communications Recent Advances in Lithium-Based Batteries Using Metal Organic Frameworks as Electrode Materials. Electrochem. Commun. 2021, 122, 106881. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef]
- Urgoiti-Rodriguez, M.; Vaquero-Vílchez, S.; Mirandona-Olaeta, A.; Fernández de Luis, R.; Goikolea, E.; Costa, C.M.; Lanceros-Mendez, S.; Fidalgo-Marijuan, A.; Ruiz de Larramendi, I. Exploring Ionic Liquid-Laden Metal-Organic Framework Composite Materials as Hybrid Electrolytes in Metal (Ion) Batteries. Front. Chem. 2022, 10, 995063. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Kumar, R.; Dhakal, G.; Shim, J.J. Recent Advances in Synthesis of Metal-Organic Frameworks (MOFs)-Derived Metal Oxides and Its Composites for Electrochemical Energy Storage Applications. J. Energy Storage 2023, 74, 109427. [Google Scholar] [CrossRef]
- Shahzad, U.; Marwani, H.M.; Saeed, M.; Asiri, A.M.; Althomali, R.H.; Rahman, M.M. Exploration of Porous Metal-Organic Frameworks (MOFs) for an Efficient Energy Storage Applications. J. Energy Storage 2023, 74, 109518. [Google Scholar] [CrossRef]
- Huo, H.; Wu, B.; Zhang, T.; Zheng, X.; Ge, L.; Xu, T. Anion-Immobilized Polymer Electrolyte Achieved by Cationic Metal-Organic Framework Fi Ller for Dendrite-Free Solid-State Batteries. Energy Storage Mater. 2019, 18, 59–67. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Alziyadi, M.O.; Gamal, H.; Hamdy, S. Solid-State Lithium-Ion Battery: The Key Components Enhance the Performance and Efficiency of Anode, Cathode, and Solid Electrolytes. J. Alloys Compd. 2023, 969, 172318. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.; Shi, L.; Jungsuttiwong, S.; Yuan, S. Ionic Liquids for High Performance Lithium Metal Batteries. J. Energy Chem. 2021, 59, 320–333. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mero, A.; Mezzetta, A.; Tofani, G.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Novel Access to Ionic Liquids Based on Trivalent Metal–EDTA Complexes and Their Thermal and Electrochemical Characterization. J. Mol. Liq. 2021, 340, 117210. [Google Scholar] [CrossRef]
- Hakim, L.; Ishii, Y.; Matsumoto, K.; Hagiwara, R.; Ohara, K.; Umebayashi, Y.; Matubayasi, N. Transport Properties of Ionic Liquid and Sodium Salt Mixtures for Sodium-Ion Battery Electrolytes from Molecular Dynamics Simulation with a Self-Consistent Atomic Charge Determination. J. Phys. Chem. B 2020, 124, 7291–7305. [Google Scholar] [CrossRef]
- Majid, M.F.; Zaid, H.F.M.; Kait, C.F.; Ahmad, A.; Jumbri, K. Ionic Liquid@Metal-Organic Framework as a Solid Electrolyte in a Lithium-Ion Battery: Current Performance and Perspective at Molecular Level. Nanomaterials 2022, 12, 1076. [Google Scholar] [CrossRef]
- Feng, L.; Li, G.Q.; Li, Y.K.; Gu, X.L.; Hu, S.Y.; Han, Y.C.; Wang, Y.F.; Zheng, J.C.; Deng, Y.H.; Wan, C.Q. MOF-Supported Crystalline Ionic Liquid: New Type of Solid Electrolyte for Enhanced and High Ionic Conductivity. Dalt. Trans. 2022, 51, 6086–6094. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater. Sci. Forum 2001, 378, 118–123. [Google Scholar] [CrossRef]
- Zhang, C.N.; Li, Y.; Fan, H.L.; Yang, C.; Wu, M.M. A Highly Reversible Sorption for Sulfur-Containing Toxic VOCs Emissions Under Ambient Temperature and Pressure. J. Inorg. Organomet. Polym. Mater. 2020, 30, 486–493. [Google Scholar] [CrossRef]
- Kiefer, J.; Fries, J.; Leipertz, A. Experimental Vibrational Study of Imidazolium-Based Ionic Liquids: Raman and Infrared Spectra of 1-Ethyl-3methylimidazolium Bis(Trifluoromethylsulfonyl) Imide and 1-Ethyl-3-Methylimidazolium Ethylsulfate. Appl. Spectrosc. 2007, 61, 1306–1311. [Google Scholar] [CrossRef]
- Rosi, N.L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O.M. Rod Packings and Metal-Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef]
- Yang, H.; Abdullah, M.; Bright, J.; Hu, W.; Kittilstved, K.; Xu, Y.; Wang, C.; Zhang, X.; Wu, N. Polymer-Ceramic Composite Electrolytes for All-Solid-State Lithium Batteries: Ionic Conductivity and Chemical Interaction Enhanced by Oxygen Vacancy in Ceramic Nanofibers. J. Power Sources 2021, 495, 229796. [Google Scholar] [CrossRef]
- Yu, X.; Grundish, N.S.; Goodenough, J.B.; Manthiram, A. Ionic Liquid (IL) Laden Metal-Organic Framework (IL-MOF) Electrolyte for Quasi-Solid-State Sodium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 24662–24669. [Google Scholar] [CrossRef]
- Nozari, V.; Calahoo, C.; Tuffnell, J.M.; Adelhelm, P.; Wondraczek, K.; Dutton, E.; Bennett, T.D.; Wondraczek, L. Sodium Ion Conductivity in Superionic IL-Impregnated Metal-Organic Frameworks: Enhancing Stability Through Structural Disorder. Sci. Rep. 2020, 10, 3532. [Google Scholar] [CrossRef]
- Goswami, S.; Hod, I.; Duan, J.D.; Kung, C.W.; Rimoldi, M.; Malliakas, C.D.; Palmer, R.H.; Farha, O.K.; Hupp, J.T. Anisotropic Redox Conductivity within a Metal-Organic Framework Material. J. Am. Chem. Soc. 2019, 141, 17696–17702. [Google Scholar] [CrossRef] [PubMed]
- Pratik, S.M.; Gagliardi, L.; Cramer, C.J. Engineering Electrical Conductivity in Stable Zirconium-Based PCN-222 MOFs with Permanent Mesoporosity. Chem. Mater. 2020, 32, 6137–6149. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Yadav, A.; Baker, J.; Saha, S. From a Collapse-Prone, Insulating Ni-MOF-74 Analogue to Crystalline, Porous, and Electrically Conducting PEDOT@MOF Composites. Inorg. Chem. 2023, 62, 18999–19005. [Google Scholar] [CrossRef]
- Wei, Y.; Dong, Y.; Ji, X.; Ullah Shah, F.; Laaksonen, A.; An, R.; Riehemann, K. Detailing Molecular Interactions of Ionic Liquids with Charged SiO2 Surfaces: A Systematic AFM Study. J. Mol. Liq. 2022, 350, 118506. [Google Scholar] [CrossRef]
- Fertig, M.P.; Skadell, K.; Schulz, M.; Dirksen, C.; Adelhelm, P.; Stelter, M. From High- to Low-Temperature: The Revival of Sodium-Beta Alumina for Sodium Solid-State Batteries. Batter. Supercaps 2022, 5, e202100131. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, F.; Zhang, X.; Li, J.R.; Chen, J.F.; Zhang, S. Combining Ionic Liquids and Sodium Salts into Metal-Organic Framework for High-Performance Ionic Conduction. ChemElectroChem 2020, 7, 183–190. [Google Scholar] [CrossRef]
- Tuffnell, J.M.; Morzy, J.K.; Kelly, N.D.; Tan, R.; Song, Q.; Ducati, C.; Bennett, T.D.; Dutton, S.E. Comparison of the Ionic Conductivity Properties of Microporous and Mesoporous MOFs Infiltrated with a Na-Ion Containing IL Mixture. Dalt. Trans. 2020, 49, 15914–15924. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.C.; Dias, L.G.; Da Silva, J.L.F. Theoretical Investigation of the Na+Transport Mechanism and the Performance of Ionic Liquid-Based Electrolytes in Sodium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4444–4458. [Google Scholar] [CrossRef]
- Kharod, R.A.; Andrews, J.L.; Dinca, M. Teaching Metal-Organic Frameworks to Conduct: Ion and Electron Transport in Metal-Organic Frameworks. Annu. Rev. Mater. Res. 2022, 52, 103–128. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic Conductivity and Ion Transport Mechanisms of Solid-State Lithium-Ion Battery Electrolytes: A Review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- Fang, C.; Mistry, A.; Srinivasan, V.; Balsara, N.P.; Wang, R. Elucidating the Molecular Origins of the Transference Number in Battery Electrolytes Using Computer Simulations. JACS Au 2023, 3, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Zugmann, S.; Fleischmann, M.; Amereller, M.; Gschwind, R.M.; Wiemhöfer, H.D.; Gores, H.J. Measurement of Transference Numbers for Lithium Ion Electrolytes via Four Different Methods, a Comparative Study. Electrochim. Acta 2011, 56, 3926–3933. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical Measurement of Transference Numbers in Polymer Electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- You, Y.; Manthiram, A. Progress in High-Voltage Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701785. [Google Scholar] [CrossRef]
- Shipitsyn, V.; Jayakumar, R.; Zuo, W.; Sun, B.; Ma, L. Understanding High-Voltage Behavior of Sodium-Ion Battery Cathode Materials Using Synchrotron X-Ray and Neutron Techniques: A Review. Batteries 2023, 9, 461. [Google Scholar] [CrossRef]



| Sample | T (°C) | σ (S cm−1) | Reference |
|---|---|---|---|
| Na-β″-alumina | RT | 1.7 × 10−3 | [35] |
| Na3Zr2Si2PO12 | RT | 2.1 × 10−3 | [8] |
| IL@MOF | 22.4 | 1.83 × 10−3 | This work |
| 70.3 | 1.71 × 10−3 | ||
| IL0.8@MOF | 18.6 | 7.93 × 10−3 | |
| 69.4 | 9.3 × 10−3 | ||
| IL0.6@MOF | 17.1 | 4.55 × 10−3 | |
| 70.3 | 6.55 × 10−3 | ||
| IL0.5@MOF | 19.1 | 4.58 × 10−3 | |
| 70.6 | 3.1 × 10−3 | ||
| [Na0.1EMIm0.9][TFSI]@ZIF-8 | RT crystalline | 2.97 × 10−3 | [30] |
| RT amorphous | 2.0 × 10−3 | ||
| Na[Bmpyr][TFSI]@ UiO-66-SO3Na | RT | 3.6 × 10−3 | [29] |
| NaTFSI-EIMS@UiO-67-MIMS | 30 | 1.24 × 10−3 | [23] |
| Na[EMIm][BF4]@MIL-101-SO3Na | 150 | 1.32 × 10−3 | [36] |
| Na[EMIm][TFSI]@ZIF-8 | RT (ZIF-8micro) | 1.6 × 10−3 | [37] |
| RT (ZIF-8meso) | 8.4 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirandona-Olaeta, A.; Goikolea, E.; Lanceros-Mendez, S.; Fidalgo-Marijuan, A.; Ruiz de Larramendi, I. Ionic Liquid-Laden Zn-MOF-74-Based Solid-State Electrolyte for Sodium Batteries. Batteries 2023, 9, 588. https://doi.org/10.3390/batteries9120588
Mirandona-Olaeta A, Goikolea E, Lanceros-Mendez S, Fidalgo-Marijuan A, Ruiz de Larramendi I. Ionic Liquid-Laden Zn-MOF-74-Based Solid-State Electrolyte for Sodium Batteries. Batteries. 2023; 9(12):588. https://doi.org/10.3390/batteries9120588
Chicago/Turabian StyleMirandona-Olaeta, Alexander, Eider Goikolea, Senentxu Lanceros-Mendez, Arkaitz Fidalgo-Marijuan, and Idoia Ruiz de Larramendi. 2023. "Ionic Liquid-Laden Zn-MOF-74-Based Solid-State Electrolyte for Sodium Batteries" Batteries 9, no. 12: 588. https://doi.org/10.3390/batteries9120588







