Impact of Surface Structure on SEI for Carbon Materials in Alkali Ion Batteries: A Review
Abstract
:1. Introduction
2. An Overview of SEI
2.1. Structure and Composition of SEI
2.2. Effect of Electrolyte on SEI
3. Effect of Carbon Surface on SEI
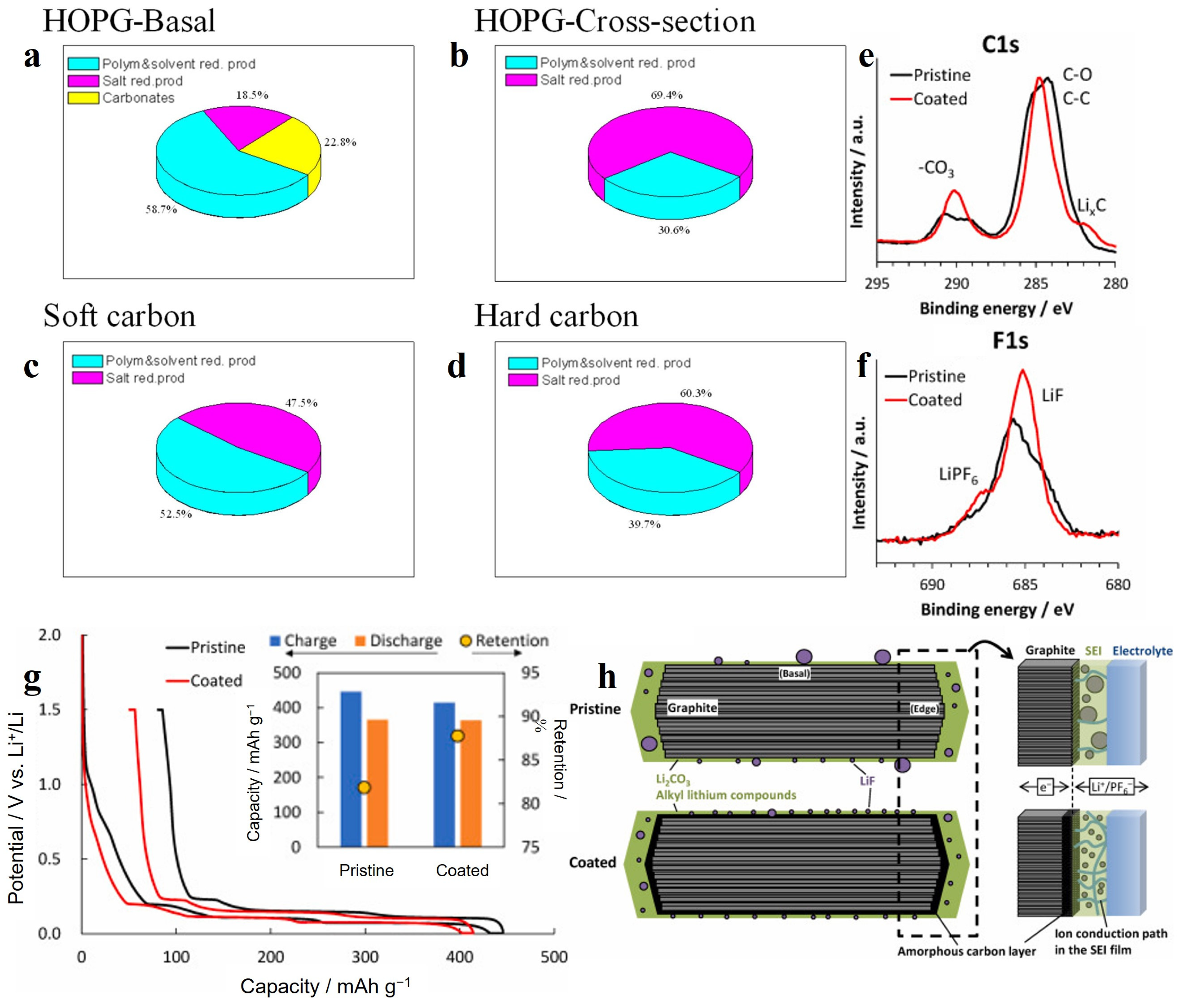
3.1. Surface Atoms Exposed
3.2. Surface Functionalization
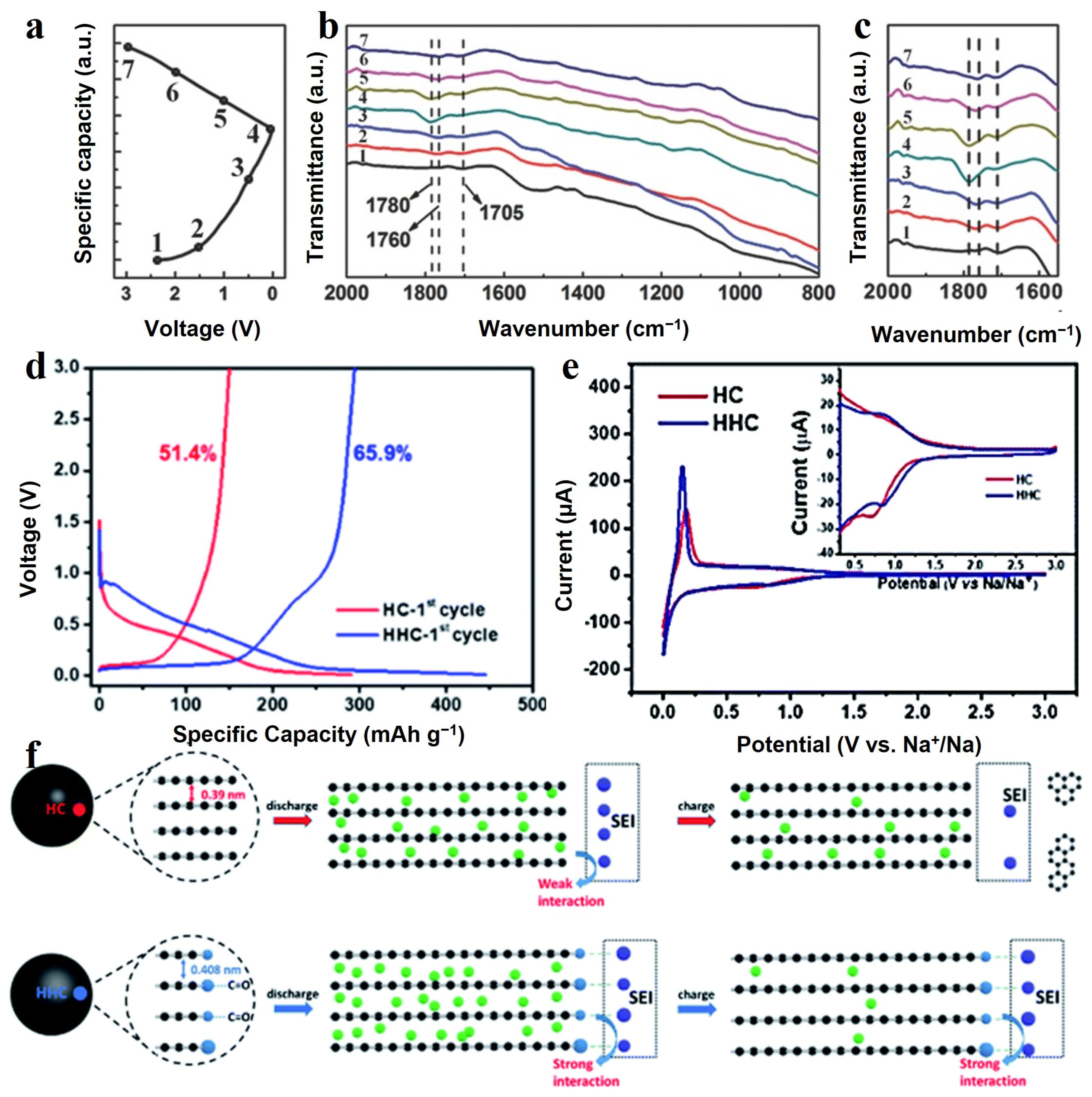
3.2.1. Surface Oxygen-Containing Groups
3.2.2. Surface Nitrogen-Containing Groups
3.3. Specific Surface Area and Pore Structure
3.3.1. Specific Surface Area
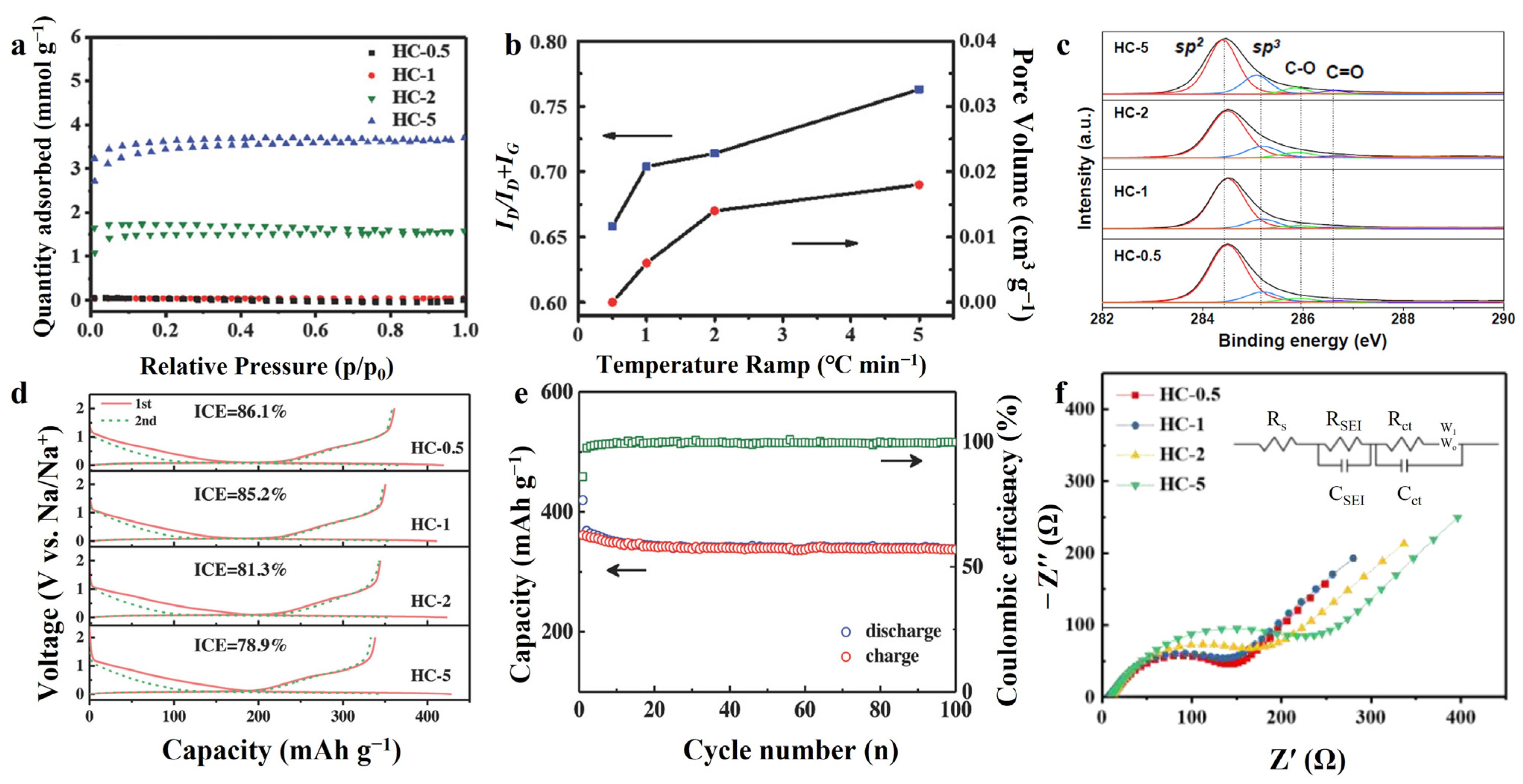
3.3.2. Pore Structure
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energ. Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Alptekin, H.; Au, H.; Jensen, A.C.S.; Olsson, E.; Goktas, M.; Headen, T.F.; Adelhelm, P.; Cai, Q.; Drew, A.J.; Titirici, M.M. Sodium storage mechanism investigations through structural changes in hard carbons. ACS Appl. Energ. Mater. 2020, 3, 9918–9927. [Google Scholar] [CrossRef]
- Irisarri, E.; Ponrouch, A.; Palacin, M.R. Review-hard carbon negative electrode materials for sodium-ion batteries. J. Electrochem. Soc. 2015, 162, A2476–A2482. [Google Scholar] [CrossRef]
- Min, X.; Xiao, J.; Fang, M.; Wang, W.; Zhao, Y.; Liu, Y.; Abdelkader, A.M.; Xi, K.; Kumar, R.V.; Huang, Z. Potassium-ion batteries: Outlook on present and future technologies. Energy Environ. Sci. 2021, 14, 2186–2243. [Google Scholar] [CrossRef]
- Pramudita, J.C.; Sehrawat, D.; Goonetilleke, D.; Sharma, N. An initial review of the status of electrode materials for potassium-ion batteries. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, C.; Yu, S.; Ge, D.; Zhou, H. Status and challenges facing representative anode materials for rechargeable lithium batteries. J. Energy Chem. 2022, 66, 260–294. [Google Scholar] [CrossRef]
- Yan, D.; Yang, H.Y.; Bai, Y. Tactics to optimize conversion-type metal fluoride/sulfide/oxide cathodes toward advanced lithium metal batteries. Nano Res. 2023, 16, 1–18. [Google Scholar] [CrossRef]
- Fedorov, R.G.; Maletti, S.; Heubner, C.; Michaelis, A.; Ein-Eli, Y. Molecular engineering approaches to fabricate artificial solid-electrolyte interphases on anodes for Li-ion batteries: A critical review. Adv. Energy Mater. 2021, 11, 2101173. [Google Scholar] [CrossRef]
- Xia, J.L.; Yan, D.; Guo, L.P.; Dong, X.L.; Li, W.C.; Lu, A.H. Hard carbon nanosheets with uniform ultramicropores and accessible functional groups showing high realistic capacity and superior rate performance for sodium-ion storage. Adv. Funct. Mater. 2020, 32, e2000447. [Google Scholar] [CrossRef]
- Bai, P.; He, Y.; Xiong, P.; Zhao, X.; Xu, K.; Xu, Y. Long cycle life and high rate sodium-ion chemistry for hard carbon anodes. Energy Storage Mater. 2018, 13, 274–282. [Google Scholar] [CrossRef]
- Ren, N.; Wang, L.; He, X.; Zhang, L.; Dong, J.; Chen, F.; Xiao, J.; Pan, B.; Chen, C. High ICE hard carbon anodes for lithium-ion batteries enabled by a high work function. ACS Appl. Mater. Inter. 2021, 13, 46813–46820. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Ponrouch, A.; Goñi, A.R.; Palacín, M.R. High capacity hard carbon anodes for sodium ion batteries in additive free electrolyte. Electrochem. Commun. 2013, 27, 85–88. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, Y.; Xu, Q.; Yu, X.; Liu, Y.; Shen, H. Low-Temperature growth of hard carbon with graphite crystal for sodium-ion storage with high initial coulombic efficiency: A general method. Adv. Energy Mater. 2019, 9, 1803648. [Google Scholar] [CrossRef]
- Hu, Q.A.; Yu, M.F.; Liao, J.Y.; Wen, Z.Y.; Chen, C.H. Porous carbon-coated NaTi(2)(PO(4))(3) with superior rate and low-temperature properties. J. Mater. Chem. A 2018, 6, 2365. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Park, J.-S.; Jung, H.-G.; Chung, K.-Y.; Aurbach, D.; Sun, Y.-K.; Myung, S.-T. NaCrO2 cathode for high-rate sodium-ion batteries. Energy Environ. Sci. 2015, 8, 2019. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the solid electrolyte interphase (SEI) in sodium ion batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.-M.; Palacín, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Yang, J.; Shi, X.; Wang, W.; Liu, Z.; Shen, C. Localized high-concentration electrolyte (LHCE) for fast charging lithium-ion batteries. Batteries 2023, 9, 155. [Google Scholar] [CrossRef]
- Ji, Y.; Li, J.; Li, J. Recent development of electrolyte engineering for sodium metal batteries. Batteries 2022, 8, 157. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, J.; Li, Q.; Li, X.; Engelhard, M.H.; Cao, R.; Zhang, J.-G.; Xu, W. Behavior of lithium metal anodes under various capacity utilization and high current density in lithium metal batteries. Joule 2018, 2, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, J.T.; Yushin, G. High temperature stabilization of lithium-sulfur cells with carbon nanotube current collector. J. Power Sources 2013, 226, 256–265. [Google Scholar] [CrossRef]
- Huang, W.; Boyle, D.T.; Li, Y.; Li, Y.; Pei, A.; Chen, H.; Cui, Y. Nanostructural and electrochemical evolution of the solid-electrolyte interphase on CuO nanowires revealed by cryogenic-electron microscopy and impedance spectroscopy. ACS Nano 2019, 13, 737–744. [Google Scholar] [CrossRef]
- Van der Ven, A.; Deng, Z.; Banerjee, S.; Ong, S.P. Rechargeable alkali-ion battery materials: Theory and computation. Chem. Rev. 2020, 120, 6977–7019. [Google Scholar] [CrossRef]
- Wu, J.; Ihsan-Ul-Haq, M.; Chen, Y.; Kim, J.-K. Understanding solid electrolyte interphases: Advanced characterization techniques and theoretical simulations. Nano Energy 2021, 89, 106489. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Penciner, J. The anode/electrolyte interface. In Handbook of Battery Materials; Besenhard, P.D.J.O., Ed.; Wiely: New York, NY, USA, 1998; pp. 419–456. [Google Scholar]
- Dey, A.N. Lithium anode film and organic and inorganic electrolyte batteries. Thin Solid Films 1977, 43, 131–171. [Google Scholar] [CrossRef]
- Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems-The solid electrolyte interphase model. J. Electrochem. Soc. 2019, 126, 2047–2051. [Google Scholar] [CrossRef]
- Zaban, A.; Aurbach, D. Impedance spectroscopy of lithium and nickel electrodes in propylene carbonate solutions of different lithium salts: A comparative study. J. Power Sources 1995, 54, 289–295. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 2019, 144, L208–L210. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, H.; Wang, R.; Ben, L.; Lu, W.; Chen, L.; Chen, L.; Li, H. 3D visualization of inhomogeneous multi-layered structure and Young’s modulus of the solid electrolyte interphase (SEI) on silicon anodes for lithium ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 13229–13238. [Google Scholar] [CrossRef]
- Shi, S.; Lu, P.; Liu, Z.; Qi, Y.; Hector, L.G., Jr.; Li, H.; Harris, S.J. Direct calculation of Li-ion transport in the solid electrolyte interphase. J. Am. Chem. Soc. 2012, 134, 15476–15487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, G.; Liu, S.; Li, X.; Wang, X.; Wang, Z.; Chen, L. Understanding the dropping of lithium plating potential in carbonate electrolyte. Nano Energy 2020, 70, 104486. [Google Scholar] [CrossRef]
- Kim, S.-P.; Van Duin, A.C.T.; Shenoy, V.B. Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: A molecular dynamics study. J. Power Sources 2011, 196, 8590–8597. [Google Scholar] [CrossRef]
- Takenaka, N.; Suzuki, Y.; Sakai, H.; Nagaoka, M. On electrolyte-dependent formation of solid electrolyte interphase film in lithium-ion batteries: Strong sensitivity to small structural difference of electrolyte molecules. J. Phys. Chem. C 2014, 118, 10874–10882. [Google Scholar] [CrossRef]
- Nie, M.; Lucht, B.L. Role of Lithium Salt on Solid Electrolyte Interface (SEI) Formation and Structure in Lithium Ion Batteries. J. Electrochem. Soc. 2014, 161, A1001–A1006. [Google Scholar] [CrossRef]
- Che, H.; Chen, S.; Xie, Y.; Wang, H.; Amine, K.; Liao, X.-Z.; Ma, Z.-F. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1075. [Google Scholar] [CrossRef]
- Rauh, R.D.; Reise, T.F.; Brummer, S.B. Efficiencies of cycling lithium on a lithium substrate in propylene carbonate. J. Electrochem. Soc. 2019, 125, 186–190. [Google Scholar] [CrossRef]
- Pistoia, G.; Rossi, M.D.; Scrosati, B. Study of the behavior of ethylene carbonate as a nonaqueous battery solvent. J. Electrochem. Soc. 1970, 117, 500–502. [Google Scholar] [CrossRef]
- Pistoia, G. Nonaqueous batteries with LiClO4-Ethylene carbonate as electrolyte. J. Electrochem. Soc. 1971, 118, 153–158. [Google Scholar] [CrossRef]
- Fong, R.; von Sacken, U.; Dahn, J.R. Studies of lithium intercalation into carbons using nonaqueous electrochemical cells. J. Electrochem. Soc. 2019, 137, 2009–2013. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Du, X.; Lin, X.; Huang, J.-Q.; Tan, H.; Zhu, Y.; Zhang, B. Nanostructures of solid electrolyte interphases and their consequences for microsized Sn anodes in sodium ion batteries. Energy Environ. Sci. 2019, 12, 1550–1557. [Google Scholar] [CrossRef]
- Parimalam, B.S.; Lucht, B.L. Reduction reactions of electrolyte salts for lithium ion batteries: LiPF(6), LiBF(4), LiDFOB, LiBOB, and LiTFSI. J. Electrochem. Soc. 2018, 165, A251–A255. [Google Scholar] [CrossRef] [Green Version]
- Abraham, K.M.; Goldman, J.L.; Natwig, D.L. Characterization of ether electrolytes for rechargeable lithium cells. J. Electrochem. Soc. 2019, 129, 2404–2409. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, S.; Dong, S.; Li, Q.; Cui, G.; Chen, L. Self-stabilized solid electrolyte interface on a host-free Li-metal anode toward high areal capacity and rate utilization. Chem. Mater. 2018, 30, 4039–4047. [Google Scholar] [CrossRef]
- Heine, J.; Hilbig, P.; Qi, X.; Niehoff, P.; Winter, M.; Bieker, P. Fluoroethylene carbonate as electrolyte additive in tetraethylene glycol dimethyl ether based electrolytes for application in lithium ion and lithium metal batteries. J. Electrochem. Soc. 2015, 162, A1094–A1101. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Cheng, X.-B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-ion batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Soto, F.A.; Yan, P.; Engelhard, M.H.; Marzouk, A.; Wang, C.; Xu, G.; Chen, Z.; Amine, K.; Liu, J.; Sprenkle, V.L.; et al. Tuning the solid electrolyte interphase for selective Li- and Na-ion storage in hard carbon. Adv. Funct. Mater. 2017, 29, 1606860. [Google Scholar] [CrossRef]
- Pan, K.; Lu, H.; Zhong, F.; Ai, X.; Yang, H.; Cao, Y. Understanding the electrochemical compatibility and reaction mechanism on Na metal and hard carbon anodes of PC-based electrolytes for sodium-ion batteries. ACS Appl. Mater. Inter. 2018, 10, 39651. [Google Scholar] [CrossRef]
- Dou, X.W.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.M.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.H.; Feng, X.L. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef] [PubMed]
- Blyth, R.I.R.; Buqa, H.; Netzer, F.P.; Ramsey, M.G.; Besenhard, J.O.; Golob, P.; Winter, M. XPS studies of graphite electrode materials for lithium ion batteries. Appl. Surf. Sci. 2000, 167, 99–106. [Google Scholar] [CrossRef]
- Peled, E.; Bar Tow, D.; Merson, A.; Gladkich, A.; Burstein, L.; Golodnitsky, D. Composition, depth profiles and lateral distribution of materials in the SEI built on HOPG-TOF SIMS and XPS studies. J. Power Sources 2001, 97–98, 52–57. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review-SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Peled, E.; Tow, D.; Merson, A.; Burstein, L. Microphase structure of SEI on HOPG. J. New Mater. Electr. Sys. 2000, 3, 321–328. [Google Scholar]
- Eshkenazi, V.; Peled, E.; Burstein, L.; Golodnitsky, D. XPS analysis of the SEI formed on carbonaceous materials. Solid State Ionics 2004, 170, 83–91. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ulus, A.; Yufit, V. Effect of carbon substrate on SEI composition and morphology. Electrochim. Acta 2004, 50, 391–395. [Google Scholar] [CrossRef]
- Oka, H.; Kadoura, H.; Takahashi, N.T.; Ikawa, T. Effect of amorphous carbon coating on the formation of solid electrolyte interphase and electrochemical properties of a graphite electrode. J. Power Sources 2022, 543, 231850. [Google Scholar] [CrossRef]
- Novák, P.; Ufheil, J.; Buqa, H.; Krumeich, F.; Spahr, M.E.; Goers, D.; Wilhelm, H.; Dentzer, J.; Gadiou, R.; Vix-Guterl, C. The importance of the active surface area of graphite materials in the first lithium intercalation. J. Power Sources 2007, 174, 1082–1085. [Google Scholar] [CrossRef]
- Spahr, M.E.; Buqa, H.; Würsig, A.; Goers, D.; Hardwick, L.; Novák, P.; Krumeich, F.; Dentzer, J.; Vix-Guterl, C. Surface reactivity of graphite materials and their surface passivation during the first electrochemical lithium insertion. J. Power Sources 2006, 153, 300–311. [Google Scholar] [CrossRef]
- Ng, S.H.; Vix-Guterl, C.; Bernardo, P.; Tran, N.; Ufheil, J.; Buqa, H.; Dentzer, J.; Gadiou, R.; Spahr, M.E.; Goers, D.; et al. Correlations between surface properties of graphite and the first cycle specific charge loss in lithium-ion batteries. Carbon 2009, 47, 705–712. [Google Scholar] [CrossRef]
- Buqa, H.; Golob, P.; Winter, M.; Besenhard, J.O. Modified carbons for improved anodes in lithium ion cells. J. Power Sources 2001, 97–98, 122–125. [Google Scholar] [CrossRef]
- Mangun, C.L.; Benak, K.R.; Daley, M.A.; Economy, J. Oxidation of activated carbon fibers: effect on pore size, surface chemistry, and adsorption properties. Chem. Mater. 1999, 11, 3476–3483. [Google Scholar] [CrossRef]
- Radovic, L.R.; Silva, I.F.; Ume, J.I.; Menéndez, J.A.; Leon, C.A.L.Y.; Scaroni, A.W. An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons. Carbon 1997, 35, 1339–1348. [Google Scholar] [CrossRef]
- Collins, J.; Ngo, T.; Qu, D.; Foster, M. Spectroscopic investigations of sequential nitric acid treatments on granulated activated carbon: Effects of surface oxygen groups on π density. Carbon 2013, 57, 174–183. [Google Scholar] [CrossRef]
- Collins, J.; Zheng, D.; Ngo, T.; Qu, D.; Foster, M. Partial graphitization of activated carbon by surface acidification. Carbon 2014, 79, 500–517. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Collins, J.; Gourdin, G.; Foster, M.; Qu, D. Carbon surface functionalities and SEI formation during Li intercalation. Carbon 2015, 92, 193–244. [Google Scholar] [CrossRef]
- Peled, E.; Menachem, C.; Bar-Tow, D.; Melman, A. Improved graphite anode for lithium-ion batteries chemically: Bonded solid electrolyte interface and nanochannel formation. J. Electrochem. Soc. 2019, 143, L4–L7. [Google Scholar] [CrossRef]
- Seredych, M.; Hulicova-Jurcakova, D.; Lu, G.Q.; Bandosz, T.J. Surface functional groups of carbons and the effects of their chemical character, density and accessibility to ions on electrochemical performance. Carbon 2008, 46, 1475–1488. [Google Scholar] [CrossRef]
- Chen, W.; Chen, C.; Xiong, X.; Hu, P.; Hao, Z.; Huang, Y. Coordination of surface-induced reaction and intercalation: Toward a high-performance carbon anode for sodium-ion batteries. Adv. Sci. 2017, 4, 1600500. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wu, Z.; Wang, Z.; Qin, N.; Li, Y.; Cao, Y.; Lu, Z. Solid electrolyte interface stabilization via surface oxygen species functionalization in hard carbon for superior performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 3606. [Google Scholar] [CrossRef]
- Yan, J.; Xia, B.-J.; Su, Y.-C.; Zhou, X.-Z.; Zhang, J.; Zhang, X.-G. Phenomenologically modeling the formation and evolution of the solid electrolyte interface on the graphite electrode for lithium-ion batteries. Electrochim. Acta 2008, 53, 7069–7078. [Google Scholar] [CrossRef]
- Spahr, M.E.; Wilhelm, H.; Palladino, T.; Dupont-Pavlovsky, N.; Goers, D.; Joho, F.; Novák, P. The role of graphite surface group chemistry on graphite exfoliation during electrochemical lithium insertion. J. Power Sources 2003, 119–121, 543–549. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.M. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Lin, C.; Yang, W.; Meng, Y.; Guo, Y.; Li, M.; Xiao, D. Hierarchically porous nitrogen-rich carbon derived from wheat straw as an ultra-high-rate anode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 9684–9690. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, Y.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-rich porous carbon derived from biomass as a high performance anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.; Li, B.; Yu, C.; Wang, H.; Li, Q. Construction of heteroatom-doped and three-dimensional graphene materials for the applications in supercapacitors: A review. J. Energy Storage 2021, 44, 103437. [Google Scholar] [CrossRef]
- Han, X.; Zhou, W.; Chen, M.; Chen, J.; Wang, G.; Liu, B.; Luo, L.; Chen, S.; Zhang, Q.; Shi, S.; et al. Interfacial nitrogen engineering of robust silicon/MXene anode toward high energy solid-state lithium-ion batteries. J. Energy Chem. 2022, 67, 727–735. [Google Scholar] [CrossRef]
- Yuan, F.; Sun, H.; Zhang, D.; Li, Z.; Wang, J.; Wang, H.; Wang, Q.; Wu, Y.; Wang, B. Enhanced electron transfer and ion storage in phosphorus/nitrogen co-doped 3D interconnected carbon nanocage toward potassium-ion battery. J. Colloid. Interf. Sci. 2022, 611, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zeng, Z.; Lian, X.; Dou, S.; Sun, L. Homologous nitrogen-doped hierarchical carbon architectures enabling compatible anode and cathode for potassium-ion hybrid capacitors. Small 2022, 18, e2107139. [Google Scholar] [CrossRef] [PubMed]
- Alvin, S.; Yoon, D.; Chandra, C.; Susanti, R.F.; Chang, W.; Ryu, C.; Kim, J. Extended flat voltage profile of hard carbon synthesized using a two-step carbonization approach as an anode in sodium ion batteries. J. Power Sources 2019, 430, 157–168. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67. [Google Scholar] [CrossRef]
- Wang, H.-l.; Shi, Z.-q.; Jin, J.; Chong, C.-b.; Wang, C.-y. Properties and sodium insertion behavior of Phenolic Resin-based hard carbon microspheres obtained by a hydrothermal method. J. Electroanal. Chem. 2015, 755, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhang, M.; Bai, Y.; Wang, X.; Dong, R.; Wu, C. Lotus seedpod-derived hard carbon with hierarchical porous structure as stable anode for sodium-ion batteries. ACS Appl. Mater. Inter. 2019, 11, 12554. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Hu, Z.; Lai, W.-H.; Tao, Y.; Peng, J.; Miao, Z.-C.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Hard carbon anodes: Fundamental understanding and commercial perspectives for Na-ion batteries beyond Li-ion and K-ion counterparts. Adv. Energy Mater. 2020, 11, 2002704. [Google Scholar] [CrossRef]
- McShane, E.J.; Bergstrom, H.K.; Weddle, P.J.; Brown, D.E.; Colclasure, A.M.; McCloskey, B.D. Quantifying graphite solid-electrolyte interphase chemistry and its impact on fast charging. ACS Energy Lett. 2022, 7, 2734–2744. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.L.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-defect and low-porosity hard carbon with high coulombic efficiency and high capacity for practical sodium ion battery anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Winter, M.; Placke, T.; Ramirez-Rico, J. Correlation of structure and performance of hard carbons as anodes for sodium ion batteries. Chem. Mater. 2019, 31, 7288. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.; Liu, Y.; Cao, Y.; Zhu, Q.; Liu, H.; Wang, Z.; Zhang, P.; Xu, B. Extended “adsorption-insertion” model: A new insight into the sodium storage mechanism of hard carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Jiang, J.M.; Zhang, Y.D.; Li, Z.W.; An, Y.F.; Zhu, Q.; Xu, Y.H.; Zang, S.; Dou, H.; Zhang, X.G. Defect-rich and N-doped hard carbon as a sustainable anode for high-energy lithium-ion capacitors. J. Colloid Interf. Sci. 2020, 567, 75–83. [Google Scholar] [CrossRef]
- Han, Y.-J.; Chung, D.; Nakabayashi, K.; Chung, J.-D.; Miyawaki, J.; Yoon, S.-H. Effect of heat pre-treatment conditions on the electrochemical properties of mangrove wood-derived hard carbon as an effective anode material for lithium-ion batteries. Electrochim. Acta 2016, 213, 432–438. [Google Scholar] [CrossRef]
- Huo, K.F.; An, W.L.; Fu, J.J.; Gao, B.; Wang, L.; Peng, X.; Cheng, G.J.; Chu, P.K. Mesoporous nitrogen-doped carbon hollow spheres as high-performance anodes for lithium-ion batteries. J. Power Sources 2016, 324, 233–238. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, S.M.; Senthil, C.; Kim, S.S.; Ju, B.K.; Jung, H.Y. Understanding excess Li storage beyond LiC6 in reduced dimensional scale graphene. ACS Nano 2021, 15, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Kensy, C.; Leistenschneider, D.; Wang, S.; Tanaka, H.; Dörfler, S.; Kaneko, K.; Kaskel, S. The role of carbon electrodes pore size distribution on the formation of the cathode-electrolyte interphase in lithium-sulfur batteries. Batter. Supercaps 2020, 4, 612–622. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and improving the initial coulombic efficiency of high-capacity anode materials for practical sodium ion batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Liu, M.; Huo, S.; Xu, M.; Wu, L.; Liu, M.; Xue, Y.; Yan, Y.-M. Structural engineering of N/S co-doped carbon material as high-performance electrode for supercapacitors. Electrochim. Acta 2018, 274, 389–399. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Li, J.; Lv, Q.; Ma, T.; Zhu, W.; Qiu, X. Improving coulombic efficiency by confinement of solid electrolyte interphase film in pores of silicon/carbon composite. J. Mater. Chem. A 2013, 1, 14075–14079. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, F.; Liu, C.; Tan, J.; Cheng, H.M. New insight into the solid electrolyte interphase with use of a focused ion beam. J. Phys. Chem. B 2005, 109, 22205–22211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Liu, S.-H.; Li, F.; Bai, S.; Liu, C.; Tan, J.; Cheng, H.-M. Electrochemical performance of pyrolytic carbon-coated natural graphite spheres. Carbon 2006, 44, 2212–2218. [Google Scholar] [CrossRef]




| Sample | d002 (nm) | La (nm) | Lc (nm) | N | IG/ID | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|---|---|---|---|---|
| HC-5 | 0.413 | 2.15 | 1.3 | 3.1 | 0.31 | 34 | 0.018 | 2.09 |
| HC-2 | 0.401 | 2.26 | 1.35 | 3.3 | 0.4 | 20 | 0.014 | 1.97 |
| HC-1 | 0.398 | 2.4 | 1.42 | 3.5 | 0.42 | 13 | 0.006 | 1.94 |
| HC-0.5 | 0.391 | 2.53 | 1.46 | 3.3 | 0.52 | 1.74 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Chen, Y.; Sun, H.; Yuan, T.; Gong, Y.; Liu, X.; Chen, T. Impact of Surface Structure on SEI for Carbon Materials in Alkali Ion Batteries: A Review. Batteries 2023, 9, 226. https://doi.org/10.3390/batteries9040226
Zhao X, Chen Y, Sun H, Yuan T, Gong Y, Liu X, Chen T. Impact of Surface Structure on SEI for Carbon Materials in Alkali Ion Batteries: A Review. Batteries. 2023; 9(4):226. https://doi.org/10.3390/batteries9040226
Chicago/Turabian StyleZhao, Xvtong, Ying Chen, Hao Sun, Tao Yuan, Yinyan Gong, Xinjuan Liu, and Taiqiang Chen. 2023. "Impact of Surface Structure on SEI for Carbon Materials in Alkali Ion Batteries: A Review" Batteries 9, no. 4: 226. https://doi.org/10.3390/batteries9040226




