Recent Progress in Electrolyte Additives for Highly Reversible Zinc Anodes in Aqueous Zinc Batteries
Abstract
:1. Introduction
2. Challenges with Zn Anode
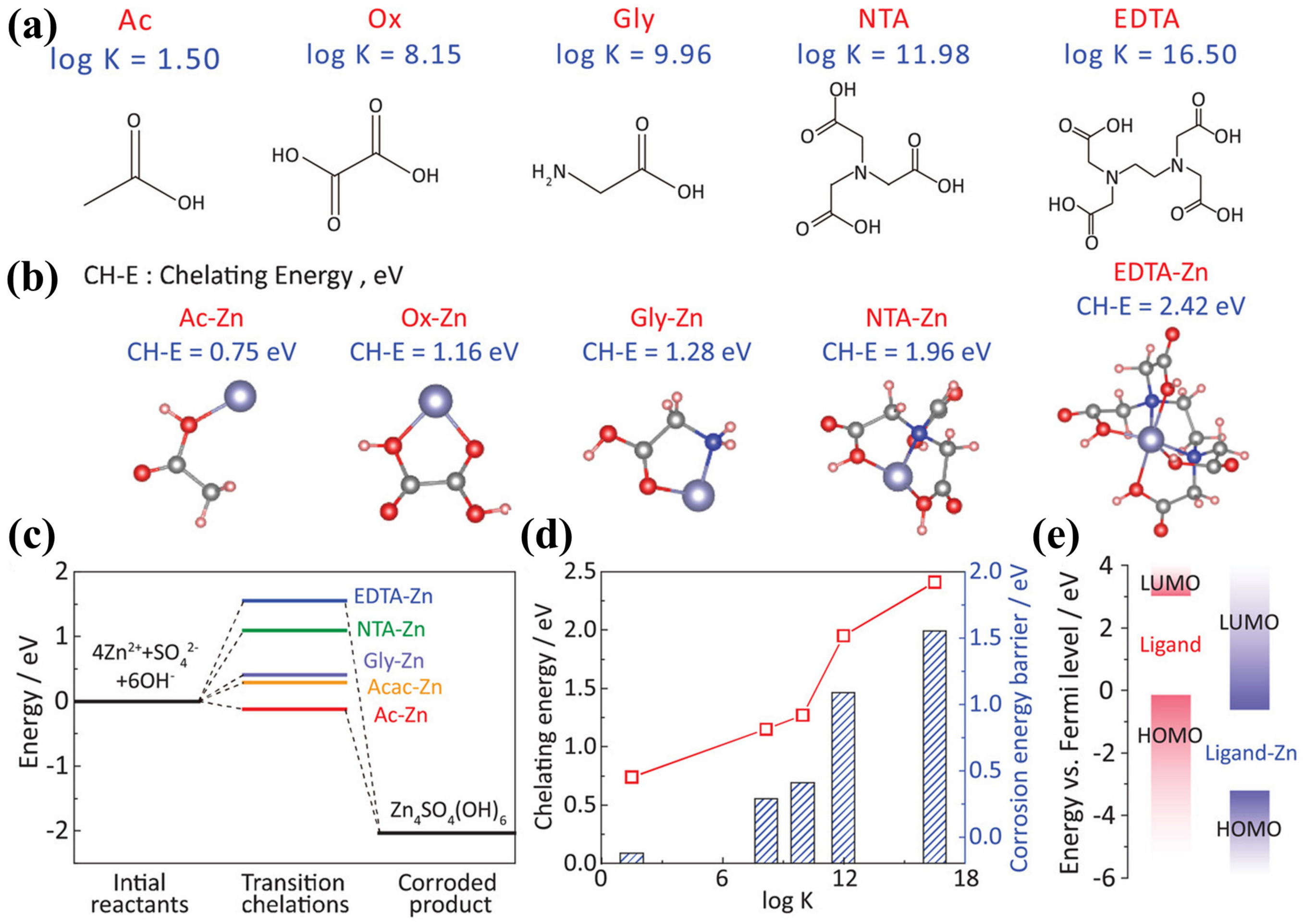
3. Electrolyte Additives
- (1)
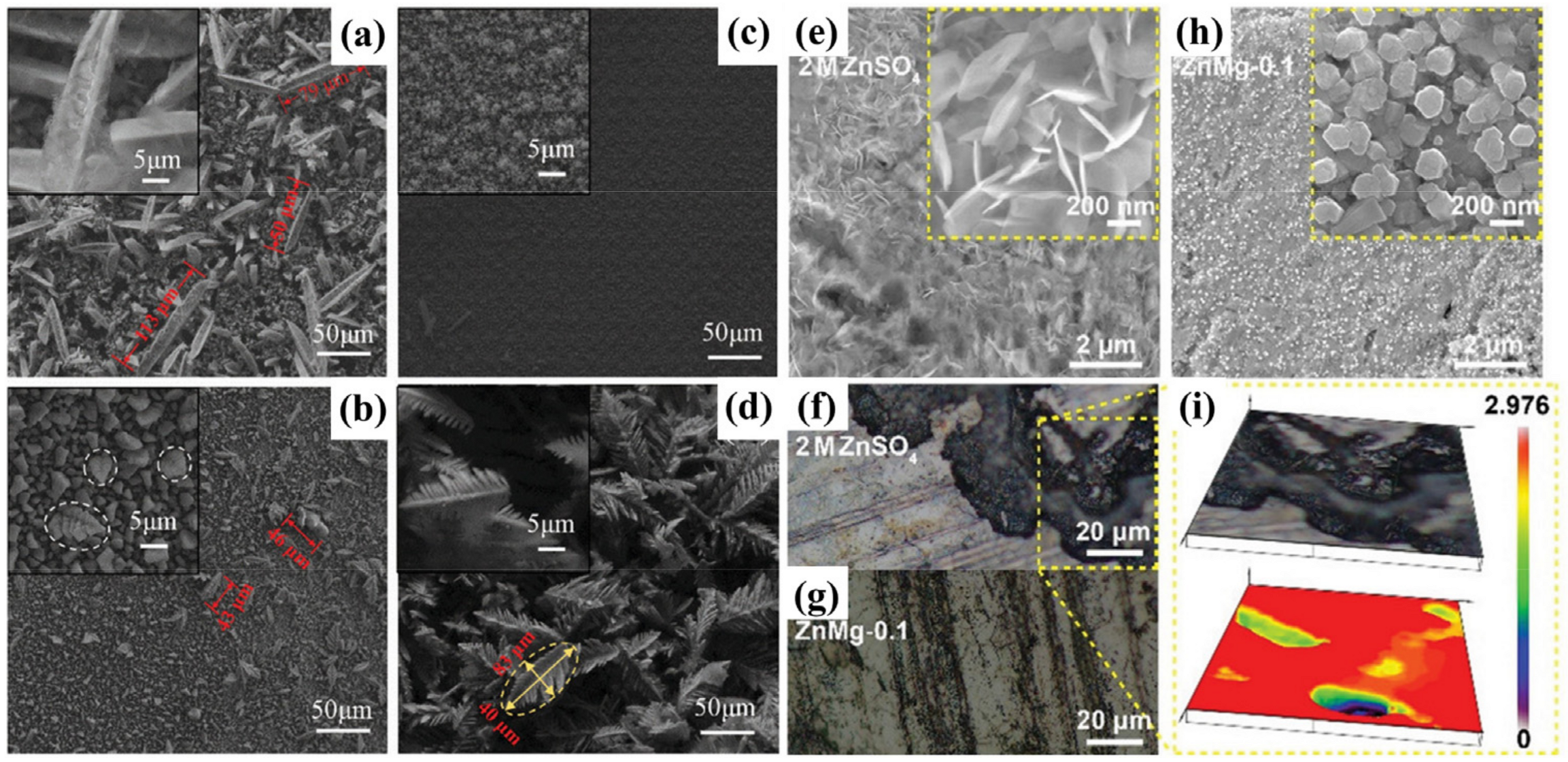
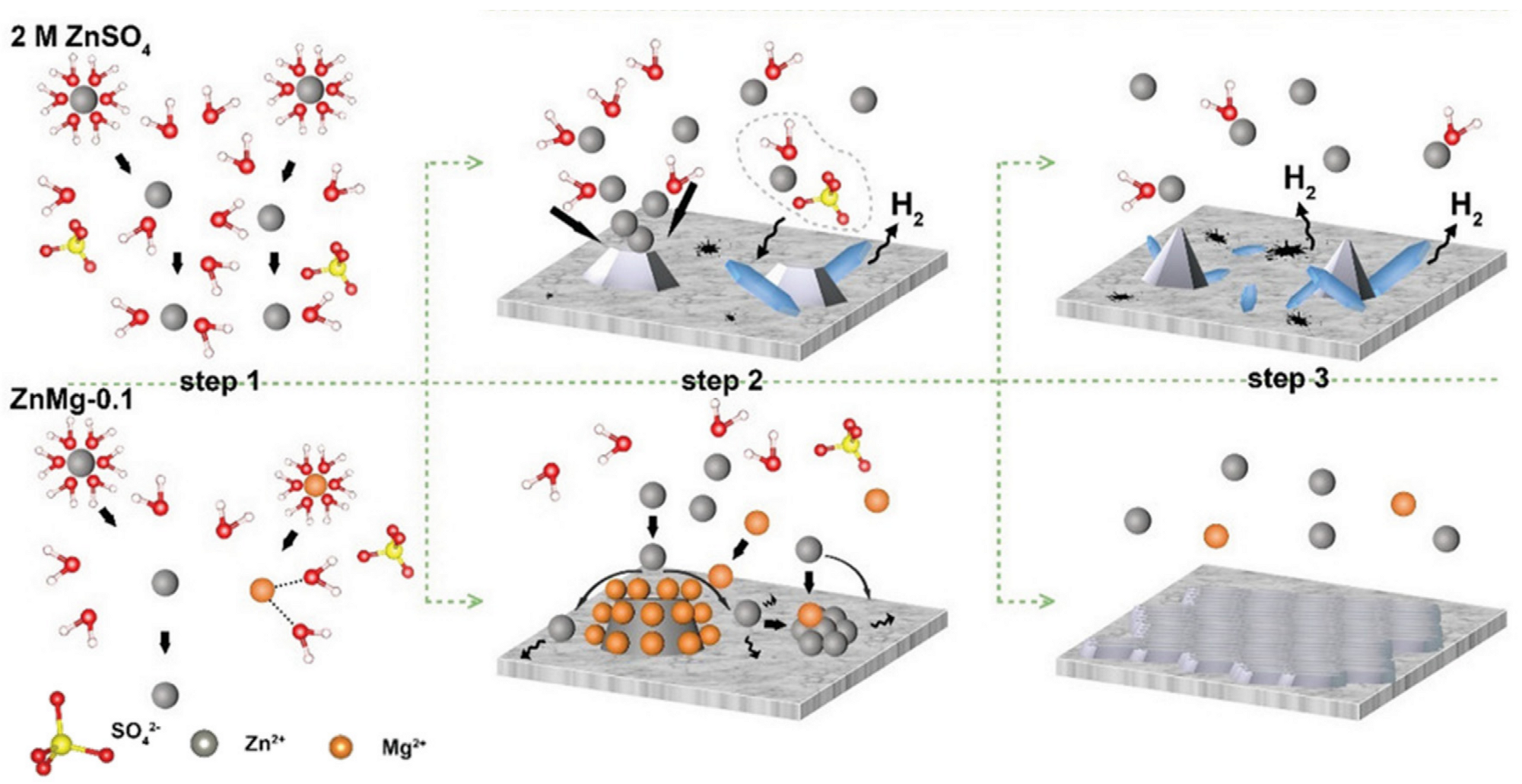
- Metal ion additives: metal ions can be reduced before zinc to provide additional deposition sites, or they cannot be reduced during zinc deposition and form an electrostatic shielding layer to suppress the tip effect.
- (2)
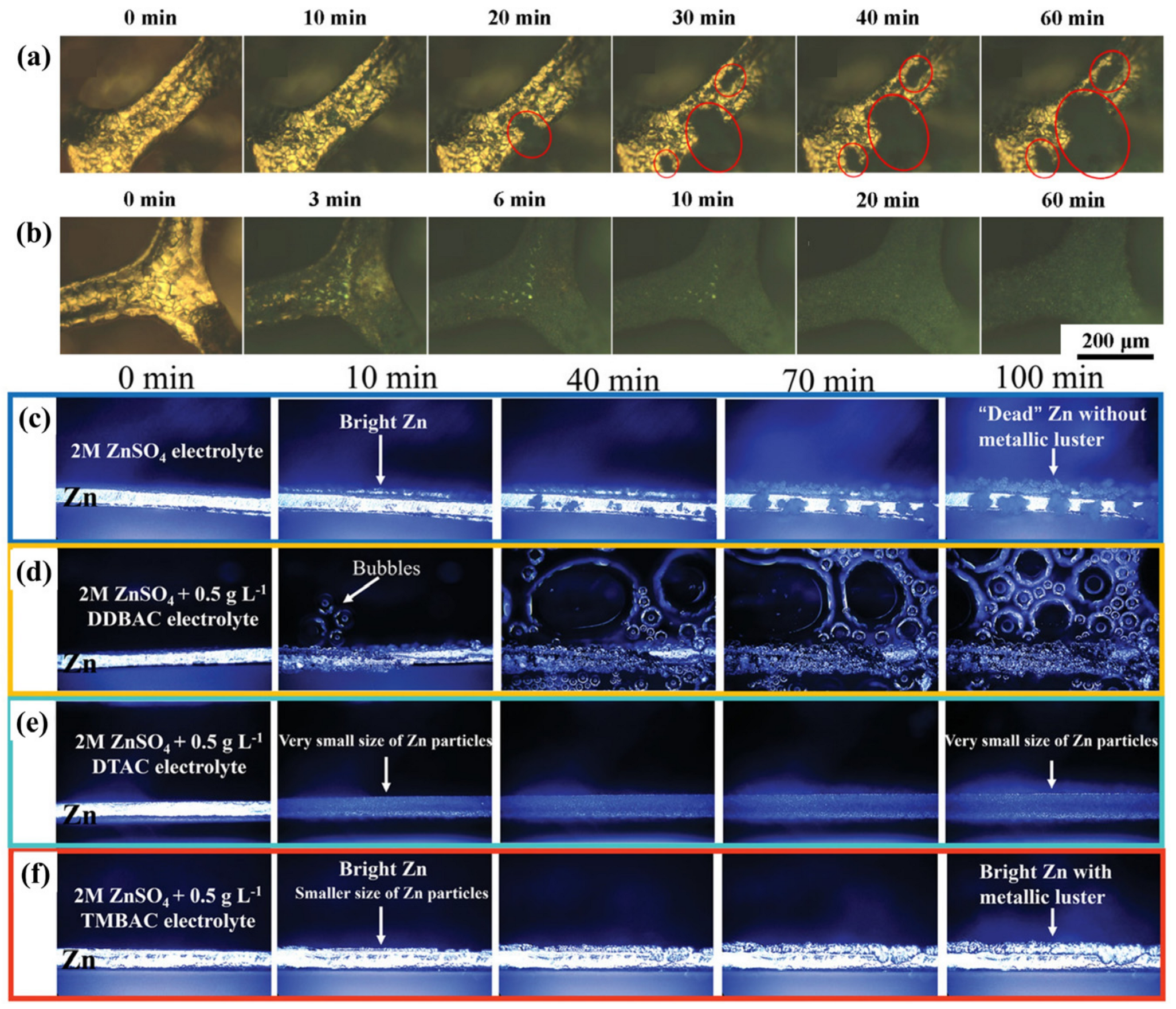
- Surfactant additives: the molecules/ions that adsorb on the electrode surface, thereby, modulate the anode/electrolyte interfacial environment and homogenize the Zn2+ flux.
- (3)
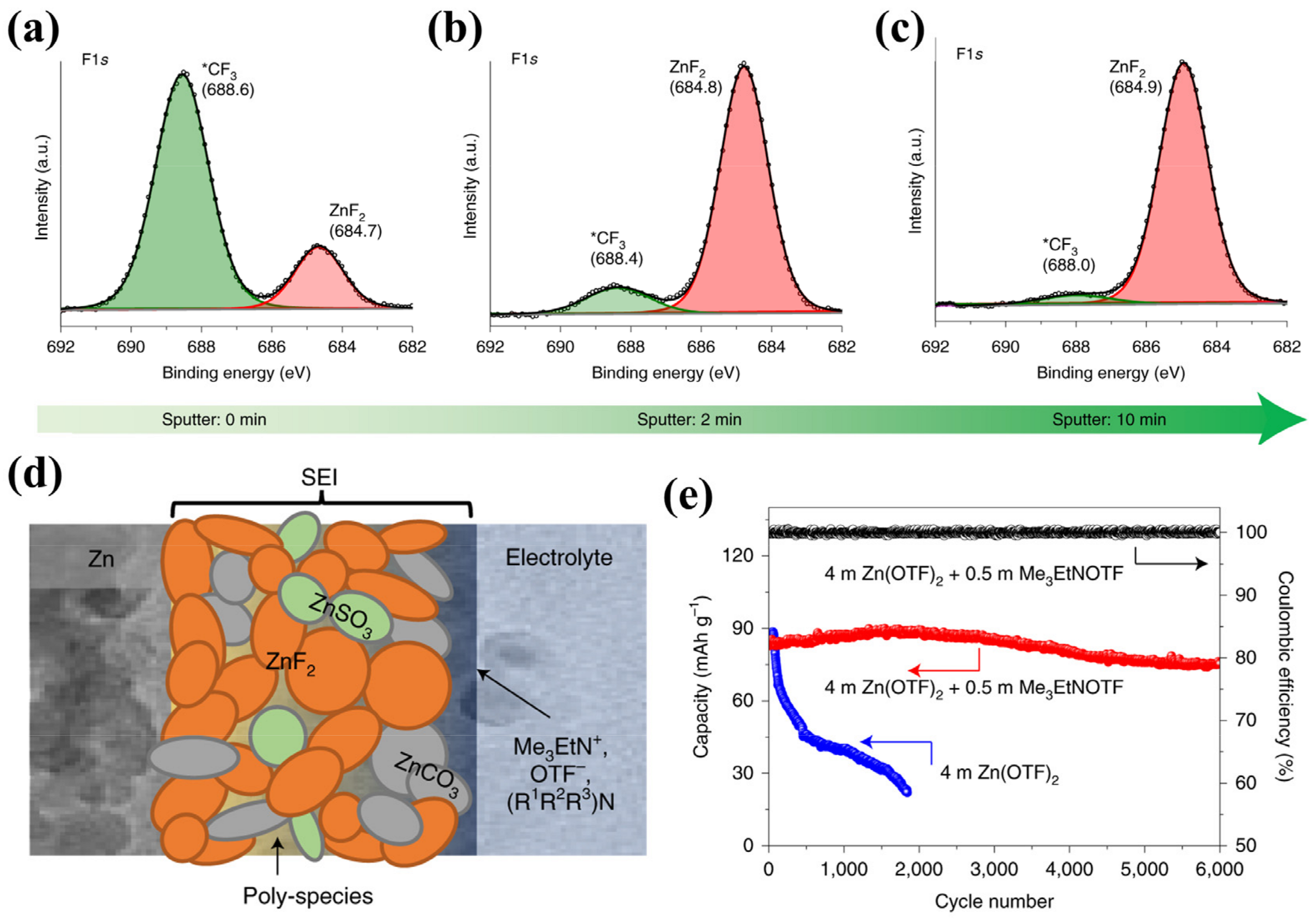
- SEI film-forming additives: the additives will be consumed by reactions to form a protective interfacial layer on the anode surface, which can prevent the hydrogen evolution reaction and dendrite formation by suppressing the 2D diffusion of Zn2+ on the substrate surface.
- (4)
- Complexing additives: the interaction between these additives and zinc ions is stronger than that between zinc ions and water, and they can enter the solvation sheath of Zn2+, weaken the activity of water molecules, and change the zinc deposition kinetics.
3.1. Metal Ion Additives
3.2. Surfactant Additives
3.3. SEI Film-Forming Additives

3.4. Complexing Additives
4. Conclusions and Perspectives
- (1)
- Developing multifunctional additives that can solve both anode and cathode issues simultaneously is of great importance. For example, some metal ion additives can suppress the dissolution of metal ions from cathodes and, simultaneously, inhibit the dendritic growth of anodes. To this end, more advanced additives are urgently needed to modify both the anode and cathode at the same time, aiming at achieving a better overall performance of the battery.
- (2)
- Exploring the working principles of additives plays a key role in guiding the future development and design of electrolyte additives. It is essential to systematically investigate the influence of additive structure on the electrochemical performance, so as to establish the relationship of structure–mechanism performance on additives.
- (3)
- Studying the interactions between different additives in electrolyte is highly desired as well, which can help realize the synergistic effects that arise from various additives added to the electrolyte, achieving further improved cell performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, D.; Yang, H.Y.; Bai, Y. Tactics to Optimize Conversion-Type Metal Fluoride/Sulfide/Oxide Cathodes toward Advanced Lithium Metal Batteries. Nano Res. 2022. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.-F.; Passerini, S.; Hahn, R. An Overview and Future Perspectives of Aluminum Batteries. Adv. Mater. 2016, 28, 7564–7579. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Z.; Lv, Y.; Tang, A.; Dai, L.; Wang, L.; He, Z. Perovskite enables high performance vanadium redox flow battery. Chem. Eng. J. 2022, 443, 136341. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Liu, H.; Wu, X.; Zhi, C. Tetragonal VO2 hollow nanospheres as robust cathode material for aqueous zinc ion batteries. Mater. Today Energy 2020, 17, 100431. [Google Scholar] [CrossRef]
- Kidanu, W.G.; Yang, H.; Park, S.; Hur, J.; Kim, I.T. Room-Temperature Liquid-Metal Coated Zn Electrode for Long Life Cycle Aqueous Rechargeable Zn-Ion Batteries. Batteries 2022, 8, 208. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Zhong, C.; Wu, T.; Chen, Z.; Hu, W.; Amine, K.; Lu, J. Recent Advances in Flexible Zinc-Based Rechargeable Batteries. Adv. Energy Mater. 2018, 9, 1802605. [Google Scholar] [CrossRef]
- Wu, X.; Markir, A.; Xu, Y.; Zhang, C.; Leonard, D.P.; Shin, W.; Ji, X. A Rechargeable Battery with an Iron Metal Anode. Adv. Funct. Mater. 2019, 29, 1900911. [Google Scholar] [CrossRef]
- Fitz, O.; Ingenhoven, S.; Bischoff, C.; Gentischer, H.; Birke, K.P.; Saracsan, D.; Biro, D. Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes. Batteries 2021, 7, 40. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat 2019, 2, 237–260. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Li, N.; Liu, Z.; Tang, Z.; Zapien, J.A.; Chen, S.; Fan, J.; Zhi, C. Hydrogen-Free and Dendrite-Free All-Solid-State Zn-Ion Batteries. Adv. Mater. 2020, 32, 1908121. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Islam, S.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.-k.; Kim, J. The dominant role of Mn2+ additive on the electrochemical reaction in ZnMn2O4 cathode for aqueous zinc-ion batteries. Energy Storage Mater. 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Thieu, N.A.; Li, W.; Chen, X.; Hu, S.; Tian, H.; Tran, H.N.N.; Li, W.; Reed, D.M.; Li, X.; Liu, X. An Overview of Challenges and Strategies for Stabilizing Zinc Anodes in Aqueous Rechargeable Zn-Ion Batteries. Batteries 2023, 9, 41. [Google Scholar] [CrossRef]
- Li, B.; Xue, J.; Han, C.; Liu, N.; Ma, K.; Zhang, R.; Wu, X.; Dai, L.; Wang, L.; He, Z. A hafnium oxide-coated dendrite-free zinc anode for rechargeable aqueous zinc-ion batteries. J. Colloid Interface Sci. 2021, 599, 467–475. [Google Scholar] [CrossRef]
- Nagy, T.; Nagy, L.; Erdélyi, Z.; Baradács, E.; Deák, G.; Zsuga, M.; Kéki, S. “In Situ” Formation of Zn Anode from Bimetallic Cu-Zn Alloy (Brass) for Dendrite-Free Operation of Zn-Air Rechargeable Battery. Batteries 2022, 8, 212. [Google Scholar] [CrossRef]
- Liu, J.; Nie, N.; Wang, J.; Hu, M.; Zhang, J.; Li, M.; Huang, Y. Initiating a wide-temperature-window yarn zinc ion battery by a highly conductive iongel. Mater. Today Energy 2020, 16, 100372. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Hao, J.; Li, X.; Zeng, X.; Li, D.; Mao, J.; Guo, Z. Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ. Sci. 2020, 13, 3917–3949. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, X.; Lai, F.; Liu, T.; Shearing, P.R.; Parkin, I.P.; He, G.; Brett, D.J.L. Rechargeable aqueous Zn-based energy storage devices. Joule 2021, 5, 2845–2903. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, X.; Yang, Z.; Xia, M.; Xu, C.; Liu, Y.; Yu, H.; Zhang, L.; Shu, J. Insight into the electrolyte strategies for aqueous zinc ion batteries. Coord. Chem. Rev. 2022, 452, 214297. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Zhao, Y.; Hong, H.; Li, H.; Huang, Z.; Liang, G.; Yang, Q.; Zhi, C. Insight on Organic Molecules in Aqueous Zn-Ion Batteries with an Emphasis on the Zn Anode Regulation. Adv. Energy Mater. 2022, 12, 2102707. [Google Scholar] [CrossRef]
- Guo, S.; Qin, L.; Zhang, T.; Zhou, M.; Zhou, J.; Fang, G.; Liang, S. Fundamentals and perspectives of electrolyte additives for aqueous zinc-ion batteries. Energy Storage Mater. 2021, 34, 545–562. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and Future Perspective on Zinc Metal Anode for Rechargeable Aqueous Zinc-ion Batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Lu, W.; Xie, C.; Zhang, H.; Li, X. Inhibition of Zinc Dendrite Growth in Zinc-Based Batteries. ChemSusChem 2018, 11, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, G.; Guo, Y.; Liu, Z.; Yan, B.; Wang, D.; Huang, Z.; Li, X.; Fan, J.; Zhi, C. Do Zinc Dendrites Exist in Neutral Zinc Batteries: A Developed Electrohealing Strategy to In Situ Rescue In-Service Batteries. Adv. Mater. 2019, 31, e1903778. [Google Scholar] [CrossRef]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Bayaguud, A.; Fu, Y.; Zhu, C. Interfacial parasitic reactions of zinc anodes in zinc ion batteries: Underestimated corrosion and hydrogen evolution reactions and their suppression strategies. J. Energy Chem. 2022, 64, 246–262. [Google Scholar] [CrossRef]
- Banik, S.J.; Akolkar, R. Suppressing Dendritic Growth during Alkaline Zinc Electrodeposition using Polyethylenimine Additive. Electrochim. Acta 2015, 179, 475–481. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhang, X.; Zeng, Z.; Qin, J.; Huang, Y. Strategies of regulating Zn2+solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries. Energy Environ. Sci. 2022, 15, 499–528. [Google Scholar] [CrossRef]
- Qin, H.; Chen, W.; Kuang, W.; Hu, N.; Zhang, X.; Weng, H.; Tang, H.; Huang, D.; Xu, J.; He, H. A Nature-Inspired Separator with Water-Confined and Kinetics-Boosted Effects for Sustainable and High-Utilization Zn Metal Batteries. Small 2023, 19, e2300130. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Huang, F.; Cai, Y.; Li, Y.; Ke, H.; Lv, P.; Wei, Q. “Water-in-Salt” Nonalkaline Gel Polymer Electrolytes Enable Flexible Zinc-Air Batteries with Ultra-Long Operating Time. Adv. Funct. Mater. 2022, 32, 2203204. [Google Scholar] [CrossRef]
- Geng, Y.; Pan, L.; Peng, Z.; Sun, Z.; Lin, H.; Mao, C.; Wang, L.; Dai, L.; Liu, H.; Pan, K.; et al. Electrolyte additive engineering for aqueous Zn ion batteries. Energy Storage Mater. 2022, 51, 733–755. [Google Scholar] [CrossRef]
- Gilman, F.; Mansfeld, S. The effect of lead ions on the dissolution and deposition characteristics of a zinc single crystal in 6M KOH. J. Electrochem. Soc. 1970, 171, 588–597. [Google Scholar]
- Wang, J.M.; Zhang, L.; Zhang, C.; Zhang, J.Q. Effects of bismuth ion and tetrabutylammonium bromide on the dendritic growth of zinc in alkaline zincate solutions. J. Power Sources 2001, 102, 139–143. [Google Scholar]
- Ma, L.; Chen, S.; Li, H.; Ruan, Z.; Tang, Z.; Liu, Z.; Wang, Z.; Huang, Y.; Pei, Z.; Zapien, J.A.; et al. Initiating a mild aqueous electrolyte Co3O4/Zn battery with 2.2 V-high voltage and 5000-cycle lifespan by a Co(iii) rich-electrode. Energy Environ. Sci. 2018, 11, 2521–2530. [Google Scholar] [CrossRef]
- Chang, G.; Liu, S.; Fu, Y.; Hao, X.; Jin, W.; Ji, X.; Hu, J. Inhibition Role of Trace Metal Ion Additives on Zinc Dendrites during Plating and Striping Processes. Adv. Mater. Interfaces 2019, 6, 1901358. [Google Scholar] [CrossRef]
- Wang, P.; Xie, X.; Xing, Z.; Chen, X.; Fang, G.; Lu, B.; Zhou, J.; Liang, S.; Fan, H.J. Mechanistic Insights of Mg2+-Electrolyte Additive for High-Energy and Long-Life Zinc-Ion Hybrid Capacitors. Adv. Energy Mater. 2021, 11, 2101158. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, F.; Zhao, Y.; Wang, H.; Huang, Y.; Wu, F.; Chen, R.; Li, L. A Self-Regulated Electrostatic Shielding Layer toward Dendrite-Free Zn Batteries. Adv. Mater. 2022, 34, e2203104. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Feng, J.; Wang, Y.; Wu, X.; Ma, P.; Zhang, X.; Wang, G.; Yan, X. A rechargeable aqueous zinc/sodium manganese oxides battery with robust performance enabled by Na2SO4 electrolyte additive. Energy Storage Mater. 2021, 38, 299–308. [Google Scholar] [CrossRef]
- Bayaguud, A.; Luo, X.; Fu, Y.; Zhu, C. Cationic Surfactant-Type Electrolyte Additive Enables Three-Dimensional Dendrite-Free Zinc Anode for Stable Zinc-Ion Batteries. ACS Energy Lett. 2020, 5, 3012–3020. [Google Scholar] [CrossRef]
- Guan, K.; Tao, L.; Yang, R.; Zhang, H.; Wang, N.; Wan, H.; Cui, J.; Zhang, J.; Wang, H.; Wang, H. Anti-Corrosion for Reversible Zinc Anode via a Hydrophobic Interface in Aqueous Zinc Batteries. Adv. Energy Mater. 2022, 12, 2103557. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Che, Y.; Cheng, L.; Zhang, H.; Chen, J.; Xie, F.; Wang, N.; Jin, Y.; Meng, H. Arginine Cations Inhibiting Charge Accumulation of Dendrites and Boosting Zn Metal Reversibility in Aqueous Rechargeable Batteries. ACS Sustain. Chem. Eng. 2021, 9, 6855–6863. [Google Scholar] [CrossRef]
- Yao, R.; Qian, L.; Sui, Y.; Zhao, G.; Guo, R.; Hu, S.; Liu, P.; Zhu, H.; Wang, F.; Zhi, C.; et al. A Versatile Cation Additive Enabled Highly Reversible Zinc Metal Anode. Adv. Energy Mater. 2021, 12, 2102780. [Google Scholar] [CrossRef]
- Qiu, Q.; Chi, X.; Huang, J.; Du, Y.; Liu, Y. Highly Stable Plating/Stripping Behavior of Zinc Metal Anodes in Aqueous Zinc Batteries Regulated by Quaternary Ammonium Cationic Salts. ChemElectroChem 2021, 8, 858–865. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, J.; Li, Y.; Zhou, X.; Ding, S.; Zheng, Q.; Lin, D.; Zhao, J.; Xu, B. Insights into Zn anode surface chemistry for dendrite-free Zn ion batteries. J. Mater. Chem. A 2022, 10, 11288–11297. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, X.; Sun, J.; Wang, W.; Wang, M.; Yuan, Y.; Chuai, M.; Chen, N.; Hu, H.; Chen, W. Nucleophilic Interfacial Layer Enables Stable Zn Anodes for Aqueous Zn Batteries. Nano Lett. 2022, 22, 3298–3306. [Google Scholar] [CrossRef]
- Lin, Y.; Mai, Z.; Liang, H.; Li, Y.; Yang, G.; Wang, C. Dendrite-free Zn anode enabled by anionic surfactant-induced horizontal growth for highly-stable aqueous Zn-ion pouch cells. Energy Environ. Sci. 2023, 16, 687–697. [Google Scholar] [CrossRef]
- Hao, J.; Long, J.; Li, B.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Yang, Z.; Pang, W.K.; Guo, Z. Toward High-Performance Hybrid Zn-Based Batteries via Deeply Understanding Their Mechanism and Using Electrolyte Additive. Adv. Funct. Mater. 2019, 29, 1903605. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Tian, Q.; Chen, J.; Xu, X.; Han, X.; Xu, J. Stabilizing zinc deposition with sodium lignosulfonate as an electrolyte additive to improve the life span of aqueous zinc-ion batteries. J. Colloid Interface Sci. 2021, 601, 486–494. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, X.; Luo, M.; Cao, K.; Lu, Y.; Xu, B.B.; Pan, H.; Tao, K.; Jiang, Y. Amino Acid-Induced Interface Charge Engineering Enables Highly Reversible Zn Anode. Adv. Funct. Mater. 2021, 31, 2103514. [Google Scholar] [CrossRef]
- Meng, Q.; Zhao, R.; Cao, P.; Bai, Q.; Tang, J.; Liu, G.; Zhou, X.; Yang, J. Stabilization of Zn anode via a multifunctional cysteine additive. Chem. Eng. J. 2022, 447, 137471. [Google Scholar] [CrossRef]
- Zhou, T.; Mu, Y.; Chen, L.; Li, D.; Liu, W.; Yang, C.; Zhang, S.; Wang, Q.; Jiang, P.; Ge, G.; et al. Toward stable zinc aqueous rechargeable batteries by anode morphology modulation via polyaspartic acid additive. Energy Storage Mater. 2022, 45, 777–785. [Google Scholar] [CrossRef]
- Miao, Z.; Liu, Q.; Wei, W.; Zhao, X.; Du, M.; Li, H.; Zhang, F.; Hao, M.; Cui, Z.; Sang, Y.; et al. Unveiling unique steric effect of threonine additive for highly reversible Zn anode. Nano Energy 2022, 97, 107145. [Google Scholar] [CrossRef]
- Niu, B.; Li, Z.; Luo, D.; Ma, X.; Yang, Q.; Liu, Y.-E.; Yu, X.; He, X.-r.; Qiao, Y.; Wang, X. Nano-scaled hydrophobic confinement of aqueous electrolyte by nonionic amphiphilic polymer for long-lasting and wide-temperature zn-based energy storage. Energy Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Jin, Y.; Han, K.S.; Shao, Y.; Sushko, M.L.; Xiao, J.; Pan, H.; Liu, J. Stabilizing Zinc Anode Reactions by Polyethylene Oxide Polymer in Mild Aqueous Electrolytes. Adv. Funct. Mater. 2020, 30, 2003932. [Google Scholar] [CrossRef]
- Bani Hashemi, A.; Kasiri, G.; La Mantia, F. The effect of polyethyleneimine as an electrolyte additive on zinc electrodeposition mechanism in aqueous zinc-ion batteries. Electrochim. Acta 2017, 258, 703–708. [Google Scholar] [CrossRef]
- Yan, M.; Xu, C.; Sun, Y.; Pan, H.; Li, H. Manipulating Zn anode reactions through salt anion involving hydrogen bonding network in aqueous electrolytes with PEO additive. Nano Energy 2021, 82, 105739. [Google Scholar] [CrossRef]
- Yan, M.; Dong, N.; Zhao, X.; Sun, Y.; Pan, H. Tailoring the Stability and Kinetics of Zn Anodes through Trace Organic Polymer Additives in Dilute Aqueous Electrolyte. ACS Energy Lett. 2021, 6, 3236–3243. [Google Scholar] [CrossRef]
- Zeng, X.; Mao, J.; Hao, J.; Liu, J.; Liu, S.; Wang, Z.; Wang, Y.; Zhang, S.; Zheng, T.; Liu, J.; et al. Electrolyte Design for In Situ Construction of Highly Zn2+-Conductive Solid Electrolyte Interphase to Enable High-Performance Aqueous Zn-Ion Batteries under Practical Conditions. Adv. Mater. 2021, 33, e2007416. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, D.; Pollard, T.; Deng, T.; Zhang, B.; Yang, C.; Chen, L.; Vatamanu, J.; Hu, E.; Hourwitz, M.J.; et al. Fluorinated interphase enables reversible aqueous zinc battery chemistries. Nat. Nanotechnol. 2021, 16, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xie, K.; Liu, S.; Zhang, S.; Hao, J.; Liu, J.; Pang, W.K.; Liu, J.; Rao, P.; Wang, Q.; et al. Bio-inspired design of anin situmultifunctional polymeric solid–electrolyte interphase for Zn metal anode cycling at 30 mA cm−2 and 30 mAh cm−2. Energy Environ. Sci. 2021, 14, 5947–5957. [Google Scholar] [CrossRef]
- Sun, C.; Wu, C.; Gu, X.; Wang, C.; Wang, Q. Interface Engineering via Ti(3)C(2)T(x) MXene Electrolyte Additive toward Dendrite-Free Zinc Deposition. Nano-Micro Lett. 2021, 13, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, S.; Yuan, Z.; Zhu, J.; Zhao, Z.; Niu, Z. Direct Self-Assembly of MXene on Zn Anodes for Dendrite-Free Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 2861–2865. [Google Scholar] [CrossRef]
- Abdulla, J.; Cao, J.; Zhang, D.; Zhang, X.; Sriprachuabwong, C.; Kheawhom, S.; Wangyao, P.; Qin, J. Elimination of Zinc Dendrites by Graphene Oxide Electrolyte Additive for Zinc-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4602–4609. [Google Scholar] [CrossRef]
- Jin, S.; Yin, J.; Gao, X.; Sharma, A.; Chen, P.; Hong, S.; Zhao, Q.; Zheng, J.; Deng, Y.; Joo, Y.L.; et al. Production of fast-charge Zn-based aqueous batteries via interfacial adsorption of ion-oligomer complexes. Nat. Commun. 2022, 13, 2283. [Google Scholar] [CrossRef]
- Han, D.; Wang, Z.; Lu, H.; Li, H.; Cui, C.; Zhang, Z.; Sun, R.; Geng, C.; Liang, Q.; Guo, X.; et al. A Self-Regulated Interface toward Highly Reversible Aqueous Zinc Batteries. Adv. Energy Mater. 2022, 12, 2102982. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Lu, Y.; Ni, Y.; Lin, L.; Hao, Z.; Yan, Z.; Zhao, Q.; Chen, J. Halogenated Zn(2+) Solvation Structure for Reversible Zn Metal Batteries. J. Am. Chem. Soc. 2022, 144, 18435–18443. [Google Scholar] [CrossRef]
- Luo, M.; Wang, C.; Lu, H.; Lu, Y.; Xu, B.B.; Sun, W.; Pan, H.; Yan, M.; Jiang, Y. Dendrite-free zinc anode enabled by zinc-chelating chemistry. Energy Storage Mater. 2021, 41, 515–521. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hao, J.; Luo, D.; Zhang, P.F.; Zhang, B.; Davey, K.; Lin, Z.; Qiao, S.Z. Dual-Function Electrolyte Additive for Highly Reversible Zn Anode. Adv. Energy Mater. 2021, 11, 2102010. [Google Scholar] [CrossRef]
- Qian, L.; Yao, W.; Yao, R.; Sui, Y.; Zhu, H.; Wang, F.; Zhao, J.; Zhi, C.; Yang, C. Cations Coordination-Regulated Reversibility Enhancement for Aqueous Zn-Ion Battery. Adv. Funct. Mater. 2021, 31, 2105736. [Google Scholar] [CrossRef]
- Meng, R.; Li, H.; Lu, Z.; Zhang, C.; Wang, Z.; Liu, Y.; Wang, W.; Ling, G.; Kang, F.; Yang, Q.H. Tuning Zn-Ion Solvation Chemistry with Chelating Ligands toward Stable Aqueous Zn Anodes. Adv. Mater. 2022, 34, e2200677. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Li, T.; Liu, X.; Yuan, Z.; Li, X. Functional complexed zincate ions enable dendrite-free long cycle alkaline zinc-based flow batteries. Nano Energy 2022, 102, 107697. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, P.; Qin, A.; Cui, G.; Mai, W. Metal-coordination chemistry guiding preferred crystallographic orientation for reversible zinc anode. Energy Storage Mater. 2022, 49, 463–470. [Google Scholar] [CrossRef]



| Type | Additive | Cycling Stability | Refs. |
|---|---|---|---|
| Cationic surfactants | 29 mg L−1 TBA2SO4 | 300 h (2.0 mA cm−2, 2.0 mA h cm−2) | [42] |
| 1 mol L−1 TMA | 2000 h (1.0 mA cm−2, 2.0 mA h cm−2) | [45] | |
| 10 mmol L−1 TEAB | 3000 h (1.0 mA cm−2, 2.0 mA h cm−2) | [46] | |
| 1 mol L−1 TS | 4500 h (0.25 mA cm−2, 0.25 mA h cm−2) | [47] | |
| 0.5 g L−1 TMBAC | 500 h (10 mA cm−2, 5.0 mA h cm−2) | [43] | |
| Anionic surfactants | 0.1 mol L−1 SDBS | 1500 h (0.5 mA cm−2, 0.5 mA h cm−2) | [50] |
| 10 mmol L−1 SPS | 4000 h (1.0 mA cm−2, 1.0 mA h cm−2) | [49] | |
| Amphoteric surfactants | 0.1 mol L−1 Arg | 2200 h (5.0 mA cm−2, 4.0 mA h cm−2) | [52] |
| 10 mmol L−1 Cys | 2300 h (5.0 mA cm−2, 5.0 mA h cm−2) | [53] | |
| 10 mmol L−1 TH | 580 h (1.0 mA cm−2, 1.0 mA h cm−2) | [55] | |
| Non-ionic surfactants | 0.5 wt% PEO | 3000 h (1.0 mA cm−2, 1.0 mA h cm−2) | [57] |
| 0.2 wt% PEO | 800 h (1.0 mA cm−2, 1.0 mA h cm−2) | [59] | |
| 1 wt% PAM | 1300 h (2.0 mA cm−2, 2.0 mA h cm−2) | [60] | |
| 1 wt% APA | 2500 h (5.0 mA cm−2, 5.0 mA h cm−2) | [56] |
| Type | Additive | Cycling Stability | Refs. |
|---|---|---|---|
| halogenated Zn salts | 1 mol L−1 ZnI2 | 5000 h (160 mA cm−2, 2.6 mA h cm−2) | [67] |
| ammonium | 1 mol L−1 NH4OAc | 3500 h (1.0 mA cm−2, 1.0 mA h cm−2) | [68] |
| 4 mol L−1 NH4 I | 200 h (1.0 mA cm−2, 1.0 mA h cm−2) | [69] | |
| Amine substituent | 0.1 mol L−1BIS-TRIS | 1000 h (1.0 mA cm−2, 1.0 mA h cm−2) | [70] |
| 75 mmol L−1 Na4EDTA | 2500 h (5.0 mA cm−2, 2.0 mA h cm−2) | [71] | |
| 0.5 mol L−1 TEHC | 1100 h (1.0 mA cm−2, 1.0 mA h cm−2) | [72] | |
| 0.1 mol L−1 EDTA | 3000 h (5.0 mA cm−2, 1.0 mA h cm−2) | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Q.; Wang, Y.; Han, G.; Li, X.; Yuan, T.; Sun, H.; Gong, Y.; Chen, T. Recent Progress in Electrolyte Additives for Highly Reversible Zinc Anodes in Aqueous Zinc Batteries. Batteries 2023, 9, 284. https://doi.org/10.3390/batteries9050284
Shen Q, Wang Y, Han G, Li X, Yuan T, Sun H, Gong Y, Chen T. Recent Progress in Electrolyte Additives for Highly Reversible Zinc Anodes in Aqueous Zinc Batteries. Batteries. 2023; 9(5):284. https://doi.org/10.3390/batteries9050284
Chicago/Turabian StyleShen, Qibin, Yuanduo Wang, Guanjie Han, Xin Li, Tao Yuan, Hao Sun, Yinyan Gong, and Taiqiang Chen. 2023. "Recent Progress in Electrolyte Additives for Highly Reversible Zinc Anodes in Aqueous Zinc Batteries" Batteries 9, no. 5: 284. https://doi.org/10.3390/batteries9050284




