How Cell Design Affects the Aging Behavior: Comparing Electrode-Individual Aging Processes of High-Energy and High-Power Lithium-Ion Batteries Using High Precision Coulometry
Abstract
:1. Introduction
1.1. Literature Review
1.2. Present Work
2. Materials and Methodology
2.1. Materials
2.2. Methods: Experiment Design
2.2.1. Cyclic Aging: Lithium Plating
2.2.2. Calendar Aging
3. Results and Discussion
3.1. Cyclic Aging: Lithium Plating
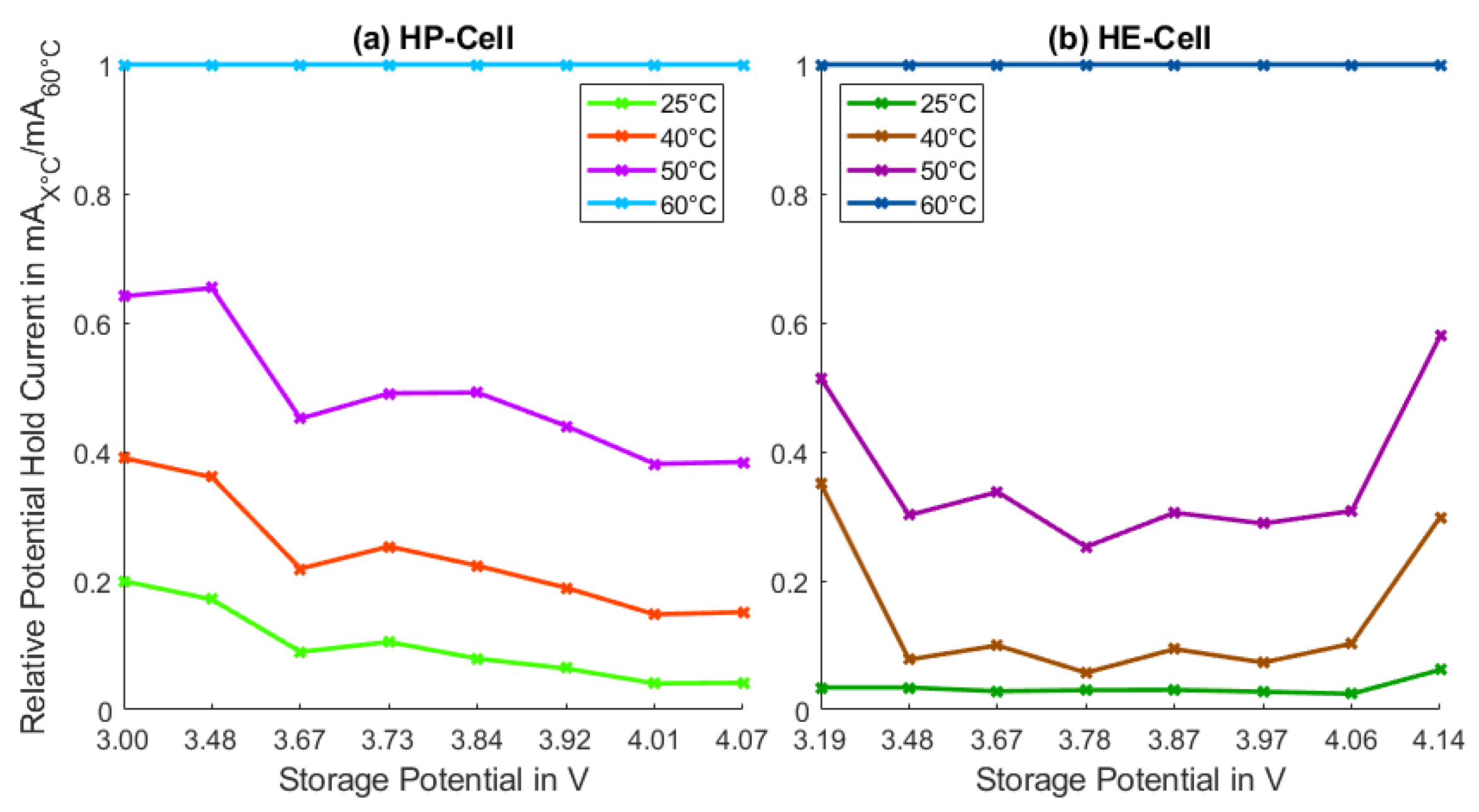
3.2. Calendar Aging
3.3. Outlook: Parameterization of Battery Aging Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DVA | Differential Voltage Analysis |
| HE cell | High-energy cell |
| HP cell | High-power cell |
| Li | Lithium |
| OCV | Open Circuit Voltage |
| SEI | Solid Electrolyte Interphase |
| SoC | State of Charge |
References
- Lain, M.J.; Brandon, J.; Kendrick, E. Design Strategies for High Power vs. High Energy Lithium Ion Cells. Batteries 2019, 5, 64. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, J.; Pan, Z.; Ouyang, M. Impedance characterization of lithium-ion batteries aging under high-temperature cycling: Importance of electrolyte-phase diffusion. J. Power Sources 2019, 426, 216–222. [Google Scholar] [CrossRef]
- Ecker, M. Lithium Plating in Lithium-Ion Batteries—An Experimental and Simulation Approach. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2016. [Google Scholar]
- Mei, W.; Jiang, L.; Liang, C.; Sun, J.; Wang, Q. Understanding of Li-plating on graphite electrode: Detection, quantification and mechanism revelation. Energy Storage Mater. 2021, 41, 209–221. [Google Scholar] [CrossRef]
- Linsenmann, F. Operando and In Situ Characterizations of Fundamental Electrochemical Processes in Lithium-and Sodium-Ion Batteries. Ph.D. Thesis, Technical University of Munich, Munich, Germany, 2021. [Google Scholar]
- Legrand, N.; Knosp, B.; Desprez, P.; Lapicque, F.; Raël, S. Physical characterization of the charging process of a Li-ion battery and prediction of Li plating by electrochemical modelling. J. Power Sources 2014, 245, 208–216. [Google Scholar] [CrossRef]
- Li, S.R.; Chen, C.H.; Camardese, J.; Dahn, J.R. High Precision Coulometry Study of LiNi0.5 Mn1.5 O4/Li Coin Cells. J. Electrochem. Soc. 2013, 160, A1517–A1523. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Bloom, I.; Cole, B.; Sohn, J.; Jones, S.; Polzin, E.; Battaglia, V.; Henriksen, G.; Motloch, C.; Richardson, R.; Unkelhaeuser, T.; et al. An accelerated calendar and cycle life study of Li-ion cells. J. Power Sources 2001, 101, 238–247. [Google Scholar] [CrossRef]
- Smith, A.J.; Burns, J.C.; Xiong, D.; Dahn, J.R. Interpreting High Precision Coulometry Results on Li-ion Cells. J. Electrochem. Soc. 2011, 158, A1136–A1142. [Google Scholar] [CrossRef]
- Smith, A.J.; Burns, J.C.; Dahn, J.R. A High Precision Study of the Coulombic Efficiency of Li-Ion Batteries. Electrochem.-Solid-State Lett. 2010, 13, A177. [Google Scholar] [CrossRef]
- Fathi, R.; Burns, J.C.; Stevens, D.A.; Ye, H.; Hu, C.; Jain, G.; Scott, E.; Schmidt, C.; Dahn, J.R. Ultra High-Precision Studies of Degradation Mechanisms in Aged LiCoO2/Graphite Li-Ion Cells. J. Electrochem. Soc. 2014, 161, A1572–A1579. [Google Scholar] [CrossRef]
- Burns, J.C.; Stevens, D.A.; Dahn, J.R. In-Situ Detection of Lithium Plating Using High Precision Coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Calendar Aging of NCA Lithium-Ion Batteries Investigated by Differential Voltage Analysis and Coulomb Tracking. J. Electrochem. Soc. 2017, 164, A6066–A6074. [Google Scholar] [CrossRef]
- Keil, P. Aging of Lithium-Ion Batteries in Electric Vehicles. Ph.D. Thesis, Technical University of Munich, Munich, Germany, 2017. [Google Scholar]
- Lygte. Battery Test-Review 18650 Comparator. 2022. Available online: https://lygte-info.dk/review/batteries2012/Common18650comparator.php (accessed on 11 April 2022).
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Zilberman, I.; Sturm, J.; Jossen, A. Reversible self-discharge and calendar aging of 18650 nickel-rich, silicon-graphite lithium-ion cells. J. Power Sources 2019, 425, 217–226. [Google Scholar] [CrossRef]
- Xie, Q.; Li, W.; Dolocan, A.; Manthiram, A. Insights into Boron-Based Polyanion-Tuned High-Nickel Cathodes for High-Energy-Density Lithium-Ion Batteries. Chem. Mater. 2019, 31, 8886–8897. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Battery Dynamics GmbH. High Resolution Battery Testers—HRT-Series M; Battery Dynamics GmbH: Munich, Germany, 2020. [Google Scholar]
- Petzl, M.; Danzer, M.A. Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries. J. Power Sources 2014, 254, 80–87. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Lewerenz, M.; Fuchs, G.; Becker, L.; Sauer, D.U. Irreversible calendar aging and quantification of the reversible capacity loss caused by anode overhang. J. Energy Storage 2018, 18, 149–159. [Google Scholar] [CrossRef]
- Streck, L.; Roth, T.; Keil, P.; Strehle, B.; Ludmann, S.; Jossen, A. A Comparison of Voltage Hold and Voltage Decay Methods for Side Reactions Characterization. J. Electrochem. Soc. 2023. submitted. [Google Scholar] [CrossRef]
- Korth, W.; Jess, A.; Kern, C. Bestimmung der elektrischen Leitfähigkeit und der Viskosität von ionischen Flüssigkeiten. Chem. Ing. Tech. 2005, 77, 985–986. [Google Scholar] [CrossRef]
- Wilhelm, J.; Seidlmayer, S.; Keil, P.; Schuster, J.; Kriele, A.; Gilles, R.; Jossen, A. Cycling capacity recovery effect: A coulombic efficiency and post-mortem study. J. Power Sources 2017, 365, 327–338. [Google Scholar] [CrossRef]
- Keil, P.; Schuster, S.F.; Wilhelm, J.; Travi, J.; Hauser, A.; Karl, R.C.; Jossen, A. Calendar Aging of Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1872–A1880. [Google Scholar] [CrossRef]
- Pieczonka, N.P.W.; Liu, Z.; Lu, P.; Olson, K.L.; Moote, J.; Powell, B.R.; Kim, J.H. Understanding Transition-Metal Dissolution Behavior in LiNi0.5Mn1.5O4 High-Voltage Spinel for Lithium Ion Batteries. J. Phys. Chem. C 2013, 117, 15947–15957. [Google Scholar] [CrossRef]
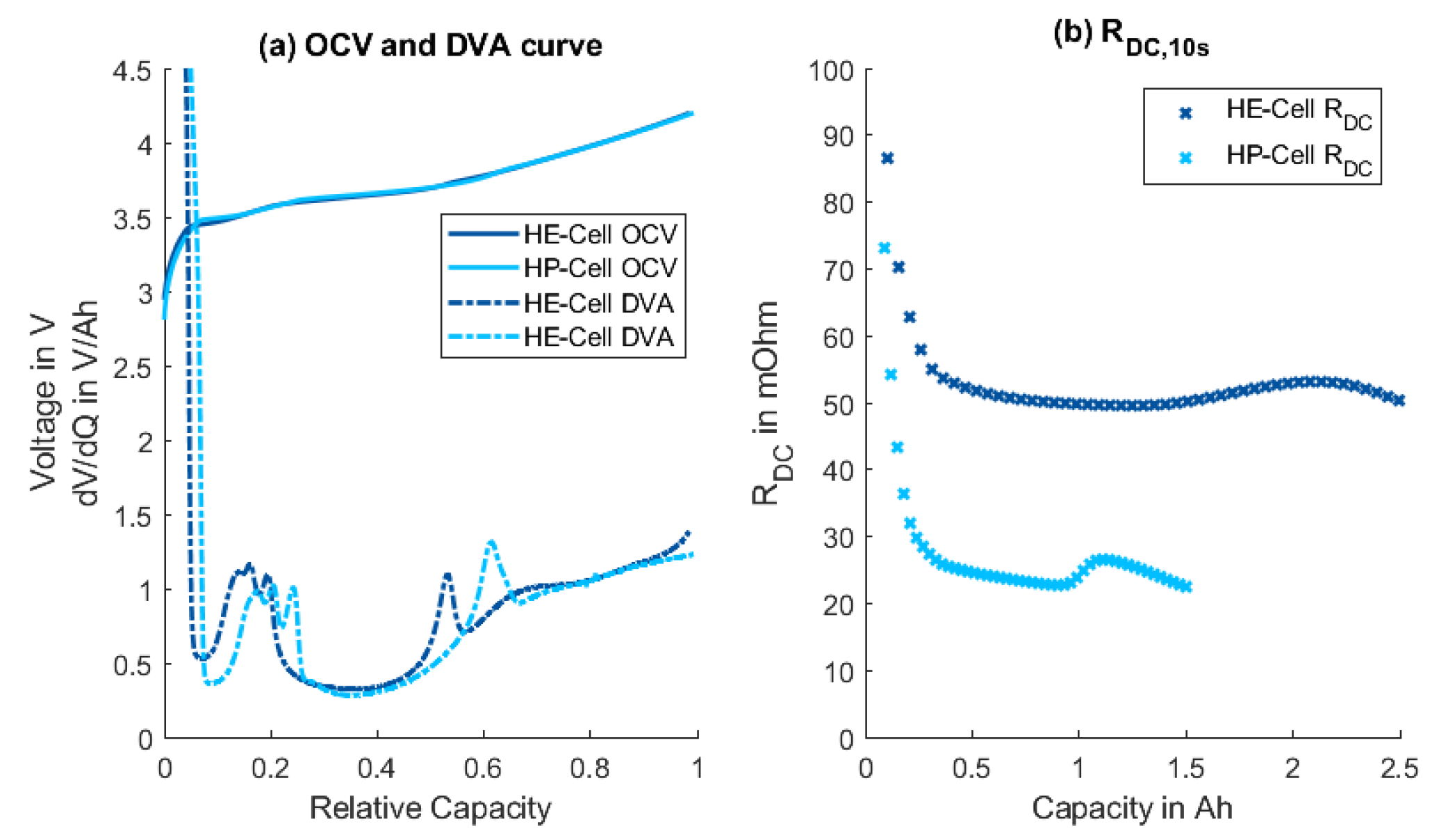
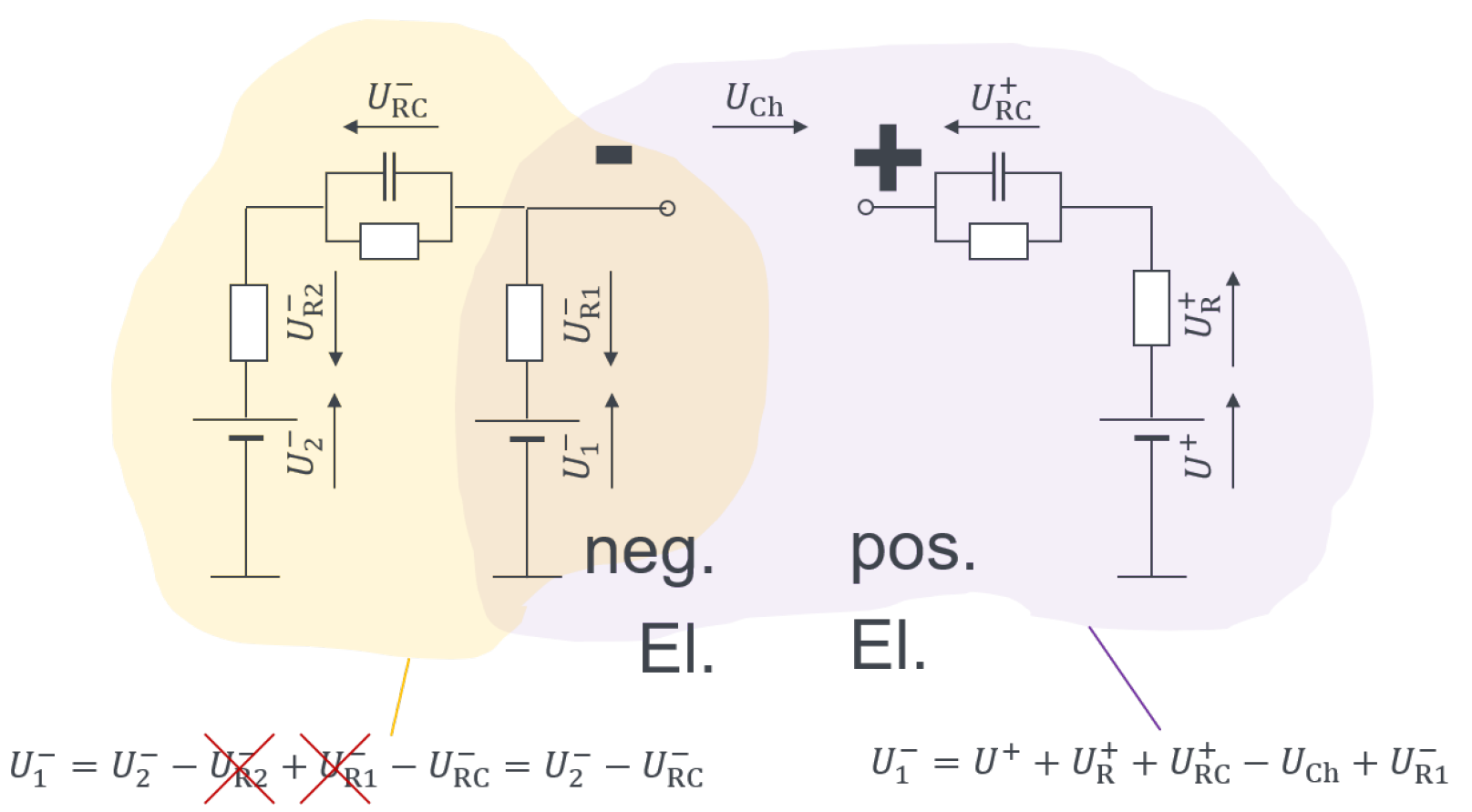

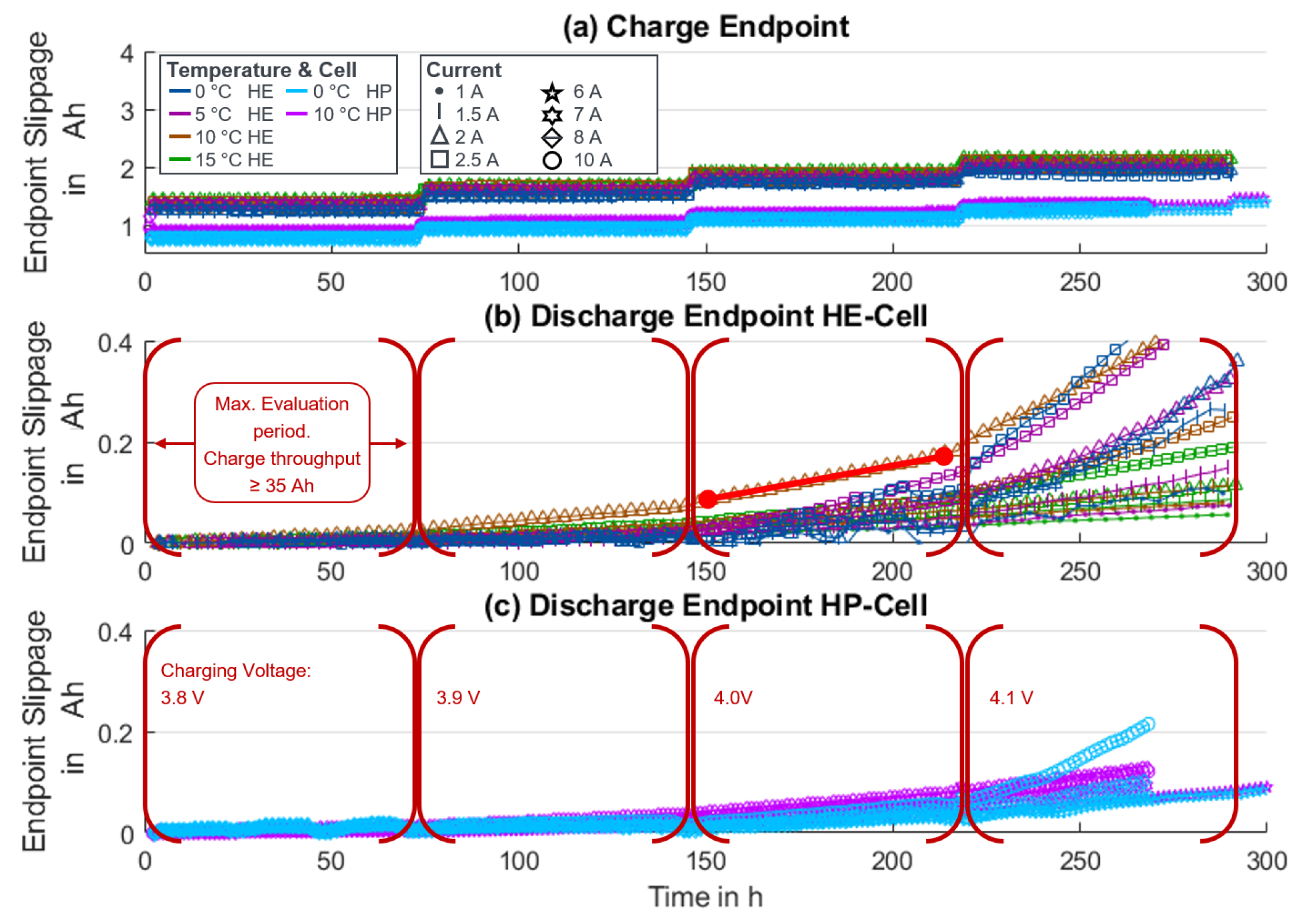

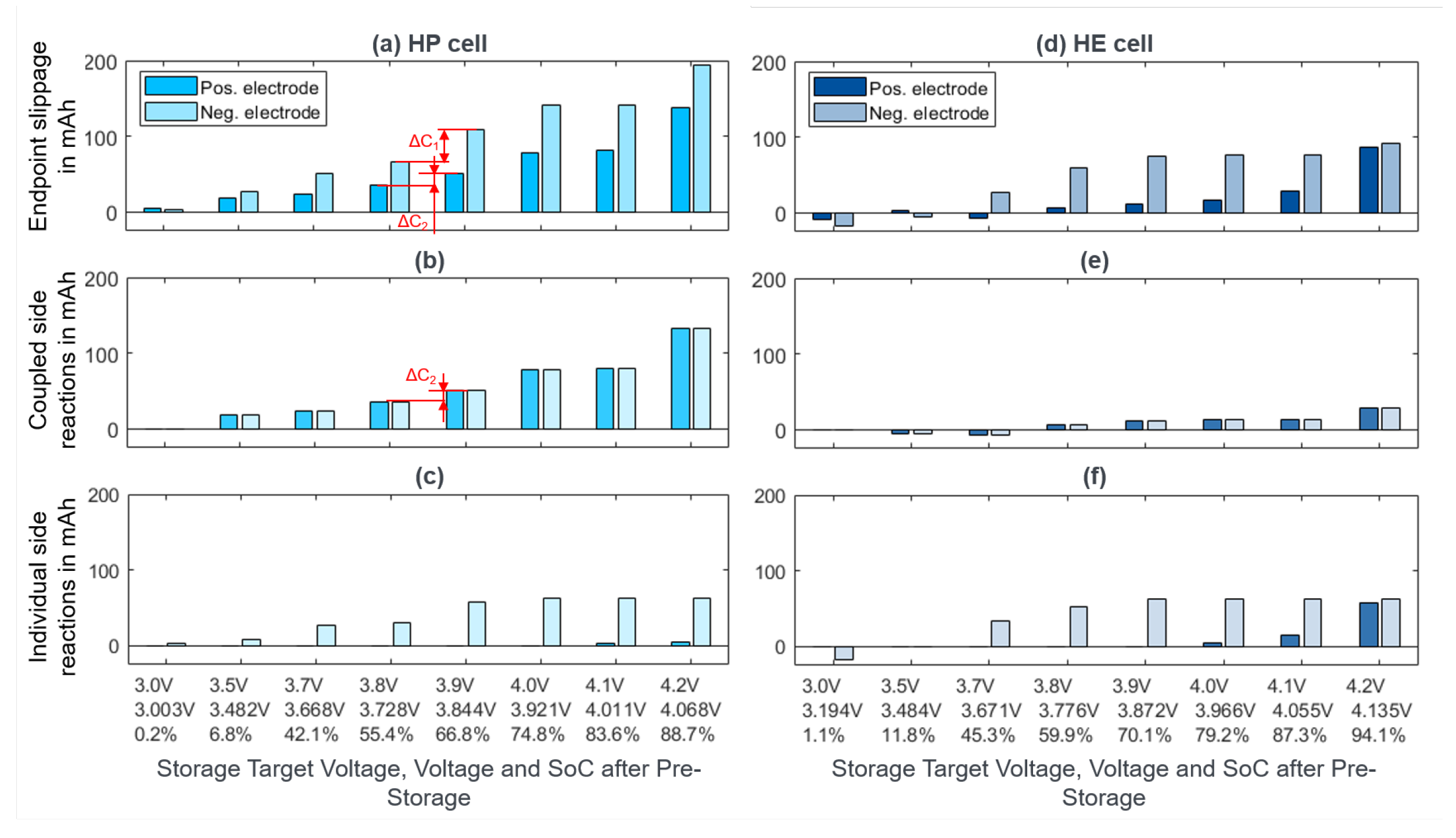
| Cell | Species | Capacity | Internal Resistance | Max. Discharge Current | Max. Charge Current | Year |
|---|---|---|---|---|---|---|
| LG INR 18650 M26 | HE | 2600 mAh | <60 m | 10 A | 2.5 A | 2013 |
| LG INR 18650 HB6 | HP | 1500 mAh | <20 m | 30 A | 4 A | 2015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagfeld, S.M.P.; Birke, K.P.; Fill, A.; Keil, P. How Cell Design Affects the Aging Behavior: Comparing Electrode-Individual Aging Processes of High-Energy and High-Power Lithium-Ion Batteries Using High Precision Coulometry. Batteries 2023, 9, 232. https://doi.org/10.3390/batteries9040232
Jagfeld SMP, Birke KP, Fill A, Keil P. How Cell Design Affects the Aging Behavior: Comparing Electrode-Individual Aging Processes of High-Energy and High-Power Lithium-Ion Batteries Using High Precision Coulometry. Batteries. 2023; 9(4):232. https://doi.org/10.3390/batteries9040232
Chicago/Turabian StyleJagfeld, Sebastian Michael Peter, Kai Peter Birke, Alexander Fill, and Peter Keil. 2023. "How Cell Design Affects the Aging Behavior: Comparing Electrode-Individual Aging Processes of High-Energy and High-Power Lithium-Ion Batteries Using High Precision Coulometry" Batteries 9, no. 4: 232. https://doi.org/10.3390/batteries9040232







