Synthesis and Performance of NaTi2(PO4)3/VGCF@C Anode Composite Material for Aqueous Sodium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Sample Preparation
2.2. Battery Assembly
2.3. Microstructure and Electrochemical Properties Analysis
3. Results and Discussion
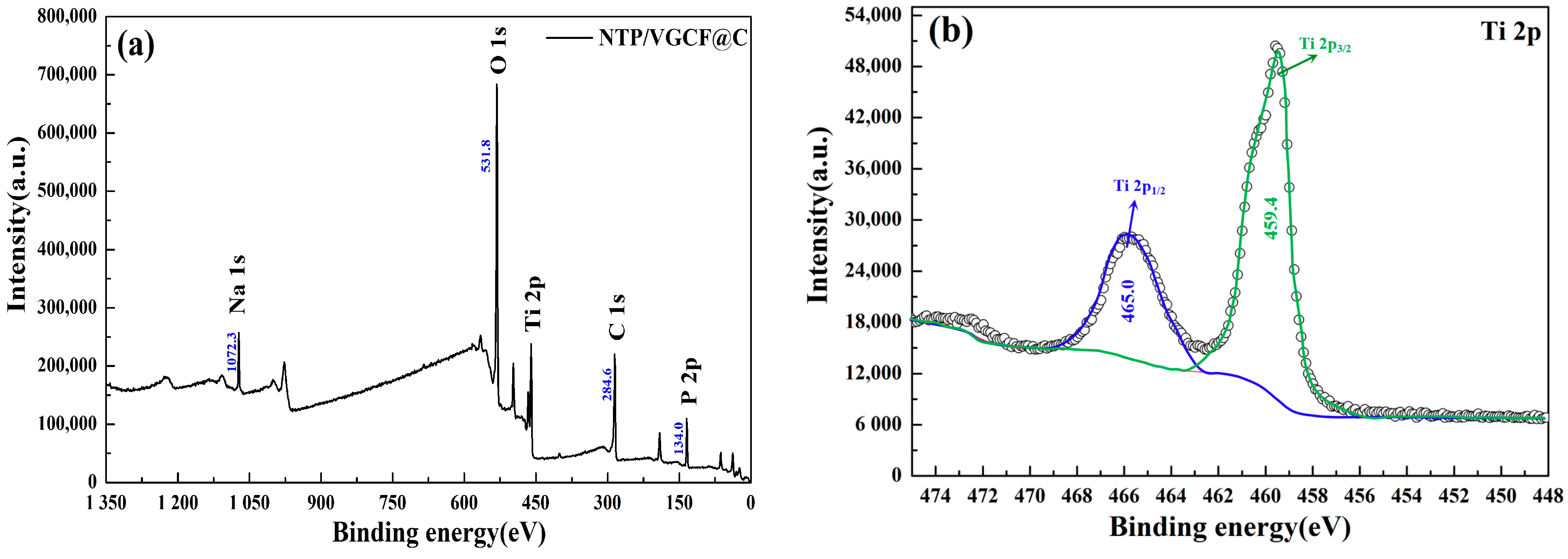
3.1. Phase and Element Analysis
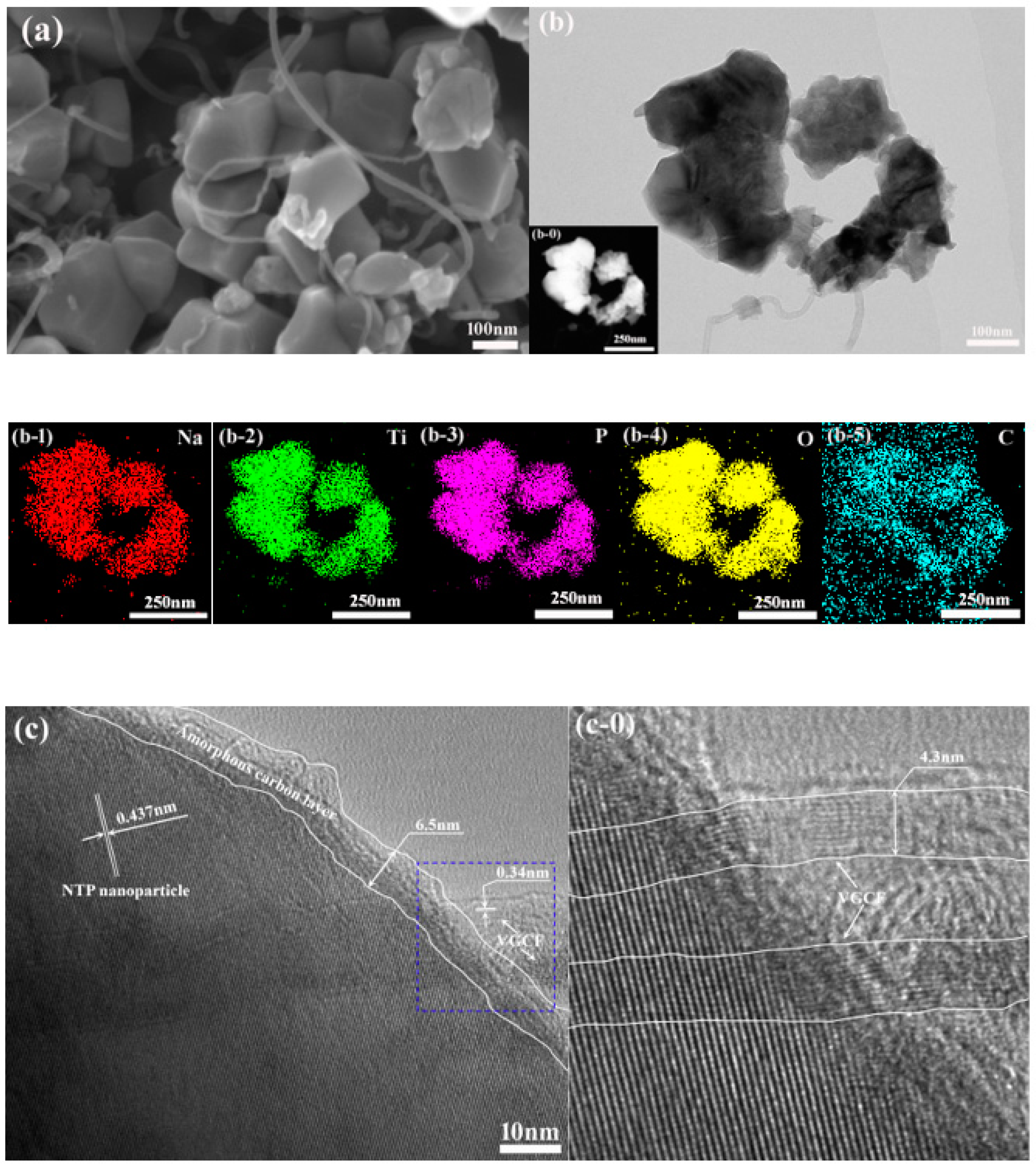
3.2. Microstructure Analysis
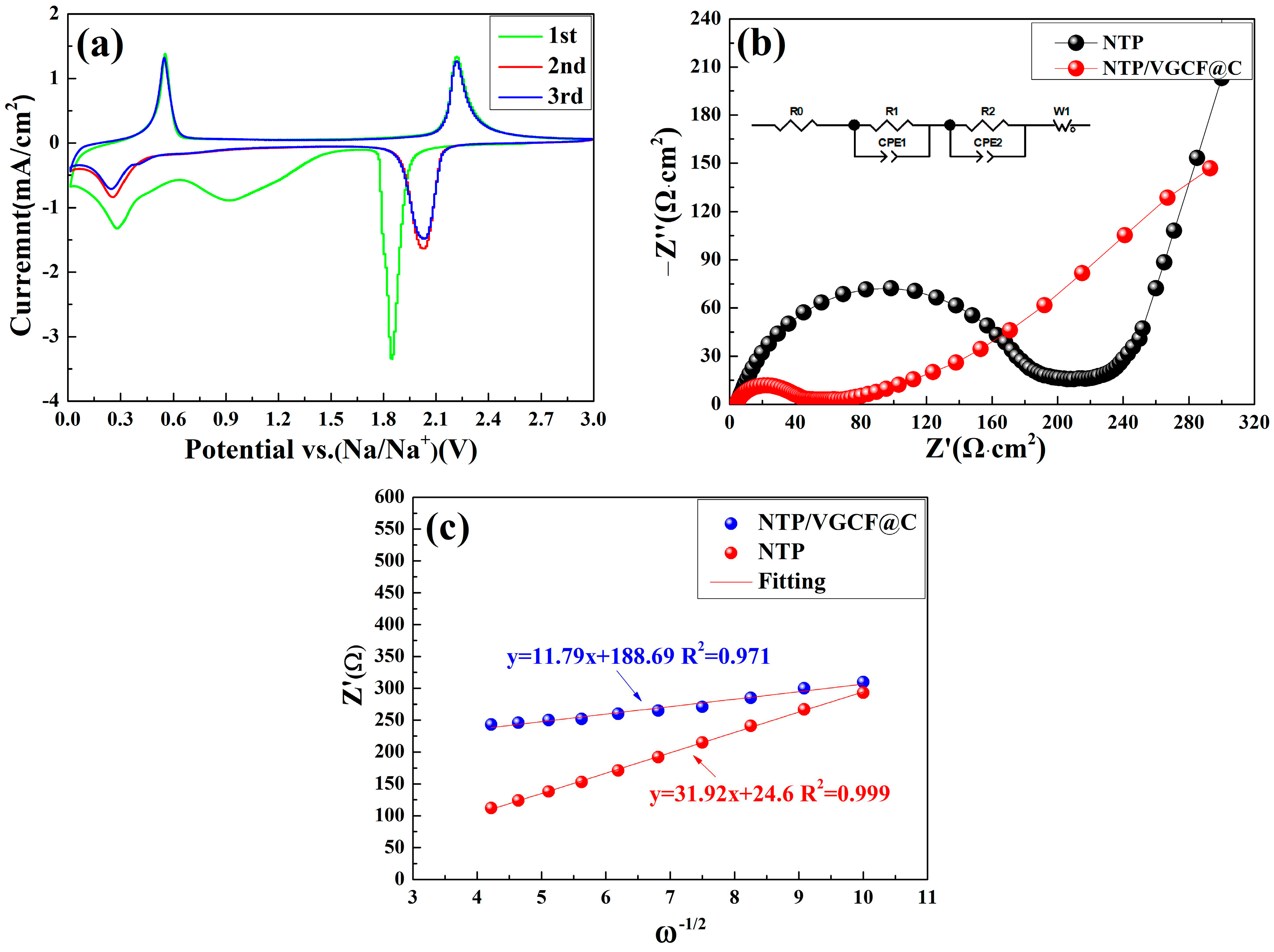
3.3. Cyclic Voltammetry and Electrochemical Impedance Spectroscopy Analysis
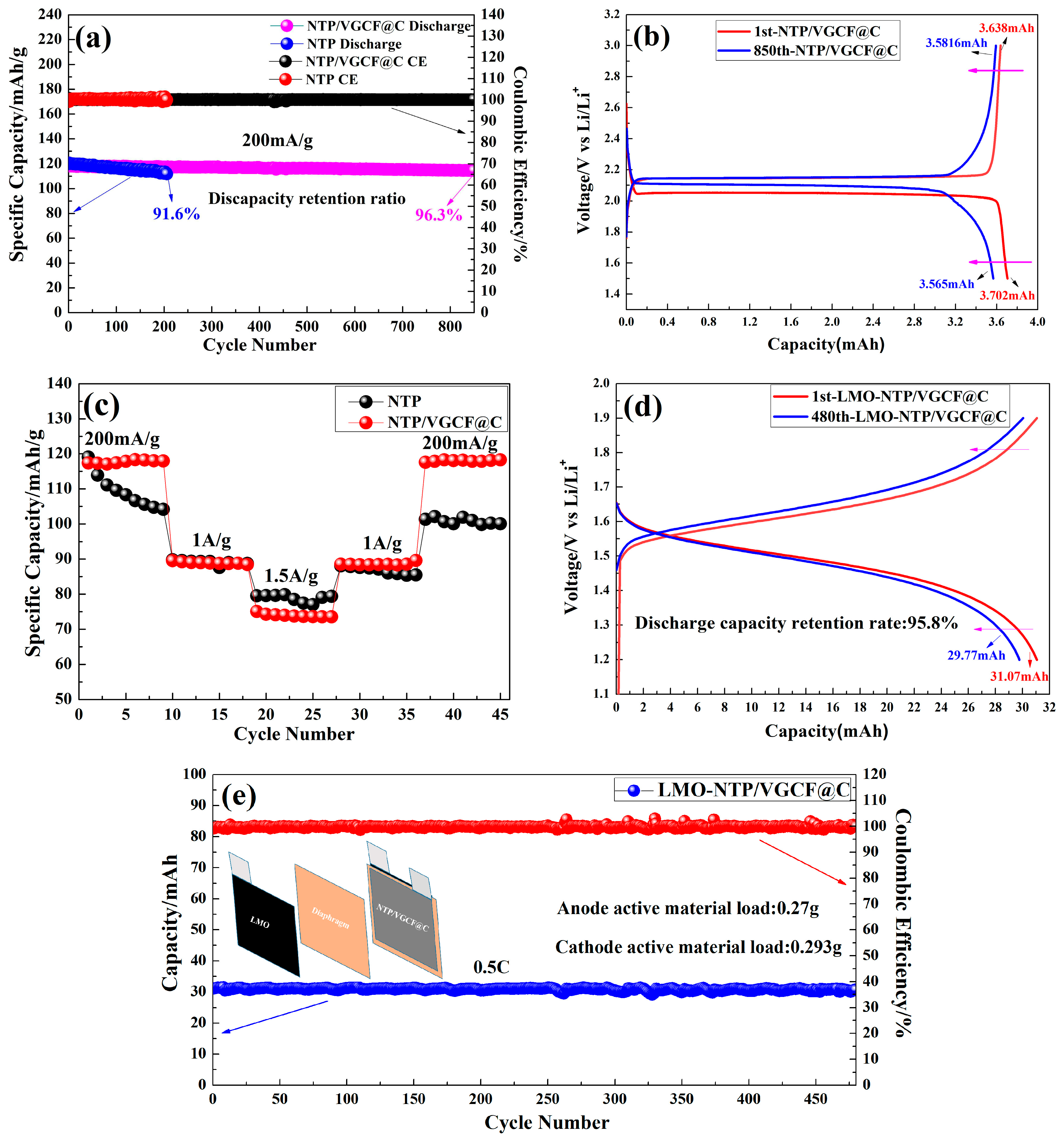
3.4. Cycling Performance
4. Conclusions
- (1)
- The synthesized anode composite material is composed of NTP nanoparticles, VGCF, and an amorphous carbon coating layer. The VGCF nanofiber has a good interfacial contact with the NTP particles in the NTP/VGCF@C composite. The combination of VGCF with carbon coating makes the composite material more robust and conductive than pure NTP material.
- (2)
- After 200 cycles of charge-discharge, the reversible capacity retention rates of NTP and NTP/VGCF@C are 91.6% and 99.2% at a current of 200 mA/g, respectively; the addition of carbon material increases the capacity retention by 7.6%, and after 850 cycles of charge-discharge, the capacity retention rate of NTP/VGCF@C is 96.3%, which is much better than that of pure NTP.
- (3)
- The initial capacity of an LMO-NTP/VGCF@C aqueous sodium-ion full battery is 31.07 mAh at a rate of 0.5C. The reversible discharge capacity is 29.77 mAh after 480 cycles, and the discharge capacity retention rate is 95.8%. According to the results of this study, the LMO-NTP/VGCF@C aqueous sodium-ion full battery can be applied for large-scale green and safe energy storage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Q.; Zheng, F.; Zhong, W.; Pan, Q.; Liu, M. P3-type K0.5Mn0.72Ni0.15Co0.13O2 Microspheres as Cathode Materials for High Performance Potassium-Ion Batteries. Chem. Eng. J. 2020, 392, 123735. [Google Scholar] [CrossRef]
- Pan, Z.H.; Yang, J.; Yang, J.; Zhang, Q.H.; Zhang, H.; Li, X.; Kou, Z.K.; Zhang, Y.F.; Chen, H.; Yan, C.L.; et al. Stitching of Zn3(OH)2V2O7•2H2O 2D nanosheets by 1D carbon nanotubes boosts ultrahigh rate for wearable quasi-solid-state zinc-ion batteries. ACS Nano 2020, 14, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, Y.; Wu, F.; Shen, L.F.; Wang, Y.; Wu, F.X.; Moudrakovski, I.; van Aken, P.A.; Maier, J.; Yu, Y. Hierarchical metal sulfide/carbon spheres: A generalized synthesis and high sodium-storage performance. Angew. Chem. Int. Ed. 2019, 58, 7238–7243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Z.; Du, Y.; Wang, Q.C.; Shen, J. A Yolk Shell Structured FePO4 Cathode for High-Rate and Long-Cycling Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 817, 17504–17510. [Google Scholar]
- Li, W.; Han, C.; Wang, W.; Xia, Q.; Chou, S.; Gu, Q.; Johannessen, B.; Liu, H.K.; Dou, S. Stress distortion restraint to boost the sodium-ion storage performance of a novel binary hexacyanoferrate. Adv. Energy Mater. 2019, 10, 1903006. [Google Scholar] [CrossRef]
- Weng, W.; Xu, J.; Lai, C.; Xu, Z.; Zhou, X. Uniform Yolk-Shell Fe7S8@C Nanoboxes as a General Host Material for the Efficient Storage of Alkali Metal Ions. J. Alloys Compd. 2019, 817, 152732. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Guo, J.Z.; Zhao, X.X.; Wang, X.T.; Xie, D.; Sun, Z.H.; Zhao, C.D.; Liang, H.J.; Li, W.H.; Wu, X.L. High-Ionicity Fuorophosphate Lattice via Aliovalent Substitution as Advanced Cathode Materials in Sodium-Ion Batteries. InfoMat 2021, 3, 694–704. [Google Scholar] [CrossRef]
- Deng, J.; Luo, W.B.; Chou, S.L.; Deng, J.Q.; Luo, W.B.; Chou, S.L.; Liu, H.K.; Dou, S.X. Sodium-ion batteries: From academic research to practical commercialization. Adv. Energy Mater. 2018, 8, 1701428. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Zheng, Y.H.; Li, X.; Adams, F.; Luo, W.; Huang, Y.H.; Hu, L.B. Electrode materials of sodium-ion batteries toward practical application. ACS Energy Lett. 2018, 3, 1604–1612. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. Engl. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Deng, Q.; Zheng, F.H.; Zhong, W.T. Nanoscale Surface Modification of P2-Type Na0.65[Mn0.70Ni0.16Co0.14]O2 Cathode Material for High-Performance Sodium-Ion Batteries. Chem. Eng. J. 2020, 404, 126446. [Google Scholar] [CrossRef]
- Fang, G.Z.; Zhou, J.; Pan, A.Q.; Liang, S.Q. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Zheng, G.Y.; Lin, Q.W.; Ma, J.B.; Zhang, J.; He, Y.B.; Tang, X.; Kang, F.Y.; Lv, W.; Yang, Q.H. Ultrafast Presodiation of Graphene Anodes for High-Efficiency and High-Rate Sodium-Ion Storage. InfoMat 2021, 3, 1445–1454. [Google Scholar] [CrossRef]
- Yan, Z.H.; Yang, Q.W.; Wang, Q.H.; Ma, J.M. Nitrogen Doped Porous Carbon as Excellent Dual Anodes for Li- and Na-Ion batteries. Chin. Chem. Lett. 2020, 31, 583–588. [Google Scholar] [CrossRef]
- Park, C.M.; Sohn, H.J. Black phosphorus and its composite for lithium rechargeable batteries. Chem. Commun. 2014, 50, 5435–5437. [Google Scholar]
- Lian, S.T.; Lv, J.S.; Yu, Q.; Hu, G.W.; Chen, Z.; Zhou, L.; Mai, L.Q. Recent progress on TiO2-Based anode materials for sodium-ion batteries. J. Electrochem. 2019, 25, 31–44. [Google Scholar]
- Massaro, A.; Muñoz-García, A.B.; Maddalena, P.; Bella, F.; Meligrana, G.; Gerbaldi, C.; Pavone, M. First-principles study of Na insertion at TiO2 anatase surfaces: New hints for Na-ion battery design. Nanoscale Adv. 2020, 2, 2745–2751. [Google Scholar] [CrossRef]
- Longoni, G.; Cabrera, R.L.P.; Polizzi, S.; D’Arienzo, M.; Mari, C.M.; Cui, Y.; Ruffo, R. Shape controlled TiO2 nanocrystals for Na-ion battery electrodes: The role of different exposed crystal facets on the electrochemical properties. Nano Lett. 2017, 17, 992–1000. [Google Scholar] [CrossRef]
- Bella, F.; Muñoz-García, A.B.; Meligrana, G.; Lamberti, A.; Destro, M.; Pavone, M.; Gerbaldi, C. Unveiling the controversial mechanism of reversible Na storage in TiO2 nanotube arrays: Amorphous versus anatase TiO2. Nano Res. 2017, 10, 2891–2903. [Google Scholar] [CrossRef]
- He, B.; Yin, K.B.; Gong, W.B.; Xiong, Y.W.; Zhang, Q.C.; Yang, J.; Wang, Z.X.; Wang, Z.; Chen, M.X.; Man, P.; et al. NaTi2(PO4)3 hollow nanoparticles encapsulated in carbon nanofibers as novel anodes for flexible aqueous rechargeable sodium-ion batteries. Nano Energy 2021, 82, 105764. [Google Scholar] [CrossRef]
- Ren, W.; Chen, X.; Zhao, C. Ultrafast aqueous potassium-ion batteries cathode for stable intermittent grid-scale energy storage. Adv. Energy Mater. 2018, 8, 1801413. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.J.; Chang, D.; Kim, J.; Moon, S.; Oh, K.; Park, K.Y.; Seong, W.M.; Park, H.; Kwon, G.; et al. Toward a low-cost high-voltage sodium aqueous rechargeable battery. Mater. Today 2019, 29, 26–36. [Google Scholar] [CrossRef]
- Fang, Y.J.; Xiao, L.F.; Qian, J.F.; Cao, Y.L.; Ai, X.P.; Huang, Y.H.; Yang, H.X. 3D graphene decorated NaTi2(PO4)3 microspheres as a superior high-rate and ultracycle-stable anode material for sodium ion batteries. Adv. Energy Mater. 2016, 6, 1502197. [Google Scholar] [CrossRef]
- Wu, M.G.; Ni, W.; Hu, J.; Ma, J.M. NASICON-structured NaTi2(PO4)3 for sustainable energy storage. Nano-Micro Lett. 2019, 11, 44. [Google Scholar] [CrossRef]
- Fu, L.; Xue, X.; Tang, Y.G.; Sun, D.; Xie, H.L.; Wang, H.Y. Size controlling and surface engineering enable NaTi2(PO4)3/C outstanding sodium storage properties. Electrochim. Acta 2018, 289, 21–28. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Fang, Y.B.; Daum, J.; Kanakaraj, S.N.; Zhang, G.Q.; Mishra, S.; Gbordzoe, S.; Shanov, V. Bio-inspired, nitrogen doped CNT-graphene hybrid with amphiphilic properties as a porous current collector for lithium-ion batteries. Carbon 2019, 145, 677–689. [Google Scholar] [CrossRef]
- Bakierska, M.; Lis, M.; Pacek, J.; Świętosławski, M.; Gajewska, M.; Tąta, A.; Proniewicz, E.; Molenda, M. Bio-derived carbon nanostructures for high-performance lithium-ion batteries. Carbon 2019, 145, 426–432. [Google Scholar] [CrossRef]
- Ji, L.W.; Meduri, P.; Agubra, V.; Xiao, X.C.; Alcoutlabi, M. Graphene-based nanocomposites for energy storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef]
- Massaro, A.; Pecoraro, A.; Muñoz-García, A.B.; Pavone, M. First-Principles study of Na intercalation and diffusion mechanisms at 2D MoS2/graphene interfaces. J. Phys. Chem. C 2021, 125, 2276–2286. [Google Scholar] [CrossRef]
- Wu, X.W.; Li, Y.H.; Zhao, S.Y.; Zeng, F.H.; Peng, X.C.; Xiang, Y.H.; Ruan, Q.Y.; Gao, F.; He, T.; Wu, J.H. Fabrication of F-doped, C-coated NiCo2O4 nanocomposites and its electrochemical performances for lithium-ion batteries. Solid State Ion. 2019, 334, 48–55. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Zhu, J.; Wang, H.Y.; Li, Y.H.; Zhou, H.Z.; Meng, W.; Dai, L.; Wang, L.; He, Z.X. N-doped carbon coated LiTi2(PO4)3 as superior anode using PANi as carbon and nitrogen bi-sources for aqueous lithium ion battery. Electrochim. Acta 2018, 279, 279–288. [Google Scholar]
- Li, L.L.; Wen, Y.H.; Zhang, H.; Ming, H.; Pang, J.; Zhao, P.C.; Cao, G.P. Core-Shell structured LiTi2(PO4)3/C anode for aqueous lithium-ion batteries. ChemElectroChem 2019, 6, 1908–1914. [Google Scholar] [CrossRef]
- Wang, D.X.; Liu, Q.; Chen, C.J.; Li, M.L.; Meng, X.; Bie, X.F.; Wei, Y.J.; Huang, Y.H.; Du, F.; Wang, C.Z.; et al. NASICON-structured NaTi2(PO4)3@C nanocomposite as the low operation-voltage anode material for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 2238–2246. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, Q.; Liu, X.Z. 3D porous Fluorine-Doped NaTi2(PO4)3@C as High-Performance Sodium-Ion battery anode with broad temperature adaptability. Chem. Eng. J. 2022, 430, 132710. [Google Scholar] [CrossRef]
- Pang, G.; Yuan, C.Z.; Nie, P.; Ding, B.; Zhu, J.J.; Zhang, X.G. Synthesis of NASICON-type NaTi2(PO4)3-graphene nanocomposite as an anode for aqueous rechargeable Na-ion batteries. Nanoscale 2014, 6, 6328–6334. [Google Scholar] [CrossRef]
- Wise, A.; Wu, W.; Yan, J. Using intimate carbon to enhance the performance of NaTi2(PO4)3 anode materials: Carbon nanotubes vs. graphite. J. Electrochem. Soc. 2014, 161, A561–A567. [Google Scholar]
- Jiang, Y.; Zeng, L.C.; Wang, J.Q.; Li, W.H.; Pan, F.S.; Yu, Y. A carbon coated NASICON structure material embedded in porous carbon enabling superior sodium storage performance: NaTi2(PO4)3 as an example. Nanoscale 2015, 7, 14723–14729. [Google Scholar] [CrossRef]
- Jung, Y.H.; Hong, S.T.; Kim, D.K. Electrochemical sodium-ion intercalation properties of Na2.7 Ru4O9 in nonaqueous and aqueous electrolytes. J. Electrochem. Soc. 2013, 160, A897–A900. [Google Scholar] [CrossRef]
- Beck, F.; Rüetschi, P. Rechargeable batteries with aqueous electrolytes. Electrochim. Acta 2000, 45, 2467–2482. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, C.Y.; Zhai, T.Y.; Li, H.Q. A high rate 1.2 V aqueous sodium-ion battery based on all NASICON structured NaTi2(PO4)3 and Na3V2(PO4)3. Electrochim. Acta 2016, 196, 470–478. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhang, T.R.; Chen, C.; Ling, M.; Lin, Z.; Zhang, S.Q.; Pan, F.; Liang, C.D. High-performance aqueous symmetric sodium-ion battery using NASICON-structured Na2VTi(PO4)3. Nano Res. 2018, 11, 490–498. [Google Scholar] [CrossRef]
- Shanbhag, S.; Bootwala, Y.; Whitacre, J.F.; Mauter, M.S. Ion transport and competition effects on NaTi2(PO4)3 and Na4Mn9O18 selective insertion electrode performance. Langmuir ACS J. Surf. Colloids 2017, 33, 12580–12591. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, F.; Tamirat, A.G.; Suo, L.M.; Wang, Y.G.; Wang, C.S.; Xia, Y.Y. Progress in aqueous rechargeable sodium-Ion batteries. Adv. Energy Mater. 2018, 8, 1703008. [Google Scholar] [CrossRef]
- Wu, X.Y.; Sun, M.Y.; Shen, Y.F.; Qian, J.F.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Energetic aqueous rechargeable sodium-ion battery based on Na2CuFe(CN)6-NaTi2(PO4)3 intercalation chemistry. ChemSusChem 2014, 7, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, J.F.; Shanbhag, S.; Mohamed, A.; Polonsky, A.; Carlisle, K.; Gulakowski, J.; Wu, W.; Smith, C.; Cooney, L.; Blackwood, D.; et al. A polyionic, large-format energy storage sevice using an aqueous electrolyte and thick-format composite NaTi2(PO4)3/activated carbon negative electrodes. Energy Technol. 2015, 3, 20–31. [Google Scholar] [CrossRef]
- Li, Z.; Young, D.; Xiang, K.; Carter, W.C.; Chiang, Y.M. Towards high power high energy aqueous sodium-ion batteries: The NaTi2(PO4)3/Na0.44MnO2 system. Adv. Energy Mater. 2013, 3, 290–294. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Z.N.; Wang, B.; Liu, G.J.; Cheng, M.; Yuan, Y.F.; Luo, H.; Gao, T.T.; Wang, D.L.; Yassar, R.S. Purifying the phase of NaTi2(PO4)3 for enhanced Na+ storage properties. ACS Appl. Mater. Interfaces 2019, 11, 10663–10671. [Google Scholar] [CrossRef]
- Vincent, M.; Avvaru, V.S.; Haranczyk, M.; Etacheri, V. High-performance Mg-Li hybrid batteries based on pseudocapacitive anatase Ti1-xCoxO2-y nanosheet cathodes. ChemSusChem 2022, 15, e202102562. [Google Scholar] [CrossRef]
- Vincent, M.; Avvaru, V.S.; Rodríguez, M.C.; Haranczyk, M.; Etacheri, V. High-rate and ultralong-life Mg-Li hybrid batteries based on highly pseudocapacitive dual-phase TiO2 nanosheet cathodes. J. Power Sources 2021, 506, 230118. [Google Scholar] [CrossRef]
- Ding, B.; Huang, X.N.; Cai, Z.F.; Ma, Y.Z.; Song, G.S.; Yang, W.D.; Wen, C. Effects of binders on electrochemical properties of high capacity silicon composite anodes. Inorg. Chem. Commun. 2020, 113, 107771. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, B.; Li, M.; Zheng, F.; Ma, Y.; Song, G.; Guan, X.; Cao, Y.; Wen, C. Synthesis and Performance of NaTi2(PO4)3/VGCF@C Anode Composite Material for Aqueous Sodium-Ion Batteries. Batteries 2023, 9, 265. https://doi.org/10.3390/batteries9050265
Ding B, Li M, Zheng F, Ma Y, Song G, Guan X, Cao Y, Wen C. Synthesis and Performance of NaTi2(PO4)3/VGCF@C Anode Composite Material for Aqueous Sodium-Ion Batteries. Batteries. 2023; 9(5):265. https://doi.org/10.3390/batteries9050265
Chicago/Turabian StyleDing, Bo, Mingzhu Li, Fuzhou Zheng, Yangzhou Ma, Guangsheng Song, Xiulong Guan, Yi Cao, and Cuie Wen. 2023. "Synthesis and Performance of NaTi2(PO4)3/VGCF@C Anode Composite Material for Aqueous Sodium-Ion Batteries" Batteries 9, no. 5: 265. https://doi.org/10.3390/batteries9050265





